Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6806
Peer-review started: May 16, 2023
First decision: August 4, 2023
Revised: August 17, 2023
Accepted: September 4, 2023
Article in press: September 4, 2023
Published online: October 6, 2023
Processing time: 131 Days and 23.8 Hours
In this paper, we present a 9-year-old boy who demonstrates a complex interplay between myopia progression, axial length (AL) extension, and retinal nerve fiber layer (RNFL) thickness loss in both eyes. Additionally, concurrent optic neuritis has directly impacted RNFL thickness in his right eye, and its potential indirect influence on RNFL and macular ganglion cell layer (mGCL) thickness in his left eye is also noteworthy.
A 9-year-old boy with bilateral myopia presented with diminished vision and pain in his right eye due to optic neuritis, while his left eye showed pseudopapilledema. Steroid therapy improved his vision in the right eye, and 16-mo follow-up revealed recovery without recurrence despite myopia progression. Follow-up optical coherence tomography conducted 16 mo later revealed a notable thinning of the RNFL in both eyes, especially along with a reduction in mGCL thickness in the left eye. This intricate interaction between optic neuritis, myopia, and retinal changes underscores the need for comprehensive mana
The progression of myopia and AL extension led to the loss of RNFL thickness in both eyes in a 9-year-old boy. Concurrently, optic neuritis directly affected RNFL thickness in his right eye and may indirectly play a role in the thickness of RNFL and mGCL in his left eye.
Core Tip: When evaluating retinal nerve fiber layer (RNFL) thickness in patients with optic neuritis and myopia, it is essential to consider both direct and indirect effects on the RNFL. Additionally, it is important to closely monitor changes in RNFL thickness over time, as it may be influenced by both myopia and optic neuritis. Further research is needed to better understand the relationship between these conditions and their impact on RNFL thickness.
- Citation: Zhao FF, Yao SQ, Wang Y, Li TP, Yang JF, Pang CP, Cen LP. Bilateral retinal nerve fiber layer thickness reduction in a 9-year-old myopic boy suffering from unilateral optic neuritis: A case report. World J Clin Cases 2023; 11(28): 6806-6811
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6806.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6806
Optical coherence tomography (OCT) imaging offers ophthalmologists a noninvasive technique to evaluate the thickness of retinal nerve fiber layer (RNFL) and macular ganglion cells (mGCL). It has been applied for both research and clinical purposes in evaluating disease progression in optic neuropathies. Thinning of RNFL has been demonstrated in glaucoma[1], optic neuritis, anterior ischemic optic neuropathy, multiple sclerosis, central nervous system diseases such as Parkinson’s disease[2], and systematic diseases involving hypertension[1]. Additionally, the diminishment of RNFL thickness has been detectable in myopic eyes, conceivably linked to elongation and thinning of the retina and sclera, thereby engendering the dispersion of nerve fibers across an expanded surface area[3]. Optic neuritis is an inflammatory process that causes demyelination of the optic nerve and damages the axon fibers, which can be measured objectively by RNFL thickness. It is speculated that RNFL thinning in optic neuritis may be due to both retrograde and anterograde neuroaxonal degeneration[4]. In this report, we present a 9-year-old boy with optic neuritis in the right eye, pseudopapilledema in the left eye, and moderate myopia in both eyes. Following steroid pulse therapy, a decrease in RNFL thickness was observed in both eyes during the 16-mo follow-up period.
Decreased right eye visual acuity with associated ocular pain upon eye movement persisting for 5 d.
Five days prior, the patient presented with a gradual decrease in vision in the right eye, accompanied by discomfort during eye movements. No specific triggering factors were identified. The patient did not exhibit symptoms of eye redness, sensitivity to light, or tearing. Seeking medical care at our institution, the patient had not undergone prior medical consultation or treatment.
Unremarkable.
A history of refractive error in both eyes.
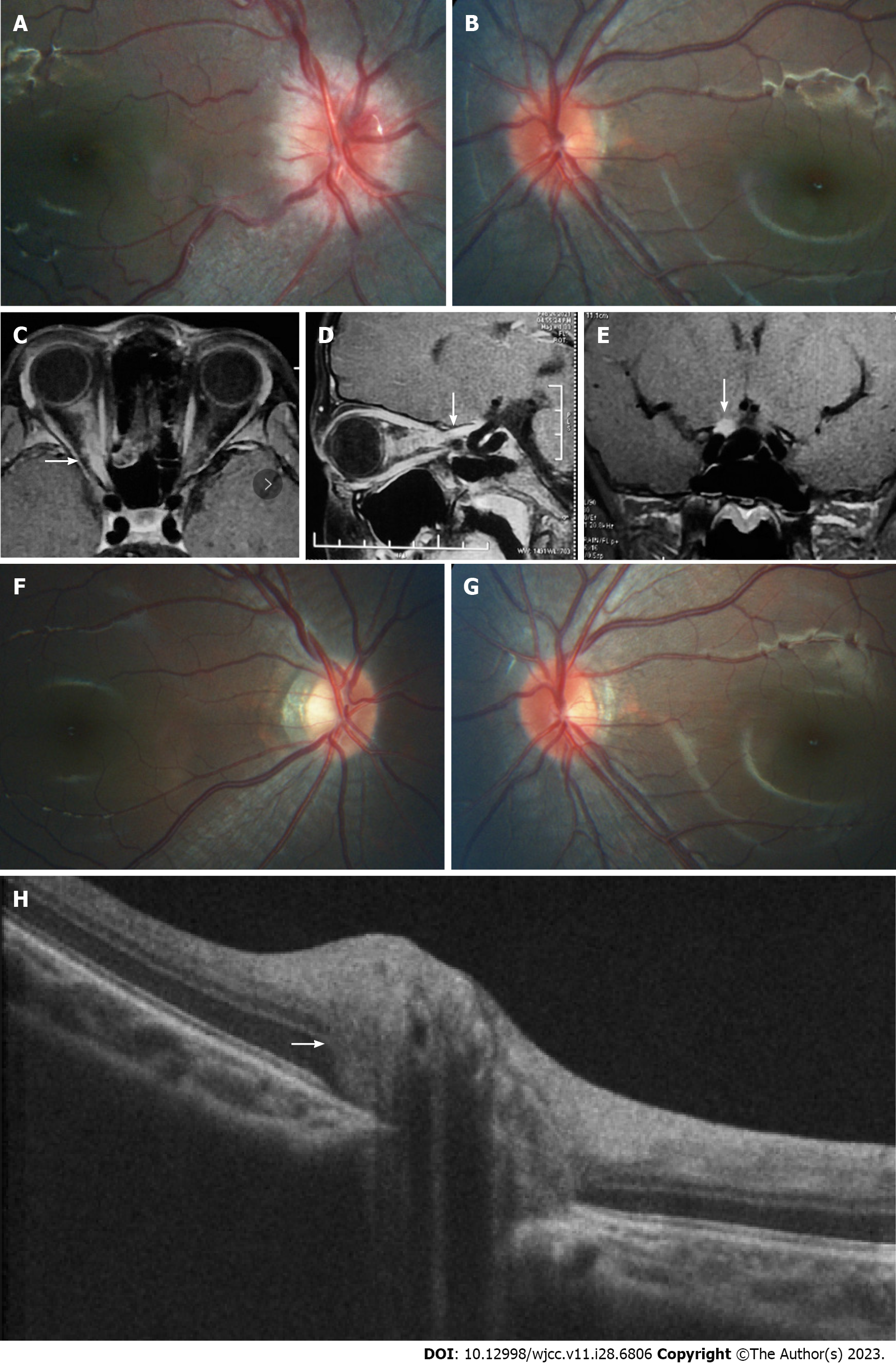
The patient had myopia in both eyes, -3.75D spherical equivalent (SE) in the right eye and -2.75D SE in the left eye. His medical history was otherwise unremarkable. At presentation, the best-corrected visual acuity (BCVA) was no light perception in the right eye with relative afferent pupillary defect and 1.0 (logarithmic visual acuity chart) in the left eye. Diffuse disc swelling and vascular tortuosity around the right optic disc, and ill-defined border of the reddish left optic disc accompanied by γ-zone peripapillary atrophy (PPA) without the optic cup were shown on fundus photography (Figure 1A and B).
The aquaporin-4 immunoglobulin and myelin oligodendrocyte glycoprotein immunoglobulin were seronegative.
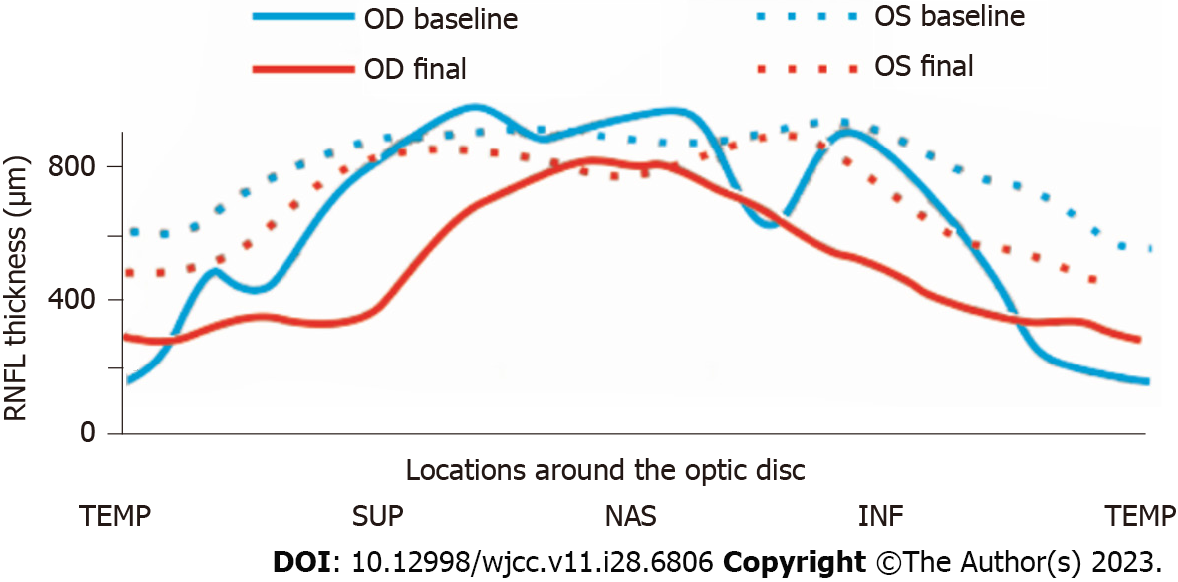
The average peripapillary RNFL thickness (Table 1 and Figure 2) was 157 μm in the right eye and 134 μm in the left eye, while the average mGCL thickness (Table 1) was 92 μm in the left eye. It was not possible to measure mGCL in the right eye due to the inability to fix vision caused by optic neuritis. Pattern visual evoked potential in the right eye failed to lead to a stable waveform but the P100 peak time and amplitude in the left eye were normal. Flash visual evoked potential in the right eye indicated delayed P2 peak and decreased P2 amplitude. Furthermore, orbital fat-suppression contrast-enhanced magnetic resonance imaging (Figure 1C-E) revealed enhancement and enlargement in the intraorbital, intracanal, and intracranial segments of the right optic nerve.
| Parameter | Right eye | Left eye | ||
| Baseline | Final | Baseline | Final | |
| BCVA | NLP | 1 | 1 | 1 |
| RNFL thickness (μm) | 157 | 74 | 134 | 106 |
| mGCL thickness (μm) | NA | 62 | 92 | 88 |
| Myopic degree (D) SE | -3.75 | -4.25 | -2.75 | -4 |
Optic neuritis in the right eye, pseudopapilledema in the left eye, and myopia in both eyes.
Steroid pulse therapy of intravenous methylprednisolone (IVMP) (20 mg/kg·d for 5 d, and halved every 1 d) was prescribed, followed by oral administration of 1 mg/kg·d prednisolone. The oral dosage was gradually tapered as the patient exhibited a favorable response to the therapeutic intervention.
A month later, the patient’s visual field returned to normal and his right BCVA improved to 1.0. Importantly, no recurrence was detected during the 16-mo follow-up period. However, the degree of myopia progressed (-4.25D SE in the right eye and -4.00D SE in the left eye) at the end of the follow-up, as well as the axial length (AL) (24.96 mm in the right eye and 24.92 mm in the left eye). The defined border of the pale right optic disc with γ-zone PPA and the ill-defined border of the reddish left optic disc accompanied by expanded γ-zone PPA without the optic cup were captured on Fundus photography (Figure 1F and G). The RNFL thickness (Table 1 and Figure 2) in the right eye was 74 μm (reduced by 157 μm) and 106 μm (reduced by 28 μm) in the left eye. Meanwhile, the left average mGCL thickness (Table 1) became thinner (88 μm, reduced by 4 μm). Enhanced depth imaging optical coherence tomography (Figure 1H) revealed a peripapillary hyperreflective ovoid mass-like structure in his left optic disc.
In this 9-year-old boy with optic neuritis in the right eye, pseudopapilledema in the left eye, and myopia in both eyes, the RNFL thickness of both eyes was reduced and myopia has progressed at the 16-mo follow-up. A significant negative correlation between RNFL thickness and deepened myopic degree has been reported in many studies[1,5,6]. The Orinda Longitudinal Study of Myopia in the United States has studied longitudinal AL data for myopic and emmetropic children and published the following equations as a function of age: Myopes up to 10.5 years: Axial Length = 18.144 + 2.391*ln(age); myopes after 10.5 years: Axial Length = 17.808 + 2.560*ln(age)[7]. Therefore, this boy’s AL might increase almost 0.55 mm according to the equations at the 16-mo follow-up. AL expansion, leading to elongation and thinning of the sclera and the retina that spread the nerve fibers over a larger surface area, could cause thinning of the RNFL in myopia[1,3]. The RNFL thickness has been reported to have decreased by 7 microns for every 1 mm of axial length, and 3 microns for every 1 Diopter sphere[8]. Moreover, the expansion of left γ-zone PPA might be significantly correlated with deepened myopic degree[9].
Certainly, it is evident that the extent of RNFL thinning in both eyes was influenced not solely by the progression of myopia and axial length elongation. As for his right eye, the RNFL thickness thinning after optic neuritis was also due to both retrograde and anterograde neuroaxonal degeneration caused by optic nerve injury[4,10,11]. In previous studies, OCT in vivo and the thinning of the RNFL had been speculated to be biomarkers of prior optic neuritis[11,12].
Simultaneously, we found that the average mGCL thickness became thinner in the patient’s contralateral unaffected left eye. It had been reported that AL expansion could cause enlargement of the γ-zone next to the optic nerve head, while the macular area was unaffected[13,14]. However, to date, there is no definitive evidence showing that the elongation of the AL could cause retinal ganglion cell (RGC) degeneration and axon fiber loss[1]. Furthermore, the patient’s left thinner average mGCL thickness was consistent with a recent report in which mGCL thickness decreased in the contralateral eye possibly by subclinical involvement[15]. The exact mechanism about subclinical involvement was not confirmed, but results of animal experiments[16,17] suggested that stress signals liberated by the damaged ret-ret RGC might cause a neurotoxic environment in the contralateral retina, the propagation of a glial reaction through the optic chiasm, a retrograde degeneration spreading from the deafferented retinorecipient areas in the brain, or even a systemic inflammatory response[16]. Meanwhile, ganglion cell axons comprise the nerve fiber layer of the retina and converge to form the optic nerve. It has been demonstrated that average RNFL thickness (per μm) was most strongly associated with average mGCL thickness and 1 μm thinner average RNFL was accompanied with 0.3 μm thinner average mGCL thickness[18]. Therefore, mGCL and RNFL would thin correspondingly after permanent injury of ganglion cells[19]. While the precise mechanism remains to be fully understood, our hypothesis is that the reduced thickness of mGCL and even RNFL thickness in his left eye could potentially be linked to the optic neuritis affecting his opposite (contralateral) eye.
The progression of myopia and AL extension led to the loss of RNFL thickness in both eyes in a 9 -year-old boy. Concurrently, optic neuritis directly affected RNFL thickness in his right eye and may indirectly play a role in the thickness of RNFL and mGCL in his left eye.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Ophthalmology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sotelo J, Mexico S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Du J, Du Y, Xue Y, Wang H, Li Y. Factors Associated with Changes in Peripapillary Retinal Nerve Fibre Layer Thickness in Healthy Myopic Eyes. J Ophthalmol. 2021;2021:3462004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Chiquita S, Rodrigues-Neves AC, Baptista FI, Carecho R, Moreira PI, Castelo-Branco M, Ambrósio AF. The Retina as a Window or Mirror of the Brain Changes Detected in Alzheimer's Disease: Critical Aspects to Unravel. Mol Neurobiol. 2019;56:5416-5435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Leung CK, Mohamed S, Leung KS, Cheung CY, Chan SL, Cheng DK, Lee AK, Leung GY, Rao SK, Lam DS. Retinal nerve fiber layer measurements in myopia: An optical coherence tomography study. Invest Ophthalmol Vis Sci. 2006;47:5171-5176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 245] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Pawlitzki M, Horbrügger M, Loewe K, Kaufmann J, Opfer R, Wagner M, Al-Nosairy KO, Meuth SG, Hoffmann MB, Schippling S. MS optic neuritis-induced long-term structural changes within the visual pathway. Neurol Neuroimmunol Neuroinflamm. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Hussein M, Ibrahim S, Taha A, Magdy R. The impact of error of refraction and retinal nerve fiber layer thickness on cognitive functions in adults with bilateral myopia. Int J Neurosci. 2023;133:290-295. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Zha Y, Zhuang J, Lin D, Feng W, Zheng H, Cai J. Evaluation of myopia on retinal nerve fiber layer thickness measured by Spectralis optical coherence tomography. Exp Ther Med. 2017;14:2716-2720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Jones LA, Mitchell GL, Mutti DO, Hayes JR, Moeschberger ML, Zadnik K. Comparison of ocular component growth curves among refractive error groups in children. Invest Ophthalmol Vis Sci. 2005;46:2317-2327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Rauscher FM, Sekhon N, Feuer WJ, Budenz DL. Myopia affects retinal nerve fiber layer measurements as determined by optical coherence tomography. J Glaucoma. 2009;18:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Cheng D, Ruan K, Wu M, Qiao Y, Gao W, Lian H, Shen M, Bao F, Yang Y, Zhu J, Huang H, Meng X, Shen L, Ye Y. Characteristics of the Optic Nerve Head in Myopic Eyes Using Swept-Source Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 2022;63:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 10. | Costello F, Pan YI, Yeh EA, Hodge W, Burton JM, Kardon R. The temporal evolution of structural and functional measures after acute optic neuritis. J Neurol Neurosurg Psychiatry. 2015;86:1369-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Kupersmith MJ, Garvin MK, Wang JK, Durbin M, Kardon R. Retinal ganglion cell layer thinning within one month of presentation for optic neuritis. Mult Scler. 2016;22:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Di Maggio G, Santangelo R, Guerrieri S, Bianco M, Ferrari L, Medaglini S, Rodegher M, Colombo B, Moiola L, Chieffo R, Del Carro U, Martinelli V, Comi G, Leocani L. Optical coherence tomography and visual evoked potentials: which is more sensitive in multiple sclerosis? Mult Scler. 2014;20:1342-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Jonas JB, Wang YX, Zhang Q, Liu Y, Xu L, Wei WB. Macular Bruch's Membrane Length and Axial Length. The Beijing Eye Study. PLoS One. 2015;10:e0136833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Jonas RA, Wang YX, Yang H, Li JJ, Xu L, Panda-Jonas S, Jonas JB. Optic Disc-Fovea Distance, Axial Length and Parapapillary Zones. The Beijing Eye Study 2011. PLoS One. 2015;10:e0138701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Wicki CA, Manogaran P, Simic T, Hanson JVM, Schippling S. Bilateral retinal pathology following a first-ever clinical episode of autoimmune optic neuritis. Neurol Neuroimmunol Neuroinflamm. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Lucas-Ruiz F, Galindo-Romero C, Albaladejo-García V, Vidal-Sanz M, Agudo-Barriuso M. Mechanisms implicated in the contralateral effect in the central nervous system after unilateral injury: focus on the visual system. Neural Regen Res. 2021;16:2125-2131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Cen LP, Han M, Zhou L, Tan L, Liang JJ, Pang CP, Zhang M. Bilateral retinal microglial response to unilateral optic nerve transection in rats. Neuroscience. 2015;311:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Tham YC, Cheung CY, Koh VT, Cheng CY, Sidhartha E, Strouthidis NG, Wong TY, Aung T. Relationship between ganglion cell-inner plexiform layer and optic disc/retinal nerve fibre layer parameters in non-glaucomatous eyes. Br J Ophthalmol. 2013;97:1592-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | De Lott LB, Bennett JL, Costello F. The changing landscape of optic neuritis: a narrative review. J Neurol. 2022;269:111-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |