Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6797
Peer-review started: July 22, 2023
First decision: August 9, 2023
Revised: August 18, 2023
Accepted: September 4, 2023
Article in press: September 4, 2023
Published online: October 6, 2023
Processing time: 64 Days and 19.9 Hours
Gastrointestinal stromal tumors (GISTs) are rare tumors of the gastrointestinal tract accounting for less than 1% of all gut tumors. GISTs occurring in the rectum are extremely rare, and these usually present at an advanced stage compared with other sites.
A 60-year-old male who presented with features of sensations of rectal tenesmus was referred to our department with a mass in the lower rectum that was detected during a routine checkup. Colonoscopy, transrectal ultrasound, perianal magnetic resonance imaging and ultrasonic contrast were used to diagnose the rectum GIST, and then the patient underwent complete transanal resection using the ultrasonic scalpel. The patient was discharged ten days after the operation and was defined as low risk. Therefore, he had no need to receive subsequent adjuvant therapies, and he had not suffered any anal dysfunction or had any evidence of recurrence at follow up.
Surgical resection with histologically negative margins is the standard curative treatment for rectal GISTs. Appropriate surgical techniques based on the location, size, and resectability of the tumor should attract great attention from clinicians.
Core Tip: Gastrointestinal stromal tumors are rare malignancies that rarely occur in the rectum. The diagnosis is established by biopsy and immunohistochemistry. Complete surgical resection with negative margins is the treatment of choice. The appropriate surgical technique should be selected based on the location, size, and resectability of the tumor and the available surgical expertise. This case is reported for its rare location and treatment with ultrasonic scalpel total resection without imatinib treatment.
- Citation: Dong RX, Wang C, Zhou H, Yin HQ, Liu Y, Liang HT, Pan YB, Wang JW, Cao YQ. Rare rectal gastrointestinal stromal tumor case: A case report and review of the literature. World J Clin Cases 2023; 11(28): 6797-6805
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6797.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6797
Gastrointestinal stromal tumors (GISTs) are the most common type of mesenchymal tumor involving the gastrointestinal tract. However, GISTs represent a rare entity that accounts for less than 1% of all alimentary tract tumors[1]. The most common sites involved are the stomach (50%–65%), followed by the small intestine (20%–30%)[2]. Esophageal GISTs are rare, but tumors may also arise in extra-gastrointestinal locations, principally in the mesentery, omentum, and retroperitoneum[3]. GISTs less frequently affect the colon and rectum, with an estimated incidence of 5%–15%[4,5]. Rectal GISTs are extremely rare with low incidence, only accounting for merely 0.1% of all kinds of rectal tumors[6]. Different surgical methods have been widely reported, including transanal excision, laparoscopic surgery, transsacral excision, and transanal endoscopic microsurgery[7-10]. Sometimes, rectal GISTs, especially those located in the lower rectum, are detected during daily physical examination.
Here, we present a case of a rectal GIST with complete resection by ultrasonic scalpel to highlight the clinical and surgical features of lower rectal GISTs.
A 60-year-old male rectal GIST patient presented with features of sensitization of the rectum with tenesmus.
The patient was referred to our department with a mass in the lower rectum that was detected during a routine checkup at two months prior. The patient had undergone a colonoscopy two months prior, which incidentally discovered a mass in the rectal submucosa (Figure 1).
The patient had a clinical history of hypertension and complete right bundle branch block, but ultrasonic cardiogram and blood tests were normal.
The patient denied any history of genetic disease or infection.
Regular physical examination showed no obvious abnormalities, except on the anorectal physical examination (inspection, palpation, digital rectal examination, careful probing, and anoscopy). A submucosal mass was found in the anterosuperior region without signs of macroscopic rectal bleeding.
Regular preoperative examination, including routine blood work, liver and kidney function tests, coagulation function and electrocardiography were normal, and biotumoural findings such as carcino-embryonic antigen, carbohydrate antigen-724, carbohydrate antigen-199, squamous cell carcinoma, carbohydrate antigen-211, carbohydrate antigen-50, carbohydrate antigen-242, alpha fetoprotein, and neuron-specific enolase were all negative.
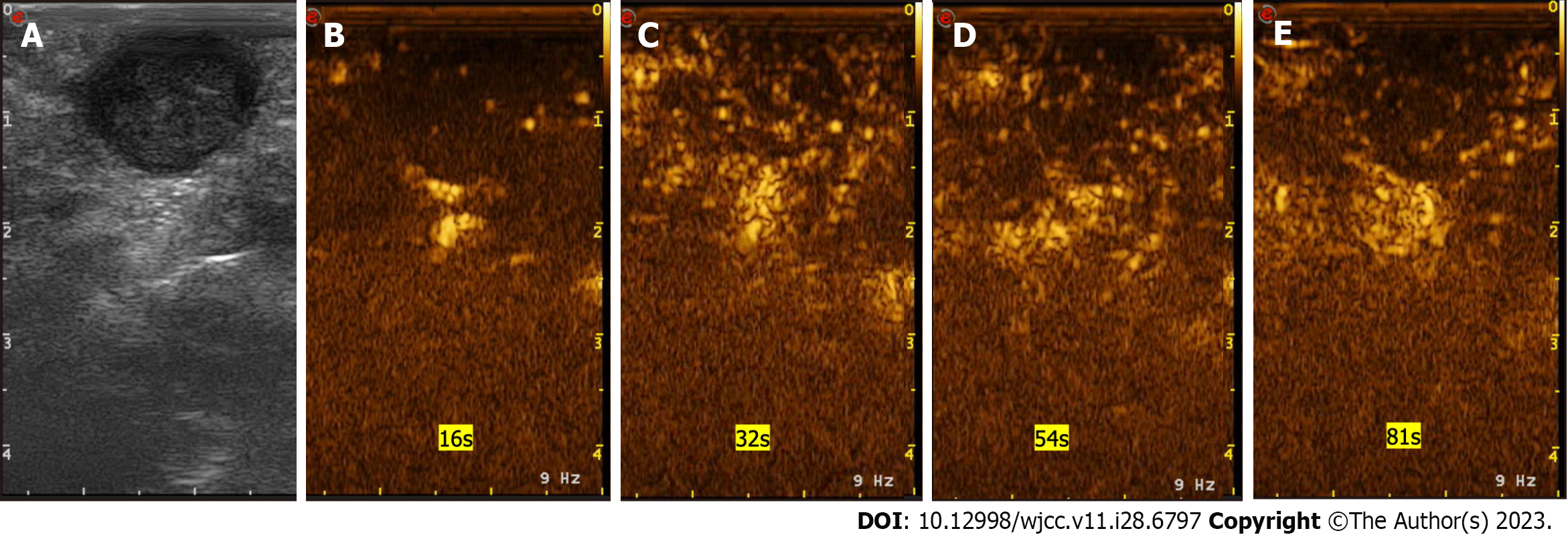
Colonoscopy (Figure 2A), transrectal ultrasound (Figure 2B), ultrasonic contrast (Figure 3) and perianal magnetic resonance imaging (MRI) (Figure 2C and D) showed that the mass involved the muscularis propria and a characteristic appearance of a well-circumscribed intramural mass involving the anterior and superior sides of the rectum located 3 cm from the anal verge without metastatic disease.
According to the results of colonoscopy, transrectal ultrasound, ultrasonic contrast, MRI and histopathological examination, the patient was eventually diagnosed with a rectal GIST.
Based on these examinations and the unusual location of this tumor, we consulted with the radiologist to evaluate whether a transanal resection could intactly excise the tumor with minimal possible surgical complications, such as incontinence of feces or anal sphincter dysfunction. The patient accepted our proposed surgery plan. Under intraspinal anaesthesia, he was required to be in the lithotomy position, and we injected 0.9% saline into the rectum submucosa for full exposure. We used an ultrasonic scalpel to incise the rectum mucosa and then separated the surrounding tissue until the submucosa was reached. During the separation, we were very careful to maintain the tumor capsule integrity. After fully exposing the tumor, we fortunately observed that the rectal GIST was centrally located in the rectum submucosa and not wrapped by the rectal muscle. We incised the rectum mucous membrane and completely separated the tumor from the submucous stratum of the rectum and then resected the intact tumor with an ultrasonic scalpel. The incision in the rectal muscle was designed to be as small as possible to maintain the integrity of the rectal mucosa. Then, we placed mattress sutures within the incision in the muscular layer of the rectum (Figure 4).
The surgical procedure lasted only approximately 20 min, and the blood loss was insignificant. No postoperative complications were observed during hospitalization. A full liquid diet was taken for five days to delay bowel movements and prevent wound infection. The first defecation occurred on the 5 d after the surgery, and the patient was discharged on the tenth postoperative day.
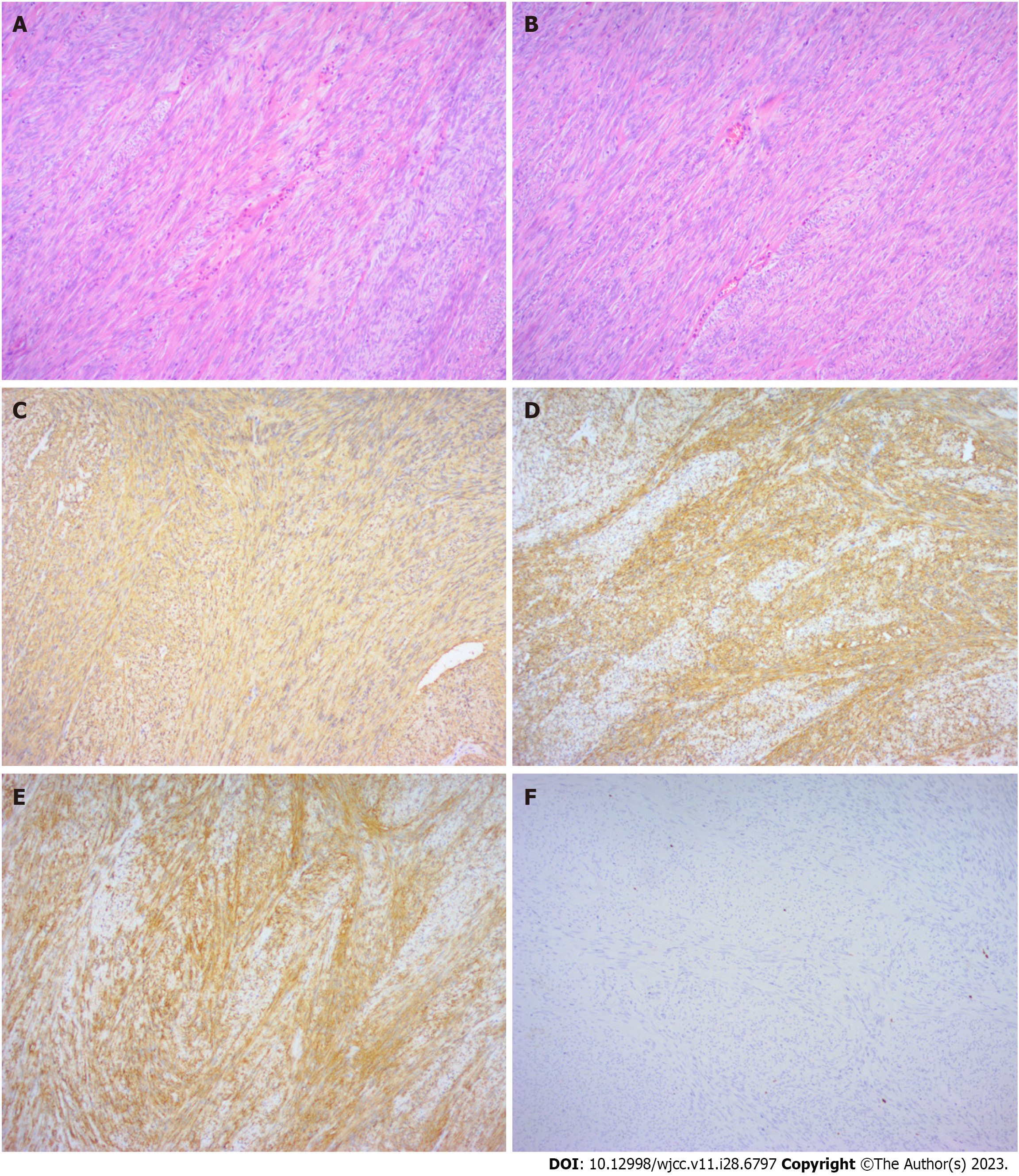
Final pathology reported a tumor of 2 cm × 1.5 cm × 1.5 cm with a complete capsule, fibrous and adipose tissue attached, and tumor-free margins. Histological examination (Figure 5A and B) confirmed a fusocelular subtype of rectal GIST. Its cellularity was low, and its mitotic index [number of mitoses per 50 high-power field (HPF)] was less than 5/50 HPF with areas of necrosis and calcification. Immunohistochemistry (IHC) demonstrated the diagnosis of GIST when the tumor was positive for CD 34 (Figure 5C), CD117 (Figure 5D), Dog-1 (Figure 5E), and Ki67 expressed approximately 3% (Figure 5F) and negative for S-100, HMB 45 (human melanoma black 45), and α-SMA (smooth muscle actin).
The recurrence risk assessment system for the primary GIST after complete resection was based on the modified National Institutes of Health classification system (2008). This patient was defined as low risk. Therefore, he had no need to receive subsequent adjuvant therapies such as imatinib treatment after surgery, and he had not suffered any anal dysfunction or evidence of recurrence at follow-up 3 months after surgery.
GISTs were first described by Mazur and Clark[11] in 1983. Rectal GISTs are usually found incidentally, either on lower abdominal computed tomography (CT), perianal MRI, colonoscopy, or clinical examination. Low-lying GISTs usually palpate as a smooth, well-demarcated, firm lump during physical examination. For patients with a suspected rectal GIST, CT scan with contrast is often the initial imaging modality of choice. It seems to be a reliable technique for the diagnosis and the stage of the disease. GISTs are believed to arise from the interstitial cells of Cajal or other pluripotential mesenchymal stem cells[12]. The morphology of the cells and the findings of IHC are the major factors for the pathological diagnosis of GIST. Regarding microscopic features, there are three types: the spindle cell type (70%), epithelioid cell type (20%), and mixed type (10%). Regarding immunohistochemical findings, 95% of GISTs are positive for CD117 and/or discovered on Dog-1, and 70% are found to be positive for CD34[13]. Although CD117 positivity is a major defining feature for a tumor that has morphological features compatible with GIST, CD117 positivity alone is not an absolute requirement for the diagnosis. When there is CD117 negativity, as in approximately 5% of GISTs, Dog-1 staining, followed by CD34 staining, is considered diagnostic.
The mitotic index, the size of tumor, the location of tumor (gastric vs. nongastric), and the tumor rupture are the four independent prognostic factors for GISTs. Additionally, tumor rupture should be considered separately with regard to whether it occurred in preoperative and intraoperative. Surgery is still considered the only treatment modality that can offer a permanent cure for GIST. There are many risk-stratification tools, for example, the 2017 American Joint Committee on Cancer recommendations on staging of rectal GISTs include both tumor size (≤ 2 cm,2-5 cm, 5-10 cm, and >10 cm) and mitotic rate (≤ 5 mitoses or > 5 mitoses per 50 high-power field) to help determine rates of disease progression[14]. For those GISTs that are inoperable, metastatic, or recurrent, imatinib mesylate was thought to be the first-line standard treatment. After accurate and complete resection, surgery is sufficient for approximately 60% of GIST patients. However, for the remaining 40% of patients with relapses, metastases or local advancement to the point where surgery may not be effective, additional targeted therapy with the tyrosine kinase inhibitor imatinib mesylate may be prescribed[15]. In rectal GISTs, radical resection is thought to be the most important determinant of prognosis[16]. However, since certain anatomical characteristics, such as a narrow, deep pelvis and the vicinity to the internal and external sphincters or invasions of the adjacent organs, there are many difficulties in surgical resection[17]. Although surgical resection without lymph node resection is still the most standard treatment for local rectal GISTs, the selection of both safe and feasible surgical procedures for rectal GISTs remains controversial[18-20]. Local resection, laparoscopic surgery, and endoscopic resection are the three mainstream surgical methods for rectal GISTs[21]. Local resection, including transanal, transacral, parasacral, perineal, and transvaginal resection, not only achieve a good clinical curative effect but aviod the abnormal appearance and dysfunction of genitourinary and anal functions[22]. However, traditional transanal local excision has limitations. Because of poor visualization, conventional transanal local excision may have difficulty controlling resection limits and allow for complete removal of the rectal wall. The conventional transanal approach is also less oncologically complete, with inherently higher recurrence rates, higher rates of tumor remnants, specimen fragmentation, and inferior overall and disease-free survival compared with radical resection[23]. The choice of rectal GISTs surgical approach should be individualized according to their location and different size of the tumor, range of local invasion, ability for sphincter preservation, and risk of malignant degeneration. The objective of surgical methods for rectal GISTs is not only to improve prognosis but also to preserve the patient’s quality of life. Therefore, in this case, we used an ultrasonic scalpel instead of excision.
The ultrasonically activated scalpel, also known as the ultrasonic scalpel, was first introduced in the early 1990s and works by generating the high-frequency harmonic motion of a metallic rod and allows for the denaturation of tissue proteins, thus producing a protein coagulum that seals coapted vessel walls[24]. These vibrations can simultaneously coagulate and cut tissues[25]. Unlike electrocoagulation, ultrasonic scalpels not only lack eschar formation over the blade and cause minimal collateral thermal damage to the surrounding tissues and less smoke[26] but also provide very effective cutting with less bleeding. There are neither transmission of electricity nor neuromuscular stimulation to patients[27].
The time spent in the operating room is expensive, and this technique can decrease both the operation time and associated costs and seems to reduce hospital costs[28]. Compared with conventional energy systems, multiple studies have demonstrated that there are many superiorities of the ultrasonic scalpel, especially in several surgical subspecialties[29-31]. The most valuable benefits were considered to be a shorter operation time and less intraoperative bleeding[32-33]. Recent studies confirmed that in settings with financial restrictions such as those encountered in low-resource countries, ultrasonic scalpels can be reused safely without any consequences to the patient’s condition or postoperative course[34]. Because the ultrasonic scalpel could cut and coagulate effectively, numerous energy exchanges between the scalpel and cautery could be eliminated. Therefore, it is extremely beneficial for surgeries in which required accurate dissection and the avoidance of issue damage from lateral heat damage, especially in anorectal surgery.
GISTs are rare malignancies that rarely occur in the rectum. The diagnosis of GISTs mainly relies on biopsy and IHC. Complete tumor excision with negative margins is often the ideal method in the clinical treatment of GISTs. Appropriate operative procedures are hard to select because accurate detection based on the location, size, and resectability of the tumor and the available surgical expertise are very difficult. This case is reported for its rare location and treatment with ultrasonic scalpel total resection without imatinib treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sano W, Japan; Tuncyurek O, Cyprus S-Editor: Lin C L-Editor: A P-Editor: Zhang XD
| 1. | Gastrointestinal Stromal Tumors Treatment (Adult) (PDQ®): Patient Version. 2020 Aug 28. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002–. [PubMed] |
| 2. | Grassi N, Cipolla C, Torcivia A, Mandala S, Graceffa G, Bottino A, Latteri F. Gastrointestinal stromal tumour of the rectum: report of a case and review of literature. World J Gastroenterol. 2008;14:1302-1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Steele SR, Hull TL, Read TE, Saclarides TJ, Senagore AJ, Whitlow CB, editor. The ASCRS Textbook of Colon and Rectal Surgery. Cham: Springer, 2016. [DOI] [Full Text] |
| 4. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1176] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 5. | Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 408] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Baik SH, Kim NK, Lee CH, Lee KY, Sohn SK, Cho CH, Kim H, Pyo HR, Rha SY, Chung HC. Gastrointestinal stromal tumor of the rectum: an analysis of seven cases. Surg Today. 2007;37:455-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Centonze D, Pulvirenti E, Pulvirenti D'Urso A, Franco S, Cinardi N, Giannone G. Local excision with adjuvant imatinib therapy for anorectal gastrointestinal stromal tumors. Tech Coloproctol. 2013;17:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Quaresima S, Balla A, Franceschilli L, La Torre M, Iafrate C, Shalaby M, Di Lorenzo N, Sileri P. Transanal Minimally Invasive Surgery for Rectal Lesions. JSLS. 2016;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Kyo K, Azuma M, Okamoto K, Nishiyama M, Shimamura T, Maema A, Kanamaru H, Shirakawa M, Nakamura T, Shinmura K, Koda K, Yokoyama H. Neoadjuvant imatinib treatment and laparoscopic anus-preserving surgery for a large gastrointestinal stromal tumor of the rectum. World J Surg Oncol. 2016;14:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Gervaz P, Huber O, Bucher P, Sappino P, Morel P. Trans-sacral (Kraske) approach for gastrointestinal stromal tumour of the lower rectum: old procedure for a new disease. Colorectal Dis. 2008;10:951-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 560] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3110] [Article Influence: 115.2] [Reference Citation Analysis (0)] |
| 13. | Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 320] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 14. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6445] [Article Influence: 429.7] [Reference Citation Analysis (0)] |
| 15. | Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 671] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 16. | Ibrahim A, Chopra S. Succinate Dehydrogenase-Deficient Gastrointestinal Stromal Tumours. Arch Pathol Lab Med. 2020;144:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Qu H, Xu Z, Ren Y, Gong Z, Ju RH, Zhang F, Kang H, Xu Y, Chen X. Recent Advancements in the Treatment of Rectal Gastrointestinal Stromal Tumour: In Era of Imatinib. Cancer Manag Res. 2022;14:1141-1152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Keller DS, Tahilramani RN, Flores-Gonzalez JR, Mahmood A, Haas EM. Transanal Minimally Invasive Surgery: Review of Indications and Outcomes from 75 Consecutive Patients. J Am Coll Surg. 2016;222:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Liu Q, Zhong G, Zhou W, Lin G. Initial application of transanal endoscopic microsurgery for high-risk lower rectal gastrointestinal stromal tumor after imatinib mesylate neoadjuvant chemotherapy: A case report. Medicine (Baltimore). 2017;96:e7538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Marcella C, Shi RH, Sarwar S. Clinical Overview of GIST and Its Latest Management by Endoscopic Resection in Upper GI: A Literature Review. Gastroenterol Res Pract. 2018;2018:6864256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Khan SI, O'Sullivan NJ, Temperley HC, Rausa E, Mehigan BJ, McCormick P, Larkin JO, Kavanagh DO, Kelly ME. Gastrointestinal Stromal Tumours (GIST) of the Rectum: A Systematic Review and Meta-Analysis. Curr Oncol. 2022;30:416-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 22. | Kane WJ, Friel CM. Diagnosis and Treatment of Rectal Gastrointestinal Stromal Tumours. Dis Colon Rectum. 2019;62:537-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Spinelli A, Carvello M, Sacchi M, Bonifacio C, Bertuzzi A, Tuynman J, Montorsi M, Foppa C. Transanal minimally invasive surgery (TAMIS) for anterior rectal GIST. Tech Coloproctol. 2019;23:501-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Sista F, Abruzzese V, Schietroma M, Cecilia EM, Mattei A, Amicucci G. New harmonic scalpel versus conventional hemostasis in right colon surgery: a prospective randomized controlled clinical trial. Dig Surg. 2013;30:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Hodgson WJ, Poddar PK, Mencer EJ, Williams J, Drew M, McElhinney AJ. Evaluation of ultrasonically powered instruments in the laboratory and in the clinical setting. Am J Gastroenterol. 1979;72:133-140. [PubMed] |
| 26. | Lee YJ, Kim HY, Han HH, Moon SH, Byeon JH, Rhie JW, Ahn ST, Oh DY. Comparison of dissection with harmonic scalpel and conventional bipolar electrocautery in deep inferior epigastric perforator flap surgery: A consecutive cohort study. J Plast Reconstr Aesthet Surg. 2017;70:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Roye GD, Monchik J, Amaral JF. Endoscopic Adrenalectomy Using Ultrasonic Cutting and Coagulating. Surg Technol Int. 2000;IX:129-138. [PubMed] |
| 28. | Kim J, Shin Y, Jeong W. Harmonic scalpels compared with electrocautery in reconstructive flap harvesting: A meta-analysis. Microsurgery. 2022;42:89-96. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Yu K, Li H, Xue P, Xie Z, Tang M, He H, Wu J. Modified ultrasound scalpel haemorrhoidectomy versus conventional haemorrhoidectomy for mixed haemorrhoids: a study protocol for a single-blind randomised controlled trial. Trials. 2023;24:140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Tsai SHL, Chang CW, Lin TY, Wang YC, Wong CB, Ghaith AK, Alvi MA, Fu TS, Bydon M. The Use of Ultrasonic Bone Scalpel (UBS) in Unilateral Biportal Endoscopic Spine Surgery (UBESS): Technical Notes and Outcomes. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Jurlina M, Pupić-Bakrač J, Pupić-Bakrač A. Harmonic Scalpel-Assisted Endonasal Endoscopic Resection of the Vascular Tumors of the Anterior Skull Base. J Craniofac Surg. 2023;34:e296-e298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Lang BH, Ng SH, Lau LL, Cowling BJ, Wong KP. A systematic review and meta-analysis comparing the efficacy and surgical outcomes of total thyroidectomy between harmonic scalpel versus ligasure. Ann Surg Oncol. 2013;20:1918-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Fitz-Gerald AL, Tan J, Chan KW, Polyakov A, Edwards GN, Najjar H, Tsaltas J, Vollenhoven B. Comparison of ultrasonic shears and traditional suture ligature for vaginal hysterectomy: randomized controlled trial. J Minim Invasive Gynecol. 2013;20:853-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Mihanović J, Šikić NL, Mrklić I, Katušić Z, Karlo R, Jukić M, Jerončić A, Pogorelić Z. Comparison of new versus reused Harmonic scalpel performance in laparoscopic appendectomy in patients with acute appendicitis-a randomized clinical trial. Langenbecks Arch Surg. 2021;406:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |