Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6782
Peer-review started: April 20, 2023
First decision: June 12, 2023
Revised: July 18, 2023
Accepted: September 12, 2023
Article in press: September 12, 2023
Published online: October 6, 2023
Processing time: 157 Days and 11.4 Hours
Ewing sarcoma (ES) is a malignant neoplasm of neuroectodermal origin and is commonly observed in children and young adults. The musculoskeletal system is the main body system impacted and ES is rarely seen in the visceral organs particularly the adrenal gland.
To present a comprehensive review of primary adrenal ES, with emphasis on diagnosis, therapy and oncological outcomes.
A systematic review of the literature was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses 2020. PubMed/ MEDLINE, EMBASE and Google Scholar bibliographic databases were searched to identify articles from 1989 to 2022 and included patients with ES/primitive neuroectodermal tumor (PNET) of the adrenal gland. PubMed, Google Scholar and EMBASE medical databases were searched, combining the terms “adrenal”, “ES” and “PNET”. Demographic, clinical, pathological and oncological data of patients were analyzed by SPSS version 29.0.
A total of 52 studies were included for review (47 case reports and 5 case series) with 66 patients reported to have primary adrenal ES. Mean age at diagnosis was 26.4 ± 15.4 years (37.9% males, 57.6% females, sex not reported in 3 cases). The most frequent complaint was abdominal/flank pain or discomfort (46.4%) followed by a palpable mass (25.0%), and the average duration of symptoms was 2.6 ± 3.1 mo. The imaging modality of choice was computed tomography scan (81.5%), followed by magnetic resonance imaging (20.4%). Preoperative staging revealed that 17 tumors (27.9%) were metastatic and 14 patients had inferior vena cava or renal vein neoplastic thrombus at initial diagnosis. Open adrenalectomy was performed in the majority of cases (80.0%), of which 27.9% required more extensive resection. Minimally invasive surgery was attempted in 8.2% of tumors. Complete surgical resection was achieved in 89.4% of the patients. Adjuvant therapy was administered to 32 patients, in the form of chemotherapy (62.5%), radiotherapy (3.1%) or combination (34.4%). Median overall survival was 15 mo and 24-mo overall survival was 40.5%. Median disease-free survival was 10 mo and 24-mo disease-free survival was 33.3%.
The significant progress in molecular biology and genetics of ES does not reflect on patient outcomes. ES remains an aggressive tumor with a poor prognosis and high mortality.
Core Tip: Primary adrenal Ewing sarcoma (ES) is uncommon with 66 cases reported in the literature since 1989. Patients usually present within the first year from the initiation of symptoms, most frequently complaining of abdominal/flank pain or discomfort, followed by a palpable mass. Open adrenalectomy is the procedure of choice, while minimally invasive techniques are anticipated to be performed more frequently in the future. Regardless of the technique, surgical resection is achieved in the vast majority of cases. The significant progress in molecular biology and genetics of ES over the past decade does not reflect on patient outcomes. ES remains an aggressive tumor with a poor prognosis and high mortality.
- Citation: Manatakis DK, Tsouknidas I, Mylonakis E, Tasis N, Antonopoulou MI, Acheimastos V, Mastoropoulou A, Korkolis DP. Primary adrenal Ewing sarcoma: A systematic review of the literature. World J Clin Cases 2023; 11(28): 6782-6791
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6782.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6782
First described by James Ewing in 1921 as “diffuse endothelioma of bone,” Ewing sarcoma (ES) is the second most common primary bone malignancy during childhood and adolescence[1,2]. Along with primitive neuroectodermal tumors (PNET) and Askin tumors, ESs belong to the ES family of tumors (ESFT). The ESFT are aggressive childhood cancers, which histologically belong to the small round blue cell sarcomas[3].
Although the cell of origin of ES remains unknown, ESFT are characterized by chromosomal translocation between the TET/FET family genes and E26 transformation specific family genes[4]. Approximately 85% of ESs exhibit the reciprocal translocation t (11; 22) (q24; q12). This genetic alteration results in the chimeric fusion protein EWS RNA binding protein 1-Friend leukemia integration 1 (EWSR1-FLI1), which allows the N-terminus of EWSR1 and the C-terminus of FLI1 to bind transcriptional complexes and target genes. The target genes are involved in tumor cell immortalization, angiogenesis, cancer stemness, tumor growth, chemotherapy resistance, transcriptional regulation, and cell-to-cell signaling. NR0B1 (DAX1), GLI1 and FOXO1 have been shown in the literature to be the genes involved in the tumorigenesis of ES[4].
The majority of ES/PNET tumors are found in the skeletal system, with only 10%-20% arising in extra-skeletal sites[5]. Visceral and particularly adrenal occurrence is even more infrequent. The morphology between ESFT may vary; however, skeletal and extra-skeletal ESFT are molecularly indistinguishable[6]. Despite recent advances in molecular biology, therapeutic protocols are not standardized due to limited knowledge and are derived from other types of sarcomas. The aim of the current study is to present an up-to-date, systematic review of the literature on primary adrenal ES/PNET, with emphasis on diagnosis, therapy and oncological outcomes.
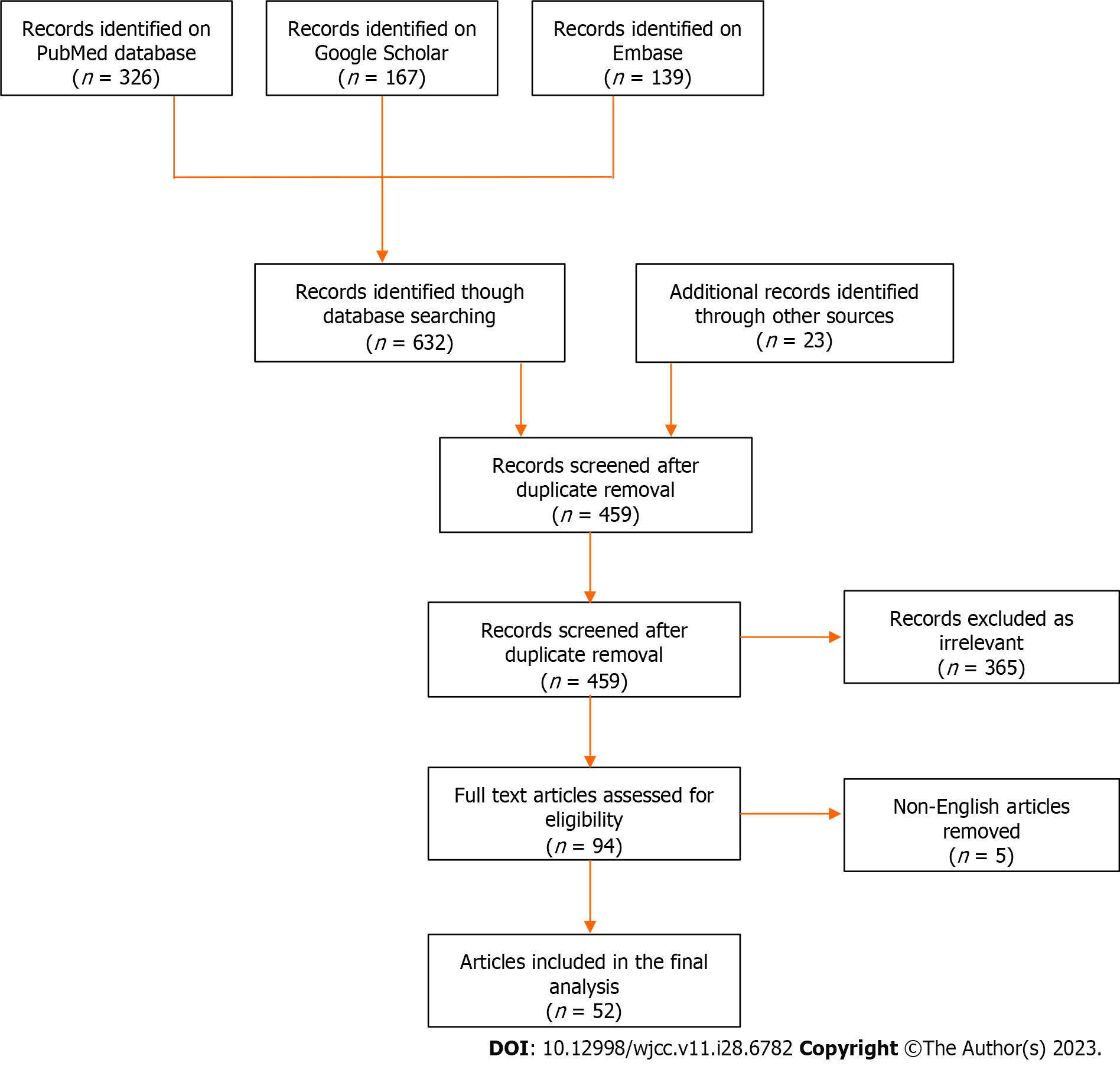
A systematic review of the English literature was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses 2020 statement[7]. PubMed/MEDLINE, EMBASE and Google Scholar bibliographic databases were searched to identify patients with ES/PNET of the adrenal gland from 1989 to 2022. The keywords “Ewing sarcoma”, “primitive neuroectodermal tumor”, “PNET” and “adrenal” were used in all possible combinations. Furthermore, the reference lists of all eligible papers were assessed for additional articles.
All study designs except conference abstracts and commentaries were considered eligible. Both adult and pediatric cases were included in the review. Non-English articles were excluded. Titles and abstracts of all articles from the initial search were independently screened by two authors. Articles, including case reports, case series, observational and clinical trial studies, were considered eligible for full text review, as long as they reported on cases of primary ES/PNET. Any discrepancies were arbitrated by all authors. A flow chart of the search strategy is shown in Figure 1.
For each eligible patient, demographic data (age, sex), clinical characteristics (presenting symptom, duration of symptoms, hormone hypersecretion, imaging modalities, size, laterality), treatment (preoperative staging, neoadjuvant treatment, extent of surgery, adjuvant treatment), histopathology (completeness of resection, immunohistochemistry, molecular testing) and oncological outcomes (overall and disease-free survival) were collected.
Statistical analysis was performed by SPSS version 29.0. All data were tabulated and outcomes were cumulatively analyzed. Continuous variables were expressed as mean±standard deviation, while categorical variables were expressed as frequencies or percentages. Kaplan-Meier survival analysis was applied to calculate 24-mo overall and disease-free survival. A descriptive approach was followed, due to limited data.
Fifty-two studies (47 case reports and 5 case series) were included in the final analysis, describing a total of 66 patients (Supplementary Table 1)[8-59]. The demographic and clinical characteristics are shown in Table 1. As shown in Figure 2, a majority of the reported cases were from China, United States and India. Twenty-five patients were males (37.9%) and 38 were females (57.6%) (sex not reported in 3 cases). Mean age at diagnosis was 26.4 ± 15.4 years (range 2-74). The most frequent complaint was abdominal/flank pain or discomfort (26 cases, 46.4%) followed by a palpable mass (14 cases, 25.0%). In 10 patients (17.9%), the tumor was an incidental finding during imaging studies for an unrelated reason. Mean duration of symptoms was 2.6 ± 3.1 mo (range 2 d to 1 year). Preoperative hormone panels were reported in 42 patients and hormonal hypersecretion was observed in 5 cases (11.9%). The imaging modality of choice was computed tomography (CT) scan (44 cases, 81.5%), followed by magnetic resonance imaging (MRI) (11 cases, 20.4%). Further imaging with positron emission tomography-CT (PET-CT) was required in 6 cases (11.1%) (data not reported in 12 cases). Mean tumor size at diagnosis was 11.4 ± 4.8 cm (range 3-24 cm). Twenty-seven patients had right-sided tumors, 28 patients had left-sided tumors (48.2% and 50.0%, respectively), and one patient had bilateral tumors (1.8%) (data not reported in 10 cases).
| Number of patients | Percentage (%) | |
| Gender | ||
| Male | 25 | 37.9 |
| Female | 38 | 57.6 |
| Not reported | 3 | 4.5 |
| Age (yr) | 26.4 ± 15.4 (range 2-74) | |
| Presentation | Reported in 56 patients | |
| Abdominal/flank pain | 26 | 46.4 |
| Palpable mass | 14 | 25.0 |
| Incidentaloma | 10 | 17.9 |
| Mean duration of symptoms | 2.6 ± 3.1 mo (range 2-365 d) | |
| Hormone hypersecretion | 5/42 | 11.9 |
| Imaging modality | Reported in 54 patients | |
| CT scan | 44 | 81.5 |
| MRI | 11 | 20.4 |
| PET-CT | 6 | 11.1 |
| Not reported | 12 | |
| Laterality | Reported in 56 patients | |
| Right | 27 | 48.2 |
| Left | 28 | 50.0 |
| Bilateral | 1 | 1.8 |
| Not reported | 10 | |
| Mean tumor size (cm) | 11.4 ± 4.8 range 3-24 |
Preoperative staging revealed that 17 tumors (27.9%) were metastatic at initial diagnosis, and 14 patients (23.0%) presented with inferior vena cava (IVC) or renal vein neoplastic thrombus. Ten patients (18.9%) received neoadjuvant chemotherapy preoperatively. Operative and pathological characteristics are shown in Table 2. Open adrenalectomy was the procedure of choice in the majority of cases (48 cases, 80.0%). Of the patients in the open adrenalectomy group, 17 patients (27.9%) required more extensive resections (usually ipsilateral nephrectomy). Minimally invasive laparoscopic and robotic approaches were attempted in 4 (6.6%) and 1 case (1.6%), respectively. Seven patients (11.7%) underwent biopsy instead of resection. Complete surgical resection (R0) was achieved in 89.4% (42/47). Immunohistochemistry staining for CD99 was positive in 98.4% (60/61) of patients. Molecular testing for the translocation EWSR1-FLI1 was performed in 27 patients, all of which were positive. Adjuvant therapy was reported in 32 patients, in the form of chemotherapy (62.5%), radiotherapy (3.1%) or chemoradiotherapy (34.4%).
| Number of patients | Percentage (%) | |
| Metastatic disease | ||
| Metastatic during initial diagnosis | 17/61 | 27.9 |
| Inferior vena cava/renal vein neoplastic thrombus | 14/61 | 23.0 |
| Treatment | ||
| Neoadjuvant chemotherapy | 10/53 | 18.9 |
| Open adrenalectomy | 48/60 | 80.0 |
| Extensive resection | 17/60 | 27.9 |
| Laparoscopic resection | 4/60 | 6.6 |
| Robotic resection | 1/60 | 1.6 |
| Biopsy without resection | 7/60 | 11.7 |
| Complete surgical resection | 42/47 | 89.4 |
| Tumor confirmation testing | ||
| Positive CD99 immunohistochemistry staining | 60/61 | 98.4 |
| Positive molecular EWSR1-FLI1translocation | 27/27 patients | 100 |
| Adjuvant therapy | Required in 32 patients | |
| Chemotherapy | 20/32 | 62.5 |
| Radiotherapy | 1/32 | 3.1 |
| Chemoradiotherapy | 11/32 | 34.4 |
| Outcomes | ||
| Survival data | 42 patients | |
| Median overall survival | 15 mo | |
| 24-mo overall survival | 17/42 | 40.5 |
| Median disease-free survival | 10 mo | |
| 24-mo disease-free survival | 14/42 | 33.3 |
Survival data were available for 42 patients. Median overall survival was 15 mo (95%CI: 9.4-20.6). 24-mo overall survival was 40.5%. Median disease-free survival was 10 mo (95%CI: 4.3-15.7). 24-mo disease-free survival was 33.3%.
ES is an aggressive primary osseous tumor[60]. Extra-skeletal sites involving soft tissue and visceral tumors constitute only 10%-20% of ESs. Soft tissue ES/PNET can be found in the muscle, connective tissue, lymph nodes, and other tissue of mesenchymal origin. Visceral ES/PNET sites include the lungs, gastrointestinal tract, prostate, brain, endometrium, thyroid and adrenal gland[45]. Our review is the first systematic review focusing on primary adrenal ES/PNET. This study yielded 66 cases between 1989 and 2022, showcasing the low prevalence of this disorder.
Clinical presentation of ES/PNET varies depending on the location of the tumor. A large, rapidly growing, solitary mass, can cause mass effects or invade nearby structures. Symptoms include constitutional symptoms, such as fever, weight loss, anorexia, pain and bleeding[61]. Our study showed that pain and discomfort were the primary complaints, followed by a palpable mass.
All patients in our study were diagnosed within one year from onset of symptoms (mean duration 2.6 mo). Half of the patients were found to have metastasis or IVC/renal vein neoplastic thrombus at the time of diagnosis. This shows that diagnosis typically occurs when these tumors have reached an advanced stage, suggesting its aggressive nature. Only 17.9% of our cases were diagnosed incidentally, which is in contrast to other adrenocortical tumors[62].
A CT scan, MRI and PET-CT are the first line imaging modalities performed to detect the tumor. Adrenal ES/PNET can present as a large size, nonfunctional mass[61]. However, imaging is limited to staging of the patient, differentiate metastatic from primary ES/PNET, and to assist with the surgical planning.
Tissue examination, immunohistochemical and genetic tests are necessary for diagnostic purposes. Histopathologically, ES/PNETs can be differentiated from other adrenocortical carcinomas. ES/PNETs are seen as small round cell tumors. The 32-kDa cell surface glycoprotein has also demonstrated significant use as a screening tool given its high sensitivity (as high as 95%), although specificity is low[63]. Immunohistochemical staining for CD99 is essential to support the diagnosis of ES/PNET from other small round cell tumors. In our review, immunohistochemistry staining for CD99 was tested in the majority of cases assisting in diagnosis (61 cases). Molecular testing to identify the most common mutations, such as t (11; 22) (q24; q12) (80% to 90% of ES/PNET), t (21; 12) (22; 12) (10%), could provide meaningful data for diagnostic purposes. However it is currently not the standard of care.
Specific instructions for the treatment of ES/PNET have not been published to date. In the majority of cases, these tumors are treated according to the soft tissue sarcoma guidelines. These guidelines are published by the European Society of Medical Oncology and the National Comprehensive Cancer Network, which includes recommendations for the treatment of visceral sarcomas[64,65]. According to the guidelines, surgical excision with negative R0 is the preferred initial treatment. If R0 margins are not obtained with the index procedure, a second excision can be performed. Planned close margins or even microscopically positive margins (R1) may be acceptable in certain cases, to preserve critical neurovascular structures. Post-operative radiation should be considered in tumors with R0 or R1 margins in order to reduce recurrence. This is especially indicated when the soft tissue tumor margin or the microscopic margin are positive proximal to bone tissue, major blood vessels or major nerves. Other adjuvant options include systemic therapy and/or chemotherapy. Neoadjuvant treatment, with radiation and/or systemic treatment, is performed in tumors deemed unresectable, to downsize the cancer or provide palliative measures.
In our review, the majority of the patients were treated surgically in accordance to the existing guidelines. Open adrenalectomy (80.0%) or more extensive resection (27.9%) were the procedures performed most frequently. Laparoscopic or robotic surgery was selected in a small number of tumors (8.2%). These were observed mostly in the last decade, although this is anticipated to become more frequent in the future, as technology advances and minimal invasive surgical techniques become more dominant. Neoadjuvant treatment was administered in 18.9% of patients, to downsize the tumor, prior to surgical excision. Negative margins were obtained in the vast majority of the patients (89.4%). Adjuvant therapy was performed, mostly with chemotherapy or chemoradiation, in order to eliminate micrometastases and increase the 5-year survival rate[66].
Despite multisystem treatment modalities, the average survival rate was found to be approximately 15 mo in our study. Less than half of the population demonstrated 24-mo survival and one-third of patients were deemed free of disease at that time. This shows the high recurrence rate of the disease.
Research in ES during the last years has focused on the identification of DNA fragments, which could potentially detect and distinguish between different cancer types and subcategories, monitor disease progression over time, as well as estimate survival and relapse probabilities at the time of diagnosis[67]. In addition, artificial intelligence has led to the development of large databases, biobanks and radiomics. In the future, both biomarkers and artificial intelligence science are anticipated to assist with stratifying patients into specific groups by creating patient profiles who share common features. These tools will lead to the development of individualized treatments and prognostic treatment-response scores in chemotherapy and/or radiotherapy[68,69].
Primary adrenal ES is uncommon with 67 cases reported in the literature since 1990. Diagnosis is usually achieved within one year from the onset of symptoms. Open adrenalectomy has been the procedure of choice, although minimally invasive techniques are anticipated to be performed more frequently in the near future. The recurrence rate within 24 mo is quite high, which proves the aggressive character of the tumor. The significant progress in molecular biology and artificial intelligence promises big achievements in the future in terms of diagnosis and treatment of ES.
Ewing sarcoma (ES) is an aggressive malignant primary osseous tumor, which is commonly observed in the young population. Visceral organs and particularly adrenal glands are rarely impacted.
Therapeutic protocols for the treatment of ES/primitive neuroectodermal tumor (PNET) are not standardized and these tumors are treated according to the soft tissue sarcoma guidelines of the European Society of Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN), due to limited knowledge.
The aim of the present study is to present an up-to-date, systematic review of the literature on primary adrenal ES/PNET, with emphasis on diagnosis, therapy and oncological outcomes.
A systematic review of the literature was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses 2020 statement. PubMed/MEDLINE, EMBASE and Google Scholar bibliographic databases were searched to identify articles that included patients with ES/PNET of the adrenal gland from 1989 to 2022. Demographic, clinical, pathological and oncological data of patients were analyzed by SPSS version 29.0.
Fifty-two studies were included in the current systematic review, describing a total of 66 patients. The mean age at diagnosis was 26.4 years and the most frequent complaint was abdominal or flank pain/discomfort. At the time of diagnosis, average tumor size was 11.4 cm. 27.9% of the tumors were metastatic and 23.0% had inferior vena cava or renal vein neoplastic thrombus. Open adrenalectomy was the procedure of choice (80.0% of patients), and a more extensive resection was required in 27.0% of these patients. Immunohistochemistry staining for CD99 was positive in 98.4%, and molecular testing for the translocation EWS RNA binding protein 1-Friend leukemia integration 1 was positive in all the patients tested. Median overall survival was 15 mo and 24-mo overall survival was 40.5%.
Primary adrenal ES/PNET is a tumor with low prevalence. Diagnosis typically occurs when the tumor has reached an advanced stage. Immunohistochemical staining for CD99 is essential to support the diagnosis of ES/PNET from other small round cell tumors. To date, these tumors are treated according to the soft tissue sarcoma guidelines of ESMO and NCCN, with surgical excision and negative surgical margins being the preferred treatment of choice, when feasible. Unfortunately, the disease has a high recurrence rate, and a relatively low survival rate.
In the future, minimally invasive techniques will be used more frequently in the surgical treatment of primary Ewing adrenal sarcoma. In addition, research in biomarkers and artificial intelligence science will assist with stratifying patients into groups, and lead to the development of individualized treatments and prognostic treatment-response scores in chemotherapy and/or radiotherapy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ma X, China; Wiemer EA, Netherlands S-Editor: Qu XL L-Editor: Webster JR P-Editor: Zhao S
| 1. | Theisen ER, Pishas KI, Saund RS, Lessnick SL. Therapeutic opportunities in Ewing sarcoma: EWS-FLI inhibition via LSD1 targeting. Oncotarget. 2016;7:17616-17630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol. 2010;11:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 456] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 3. | Machado I, Navarro L, Pellin A, Navarro S, Agaimy A, Tardío JC, Karseladze A, Petrov S, Scotlandi K, Picci P, Llombart-Bosch A. Defining Ewing and Ewing-like small round cell tumors (SRCT): The need for molecular techniques in their categorization and differential diagnosis. A study of 200 cases. Ann Diagn Pathol. 2016;22:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Kim SK, Park YK. Ewing sarcoma: a chronicle of molecular pathogenesis. Hum Pathol. 2016;55:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Koscielniak E, Sparber-Sauer M, Scheer M, Vokuhl C, Kazanowska B, Ladenstein R, Niggli F, Ljungman G, Paulussen M, Bielack SS, Seitz G, Fuchs J, Hallmen E, Klingebiel T; On Behalf Of The Cws Study Group. Extraskeletal Ewing sarcoma in children, adolescents, and young adults. An analysis of three prospective studies of the Cooperative Weichteilsarkomstudiengruppe (CWS). Pediatr Blood Cancer. 2021;68:e29145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Murthy SS, Challa S, Raju K, Rajappa SJ, Fonseca D, Gundimeda SD, Rao BV, Ahmed F, Kodandapani S, Nambaru L, Mundada MC, Sharma R, Mallavarapu KM, Koppula VC, Rao TS. Ewing Sarcoma With Emphasis on Extra-skeletal Ewing Sarcoma: A Decade's Experience From a Single Centre in India. Clin Pathol. 2020;13:2632010X20970210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40601] [Article Influence: 10150.3] [Reference Citation Analysis (2)] |
| 8. | Marina NM, Etcubanas E, Parham DM, Bowman LC, Green A. Peripheral primitive neuroectodermal tumor (peripheral neuroepithelioma) in children. A review of the St. Jude experience and controversies in diagnosis and management. Cancer. 1989;64:1952-1960. [PubMed] [DOI] [Full Text] |
| 9. | Renshaw AA, Perez-Atayde AR, Fletcher JA, Granter SR. Cytology of typical and atypical Ewing's sarcoma/PNET. Am J Clin Pathol. 1996;106:620-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Kim MS, Kim B, Park CS, Song SY, Lee EJ, Park NH, Kim HS, Kim SH, Cho KS. Radiologic findings of peripheral primitive neuroectodermal tumor arising in the retroperitoneum. AJR Am J Roentgenol. 2006;186:1125-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Li H. Primitive neuroectodermal tumors of adrenal gland. Jpn J Clin Oncol. 2010;40:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Gonin J, Larousserie F, Dousset B, Rousseau J, Delattre O, Waintrop C, Tsatsaris V, Pierga JY, Vacher-Lavenu MC, Tissier F. [An unusual adrenal tumor: Ewing tumor]. Ann Pathol. 2011;31:28-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Mohsin R, Hashmi A, Mubarak M, Sultan G, Shehzad A, Qayum A, Naqvi SA, Rizvi SA. Primitive neuroectodermal tumor/Ewing's sarcoma in adult uro-oncology: A case series from a developing country. Urol Ann. 2011;3:103-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Stephenson J, Gow KW, Meehan J, Hawkins DS, Avansino J. Ewing sarcoma/primitive neuroectodermal tumor arising from the adrenal gland in an adolescent. Pediatr Blood Cancer. 2011;57:691-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Saboo SS, Krajewski KM, Jagannathan JP, Ramaiya N. IVC tumor thrombus: an advanced case of rare extraosseous Ewing sarcoma of the adrenal gland. Urology. 2012;79:e77-e78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Abi-Raad R, Manetti GJ, Colberg JW, Hornick JL, Shah JG, Prasad ML. Ewing sarcoma/primitive neuroectodermal tumor arising in the adrenal gland. Pathol Int. 2013;63:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Blas JV, Smith ML, Wasif N, Cook CB, Schlinkert RT. Ewing sarcoma of the adrenal gland: a rare entity. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Dutta D, Shivaprasad KS, Das RN, Ghosh S, Chowdhury S. Primitive neuroectodermal tumor of adrenal: clinical presentation and outcomes. J Cancer Res Ther. 2013;9:709-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Lim SH, Lee JY, Kim JH, Choi KH, Hyun JY, Ko YH, Lee J, Kim SJ, Kim WS. Unusual presentation of Ewing sarcoma in the adrenal gland: a secondary malignancy from a survivor of Burkitt lymphoma. Jpn J Clin Oncol. 2013;43:676-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Matsuoka Y, Fujii Y, Akashi T, Gosehi N, Kihara K. Primitive neuroectodermal tumour of the adrenal gland. BJU Int. 1999;83:515-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Phukan C, Nirmal TJ, Kumar RM, Kekre NS. Peripheral primitive neuroectodermal tumor of the adrenal gland: A rare entity. Indian J Urol. 2013;29:357-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Sasaki T, Onishi T, Yabana T, Hoshina A. Ewing's sarcoma/primitive neuroectodermal tumor arising from the adrenal gland: a case report and literature review. Tumori. 2013;99:e104-e106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Yamamoto T, Takasu K, Emoto Y, Umehara T, Ikematsu K, Shikata N, Iino M, Matoba R. Latent adrenal Ewing sarcoma family of tumors: A case report. Leg Med (Tokyo). 2013;15:96-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Zahir MN, Ansari TZ, Moatter T, Memon W, Pervez S. Ewing's sarcoma arising from the adrenal gland in a young male: a case report. BMC Res Notes. 2013;6:533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Somarouthu BS, Shinagare AB, Rosenthal MH, Tirumani H, Hornick JL, Ramaiya NH, Tirumani SH. Multimodality imaging features, metastatic pattern and clinical outcome in adult extraskeletal Ewing sarcoma: experience in 26 patients. Br J Radiol. 2014;87:20140123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Tsang YP, Lang BH, Tam SC, Wong KP. Primitive neuroectodermal adrenal gland tumour. Hong Kong Med J. 2014;20:444-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Yoon JH, Kim H, Lee JW, Kang HJ, Park HJ, Park KD, Park BK, Shin HY, Park JD, Park SH, Ahn HS. Ewing sarcoma/peripheral primitive neuroectodermal tumor in the adrenal gland of an adolescent: a case report and review of the literature. J Pediatr Hematol Oncol. 2014;36:e456-e459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Priya D, Kumar RV, Appaji L, Aruna Kumari BS, Padma M, Kumari P. Histological diversity and clinical characteristics of Ewing sarcoma family of tumors in children: A series from a tertiary care center in South India. Indian J Cancer. 2015;52:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Batahar SA, Elidrissi S, Berrada S, Rais H, Amro L. Extraskeletal Ewing’s sarcoma: An adrenal localization. Int J Case Reports Images. 2016;7:770-773. [DOI] [Full Text] |
| 30. | Kumar S, Govinda V, Singh SK, Singh J. Bilateral Adrenal PNET: A Rare Presentation. J Clin Diagn Res. 2016;10:XD01-XD02. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Pirani JF, Woolums CS, Dishop MK, Herman JR. Primitive neuroectodermal tumor of the adrenal gland. J Urol. 2000;163:1855-1856. [PubMed] |
| 32. | Pal DK, Chandra V, Ranjan KR, Chakrabortty D, Banerjee M. Ewing's Sarcoma of the Adrenal Gland. APSP J Case Rep. 2016;7:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Zhang L, Yao M, Hisaoka M, Sasano H, Gao H. Primary Ewing sarcoma/primitive neuroectodermal tumor in the adrenal gland. APMIS. 2016;124:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | de Groot S, Gelderblom H, Fiocco M, Bovée JV, van der Hoeven JJ, Pijl H, Kroep JR. Serum levels of IGF-1 and IGF-BP3 are associated with event-free survival in adult Ewing sarcoma patients treated with chemotherapy. Onco Targets Ther. 2017;10:2963-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Guo H, Chen S, Liu S, Wang K, Liu E, Li F, Hou Y. Rare adrenal gland incidentaloma: an unusual Ewing's sarcoma family of tumor presentation and literature review. BMC Urol. 2017;17:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Soydan L, Demir AA, Sayman E, Onomay Celik B, Oven Ustaalioglu BB. Adrenal mass of unusual etiology: Ewing sarcoma in a young man. Radiol Case Rep. 2017;12:838-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Zhang Y, Cai P, Chen M, Yi X, Li L, Xiao D, Liu W, Li W, Li Y. Imaging findings of adrenal primitive neuroectodermal tumors: a series of seven cases. Clin Transl Oncol. 2017;19:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Afridi ZU, Haleem A, Khan MA, Ahmad R, Rashid M, Arooj S, Arifeen S U, Ullah A. EWING SARCOMA OF ADRENAL GLAND CAUSING CUSHING’S SYNDROME; AN EXCEPTIONALLY RARE TUMOR. J Med Sci. 2018;26:178-180 Available from: https://jmedsci.com/index.php/Jmedsci/article/view/548. |
| 39. | Eddaoualline H, Mazouz K, Rafiq B, El Mghari Tabib G, El Ansari N, Belbaraka R, El Omrani A, Khouchani M. Ewing sarcoma of the adrenal gland: a case report and review of the literature. J Med Case Rep. 2018;12:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Goudarzipour K, Farahmandi F, Mohammadi A, Taherian R. Ewing Sarcoma/Peripheral Primitive Neuroectodermal Tumor in the Adrenal Gland of a Child. Iran J Kidney Dis. 2018;12:190-192. [PubMed] |
| 41. | Toda K, Ishii S, Yasuoka H, Nishioka M, Kobayashi T, Horiguchi K, Tomaru T, Ozawa A, Shibusawa N, Satoh T, Koshi H, Segawa A, Shimizu SI, Oyama T, Yamada M. Adrenal Ewing's Sarcoma in an Elderly Man. Intern Med. 2018;57:551-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Jung SP, Oh CG, Lim IS, Lee DK, Yoo BH. A Case of Primitive Neuroectodermal Tumor of the Adrenal Gland. J Korean Pediatr Soc 2001; 44: 1459-1462. |
| 43. | Patnaik S, Yarlagadda J, Susarla R. Imaging features of Ewing's sarcoma: Special reference to uncommon features and rare sites of presentation. J Cancer Res Ther. 2018;14:1014-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Koufopoulos N, Kokkali S, Manatakis D, Balalis D, Nasi D, Ardavanis A, Korkolis D, Khaldi L. Primary peripheral neuroectodermal tumor (PNET) of the adrenal gland: a rare entity. J BUON. 2019;24:770-778. [PubMed] |
| 45. | Ibabao C, Tsetse C, Sheth Y, Maitland C, Mohammed M. Primary Ewing sarcoma of the adrenal gland: A rare cause of abdominal mass. Radiol Case Rep. 2020;15:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Maity K, Agrawal A, Datta C, Pal DK. Primary Ewing’s Sarcoma of Adrenal Gland-A Rare Case. J Clin Diagnostic Res. 2019;13:PD01-02. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 47. | Dai J, He HC, Huang X, Sun FK, Zhu Y, Xu DF. Long-term survival of a patient with a large adrenal primitive neuroectodermal tumor: A case report. World J Clin Cases. 2019;7:340-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Gaujoux S, Hain É, Marcellin L, de Carbonnieres A, Goffinet F, Bertherat J, Dousset B. Adrenalectomy during pregnancy: A 15-year experience at a tertiary referral center. Surgery. 2020;168:335-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Sari M, Ekenei M, Ozluk MY, Basaran M. Clinical and Pathological Features of Ewing Sacoma Family Tumors in Uro-oncology: A Single-Institute Experience. Eurasian J Med Investig. 2020;4: 247-252. [DOI] [Full Text] |
| 50. | Bradford K, Nobori A, Johnson B, Allen-Rhoades W, Naik-Mathuria B, Panosyan EH, Gotesman M, Lasky J, Cheng J, Ikeda A, Goldstein J, Singh A, Federman N. Primary Renal Ewing Sarcoma in Children and Young Adults. J Pediatr Hematol Oncol. 2020;42:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Salah S, Abuhijla F, Ismail T, Yaser S, Sultan I, Halalsheh H, Shehadeh A, Abdelal S, Almousa A, Jaber O, Abu-Hijlih R. Outcomes of extraskeletal vs. skeletal Ewing sarcoma patients treated with standard chemotherapy protocol. Clin Transl Oncol. 2020;22:878-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Jagdale R V, Pol JN. Primary primitive neuroectodermal tumor of the adrenal gland: A unique tumor at an unusual site. Indian J Pathol Oncol. 2021;8:420-423. [DOI] [Full Text] |
| 53. | Kato K, Kato Y, Ijiri R, Misugi K, Nanba I, Nagai J, Nagahara N, Kigasawa H, Toyoda Y, Nishi T, Tanaka Y. Ewing's sarcoma family of tumor arising in the adrenal gland--possible diagnostic pitfall in pediatric pathology: histologic, immunohistochemical, ultrastructural, and molecular study. Hum Pathol. 2001;32:1012-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Wang JL, Xu CY, Geng CJ, Liu L, Zhang MZ, Wang H, Xiao RT, Zhang G, Ni C, Guo XY. Anesthesia and perioperative management for giant adrenal Ewing's sarcoma with inferior vena cava and right atrium tumor thrombus: A case report. World J Clin Cases. 2022;10:643-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (2)] |
| 55. | Roy D, Pereira M, Shivdasani D, Singh N. (18) F-FDG PET-CT Evaluation of Primary Adrenal Ewing Sarcoma with Venous Thrombosis: An Unusual Presentation. World J Nucl Med. 2023;22:26-28. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 56. | Girot V, Johann M, Sahyoun A, Platini C, Fery N, Khalife K. [Primitive Ewing sarcoma presenting as a left adrenal mass associated with a vena cava thrombus]. Prog Urol. 2002;12:668-671. [PubMed] |
| 57. | Khong PL, Chan GC, Shek TW, Tam PK, Chan FL. Imaging of peripheral PNET: common and uncommon locations. Clin Radiol. 2002;57:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Ahmed AA, Nava VE, Pham T, Taubenberger JK, Lichy JH, Sorbara L, Raffeld M, Mackall CL, Tsokos M. Ewing sarcoma family of tumors in unusual sites: confirmation by rt-PCR. Pediatr Dev Pathol. 2006;9:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Komatsu S, Watanabe R, Naito M, Mizusawa T, Obara K, Nishiyama T, Takahashi K. Primitive neuroectodermal tumor of the adrenal gland. Int J Urol. 2006;13:606-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Grünewald TGP, Cidre-Aranaz F, Surdez D, Tomazou EM, de Álava E, Kovar H, Sorensen PH, Delattre O, Dirksen U. Ewing sarcoma. Nat Rev Dis Primers. 2018;4:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 523] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 61. | Wright A, Desai M, Bolan CW, Badawy M, Guccione J, Rao Korivi B, Pickhardt PJ, Mellnick VM, Lubner MG, Chen L, Elsayes KM. Extraskeletal Ewing Sarcoma from Head to Toe: Multimodality Imaging Review. Radiographics. 2022;42:1145-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 62. | Latronico AC, Chrousos GP. Extensive personal experience: adrenocortical tumors. J Clin Endocrinol Metab. 1997;82:1317-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Yan D, Zhang J, Zhong D. Ewing's sarcoma in the spinal canal of T12-L3: A case report and review of the literature. Oncol Lett. 2019;18:6157-6163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Blay JY, Bolle S, Bonvalot S, Boukovinas I, Bovee JVMG, Boye K, Brennan B, Brodowicz T, Buonadonna A, De Álava E, Del Muro XG, Dufresne A, Eriksson M, Fagioli F, Fedenko A, Ferraresi V, Ferrari A, Frezza AM, Gasperoni S, Gelderblom H, Gouin F, Grignani G, Haas R, Hassan AB, Hecker-Nolting S, Hindi N, Hohenberger P, Joensuu H, Jones RL, Jungels C, Jutte P, Kager L, Kasper B, Kawai A, Kopeckova K, Krákorová DA, Le Cesne A, Le Grange F, Legius E, Leithner A, Lopez-Pousa A, Martin-Broto J, Merimsky O, Messiou C, Mir O, Montemurro M, Morland B, Morosi C, Palmerini E, Pantaleo MA, Piana R, Piperno-Neumann S, Reichardt P, Rutkowski P, Safwat AA, Sangalli C, Sbaraglia M, Scheipl S, Schöffski P, Sleijfer S, Strauss D, Strauss S, Sundby Hall K, Trama A, Unk M, van de Sande MAJ, van der Graaf WTA, van Houdt WJ, Frebourg T, Casali PG, Stacchiotti S; ESMO Guidelines Committee, EURACAN and GENTURIS. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:1348-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 585] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 65. | National Comprehensive Cancer Network. Soft Tissue Sarcoma (version 2.2023). Available from: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf. |
| 66. | Abboud A, Masrouha K, Saliba M, Haidar R, Saab R, Khoury N, Tawil A, Saghieh S. Extraskeletal Ewing sarcoma: Diagnosis, management and prognosis. Oncol Lett. 2021;21:354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 67. | Peneder P, Stütz AM, Surdez D, Krumbholz M, Semper S, Chicard M, Sheffield NC, Pierron G, Lapouble E, Tötzl M, Ergüner B, Barreca D, Rendeiro AF, Agaimy A, Boztug H, Engstler G, Dworzak M, Bernkopf M, Taschner-Mandl S, Ambros IM, Myklebost O, Marec-Bérard P, Burchill SA, Brennan B, Strauss SJ, Whelan J, Schleiermacher G, Schaefer C, Dirksen U, Hutter C, Boye K, Ambros PF, Delattre O, Metzler M, Bock C, Tomazou EM. Multimodal analysis of cell-free DNA whole-genome sequencing for pediatric cancers with low mutational burden. Nat Commun. 2021;12:3230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 68. | Zöllner SK, Amatruda JF, Bauer S, Collaud S, de Álava E, DuBois SG, Hardes J, Hartmann W, Kovar H, Metzler M, Shulman DS, Streitbürger A, Timmermann B, Toretsky JA, Uhlenbruch Y, Vieth V, Grünewald TGP, Dirksen U. Ewing Sarcoma-Diagnosis, Treatment, Clinical Challenges and Future Perspectives. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 69. | Li G, Wu X, Ma X. Artificial intelligence in radiotherapy. Semin Cancer Biol. 2022;86:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |