Published online Sep 26, 2023. doi: 10.12998/wjcc.v11.i27.6646
Peer-review started: July 18, 2023
First decision: August 16, 2023
Revised: August 22, 2023
Accepted: September 1, 2023
Article in press: September 1, 2023
Published online: September 26, 2023
Processing time: 64 Days and 14.2 Hours
Orthopedic surgeries after device implantation are susceptible to infections and may require device removal in the worst cases. For this reason, many efforts are being made to control infections after spinal surgery; however, the number of infec
A 75-year-old male with a chronic spinal defect due to previous spine surgery underwent reconstruction using a perforator-based island flap. After bursectomy and confirmation that there was no connection with the deep tissue, reconstru
Infection is one of the most common causes of surgical wound dehiscence and is associated with devastating results if not controlled promptly and definitively. Surgeons should always suspect delayed infections when reconstructing chronic soft tissue defects.
Core Tip: Reasonably, several patients with surgical site dehiscence may require flap surgery after spinal surgery. Although surgeons pay close attention to signs of infection during reconstruction, there can be neglected delayed infection around deep devices. Reconstructive surgeons should be cautious when planning flap surgery after implant surgery.
- Citation: Kim D, Lim S, Eo S, Yoon JS. Reconstruction of the lower back wound with delayed infection after spinal surgery: A case report. World J Clin Cases 2023; 11(27): 6646-6652
- URL: https://www.wjgnet.com/2307-8960/full/v11/i27/6646.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i27.6646
Surgical wound dehiscence (SWD) is an uncommon but inevitable complication for surgeons. Various factors such as poor general condition, old age, repetitive surgeries, obesity, diabetes, and poor nutrition may contribute to SWD. Among these, surgical site infections (SSIs) are the most frequently reported[1]. A previous study on SWD suggested that longer healing times require more topical antimicrobial dressings or treatment solutions for infections[2].
Recently, the commonly applied management of SSI cases is based on preliminary wound swab culture to detect bacterial infection. Surgical debridement is performed, and intravenous antibiotics are administered. Modern technological developments suggest treating SWD using negative-pressure wound therapy and biosynthetic materials. Vacuum-assisted closure not only cleanses necrotic tissues, but also helps in granulation tissue formation and gradual skin margin approximation[3-5].
Orthopedic surgery, including spinal surgery, is one of the most common causes of SSI. If the infection is related to foreign bodies such as implants, it is associated with high morbidity and may require secondary surgery and delay recovery. Spinal infections with implants are reported to be approximately 8.5%, but < 1% in open surgery without devices[6]. The treatment course is challenging considering that the surgical and medical multidisciplinary approach is essentially based on the type and location of the implant and the patient’s comorbidities[7].
By sharing a case of unexpected SSI after reconstruction of a chronic SWD in the lumbar region, the authors want to emphasize a more careful approach to SSI management and the usefulness of perforator flaps for median or paramedian spinal defects. Written informed consent was obtained from the patient.
A 75-year-old male was referred to our unit from a local clinic complaining of chronic wounds on his back after under
There seemed to be no problem postoperatively for 2 years; however, approximately 2 mo prior, he noticed wound discharge from the back. The neurosurgeon who performed his spine surgery at the local clinic tried debridement and wound revision three times. Radiology at a local clinic revealed no evidence of deep wound infection, and in wound culture, Pseudomonas spp. were cultivated. This led to the use of piperacillin/tazobactam; however, the patient developed a severe allergic reaction, and ciprofloxacin was administered instead. The patient was referred to us because of no im
He had herniation of the nucleus purposus and had undergone open lumbar-assisted microdiscectomy 3 years previously. At that time, the patient underwent wound revision for SWD. He also underwent anterior lumbar interbody fusion 2 years prior and direct lateral interbody fusion 3 mo later. SWD was observed again in both the second and third surgeries.
His body mass index was 27.67 kg/m² and he had been taking diabetic medications for approximately 20 years. There were no other remarkable comorbidities.
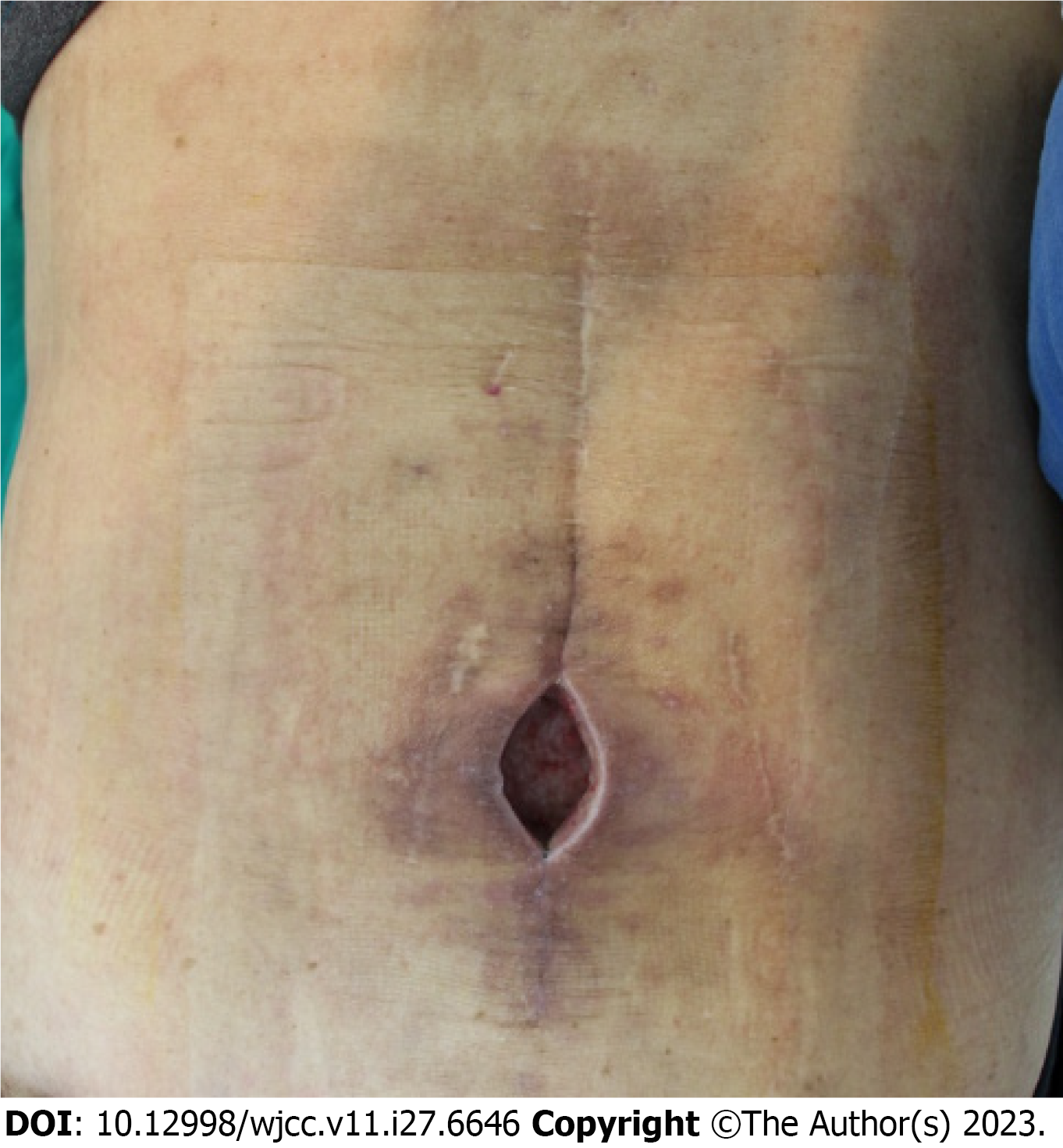
On physical examination, approximately 5 cm × 4 cm sized elliptical soft tissue defect was discovered with bursa for
C-reactive protein (CRP) level at the time of admission was 0.50 mg/dL, within the normal range. In addition, no abnor
Simple spine X-ray revealed degenerative spondylosis and further imaging study was not necessary at the time of visit.
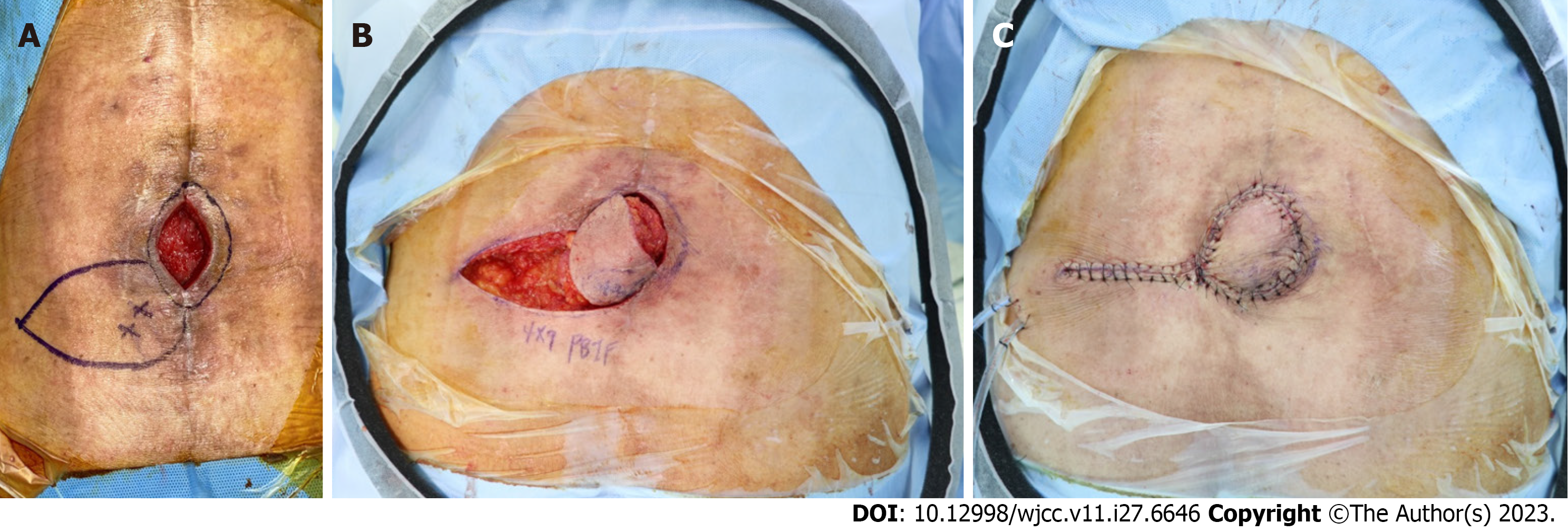
Soft tissue defects with chronic infections were diagnosed after spinal surgery. After debridement with curettage of the infected granulation tissue, negative-pressure wound therapy was planned for one week, and a perforator-based island flap was scheduled under general anesthesia.
After a thorough bursectomy, no connection with the deep tissue was confirmed based on gross findings, and recon
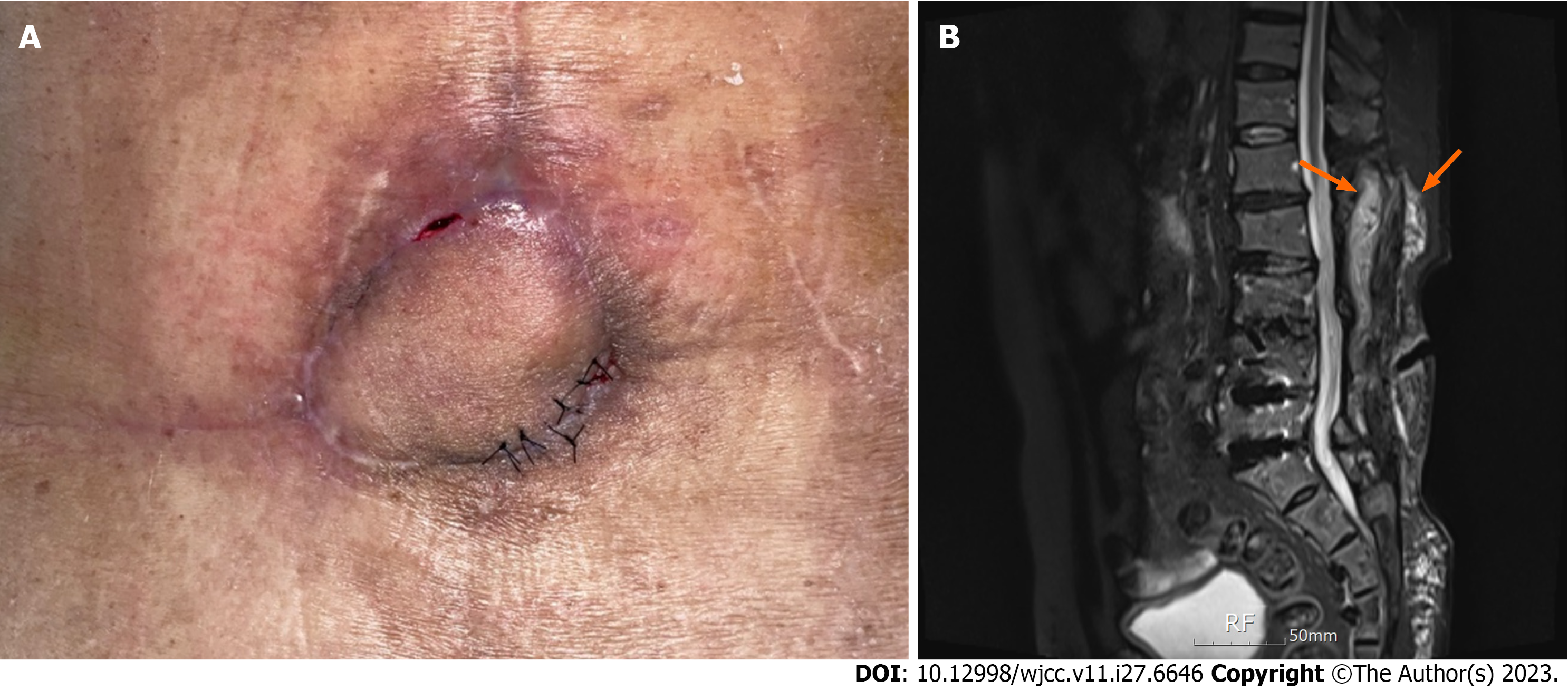
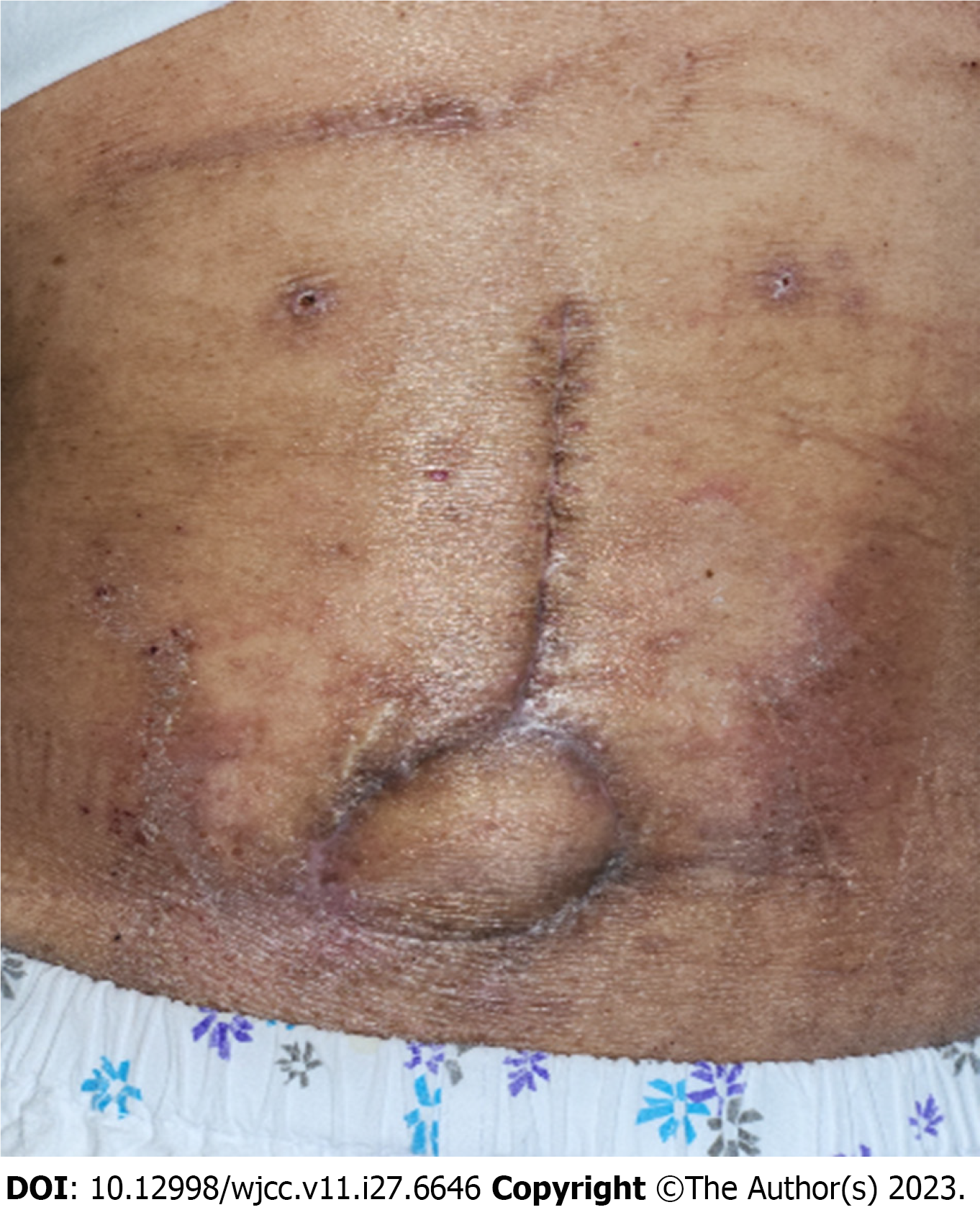
On postoperative day 29, when stitches were removed partially, skin thinning with bulging was observed in the flap’s 5 o’clock region. A pus-like discharge was made out of the lesion using a slit incision. A betadine-soaked gauze packing dressing with massive irrigation was applied for 10 d, and wound closure with 4-0 nylon was attempted. Nine days after resuturing, another abscess formation was observed in the 11 o’clock region of the flap (Figure 3A).
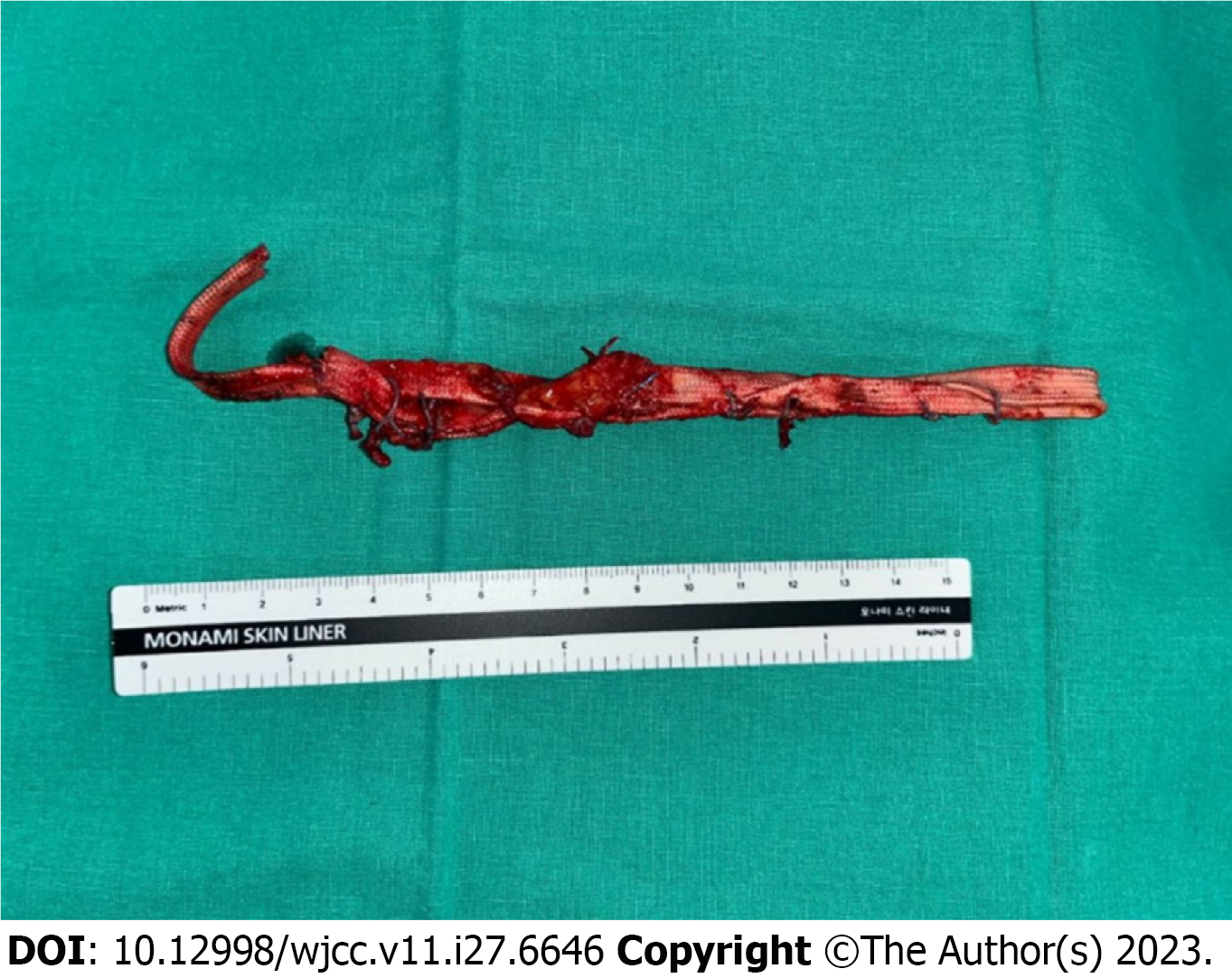
Therefore, we rechecked the imaging results. L-spine computed tomography and enhanced L-spine magnetic reso
On postoperative day 18, all sutures were removed after the wire removal, and the patient was discharged without fur
Delayed wound healing and compromised granulation tissue formation result from numerous causes. The host factors include age, tobacco use, comorbidities, and obesity[7]. Mechanical factors include preoperative skin antisepsis, suture breakage, infection, hematoma, seroma, and mechanical stress[1,7,8]. Wound healing requires sufficient oxygen and nutrients from an appropriate diet and vascular supply. Therefore, any factor that impedes them might compromise wound healing and increase the risk of dehiscence (SWD). They deteriorate quality of life, prolong hospital stays, and increase medical costs. Infection (SSI) is one of the most frequent causes of all the factors. Some surgeons consider SSD as an SSI because of its inseparable association[9].
Orthopedic implants are increasingly being used by senior citizens and those with moderate-to-severe medical problems[9]. Orthopedic implants are classified into two categories. One is a prosthesis designed to replace joints, and the other is hardware, such as plates, screws, and wires, designed to fix broken bones. Both are highly susceptible to infec
Staphylococcus aureus and Staphylococcus epidermidis are the most commonly reported pathogens associated with orthopedic device-related infections[14]. Pseudomonas aeruginosa is known to develop sturdy biofilms that easily lead to chronic infections. It resides in a deeper layer of wounds than Staphylococcus aureus, and the infection tends to be more severe, broader, and harder to recover from[15]. This mechanism involves interactions between the host, implant, and the microorganism[10]. During surgery, the bacteria on the patient’s skin enter through the incision site and establish colonies on the device[14]. Implants lack microcirculation, which is required for host defense and antibiotic distribution. Biofilms formed by bacterial colonization consist of aggregated microbial cells that continuously produce a self-made polymeric matrix, including host components[2]. It can be observed in chronic wounds and most implant-associated infections. Biofilms defeat antibiotics and host defenses and help bacteria prosper on the device[2,14].
Diagnosis is made considering all clinical, laboratory, histopathological, microbiological, and imaging findings. If the patient has no symptoms such as continuous back pain, fever, or shivers, the surgeon should carefully inspect the wound for any local inflammatory signs[13]. Repetitive postoperative CRP measurements can provide valuable information during the postoperative period. Histopathological analysis of periprosthetic tissue is reported to have more than 80% of sensitivity and more than 90% of specificity in the literature[10]. The rapid detection of infection is critical for preventing osteomyelitis and device loss. Radiological assessment is crucial for detecting mechanical complications, such as spinal instability and inflammation, abscess formation, or fluid collection[13].
Despite the devastating results, the optimal management of SSI after spinal instrumentation remains controversial[13]. A multidisciplinary approach involving surgeons, microbiologists, and infectious disease specialists is essential to achieve the best outcome. When the microorganism causing the infection is of low virulence and is reactive to antibiotics, mostly in postoperative three to six weeks, device retention may be possible. Surgical debridement may be needed, and intra
Flap reconstruction of the back is not commonly compared with other regions; therefore, related literature is limited[16]. Surgical reconstruction in this region remains challenging because the lower back has low skin laxity and is securely attached to underlying vital structures. Pressure wounds, tumor resection, and spine surgeries such as laminectomy, discectomy, and spinal arthrodesis can cause soft tissue defects in the lower back. When bones or implants are exposed, because there are few bulky muscles available, reconstruction options are traditionally limited to the paraspinal muscle flap, turnover latissimus dorsi muscle flap, bipedicled paraspinous muscle flap, and free flaps[16]. Perforator flaps have gained popularity due to advances in anatomy. They are widely used in back-defect repair, in preference to local random or free flaps. Even large lumbar defects can be reconstructed using intercostal artery, lumbar artery, and superior gluteal artery perforators, and pedicled flaps[3]. A single perforator supplies a wide base of tissue[16]. It is advantageous in many ways, including mobility, desired shape, and shorter operative time. The biggest advantage is low donor site morbidity and preservation of the major back muscles, which preserves shoulder and arm function[16]. It also decreases the operation time compared to traditional flaps, which is essential considering that the surgical position is inevitably limited to the prone or lateral positions. Other options include lumbar artery and superior gluteal artery perforator flaps. They are the two major perforasomes in the lumbosacral region and have large interactions[4].
In the aforementioned case, a delayed chronic infection occurred after spinal surgery. Chronic infection was not easily noticed because the patient had no symptoms, including pain or fever, and all laboratory results were within the normal range. It might have been better if the authors suspected a neglected or subclinical infection, and the wire was removed before flap surgery. However, evidence supporting the removal of deep device was insufficient at that time. We assume that previous hematoma, wound infection at the first surgery, and repetitive spine surgery may have induced biofilm infection in the deep tissue and implant devices. Consequently, biofilm formation may have caused the chronic infection observed in our case.
Repetitive surgical wound infections and dehiscence can induce delayed infection, making complete wound healing challenging. Imaging studies and other laboratory results do not reveal significant infection; however, deep tissue
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Collart-Dutilleul PY, France; Long X, China; Mostafavinia A, Iran S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Sandy-Hodgetts K, Carville K, Leslie GD. Surgical wound dehiscence: a conceptual framework for patient assessment. J Wound Care. 2018;27:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Breder JSC, Tsukumo DML, Pereira E, Lima MH. Surgical Wound Dehiscence Treatment With Low-Level Laser Therapy and Barbatimão: A Case Report. Wound Manag Prev. 2021;67:18-22. [PubMed] |
| 3. | Ahmed O, Storey CM, Zhang S, Chelly MR, Yeoh MS, Nanda A. Vacuum-assisted closure of necrotic and infected cranial wound with loss of dura mater: A technical note. Surg Neurol Int. 2015;6:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Ploumis A, Mehbod AA, Dressel TD, Dykes DC, Transfeldt EE, Lonstein JE. Therapy of spinal wound infections using vacuum-assisted wound closure: risk factors leading to resistance to treatment. J Spinal Disord Tech. 2008;21:320-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Labler L, Keel M, Trentz O, Heinzelmann M. Wound conditioning by vacuum assisted closure (V.A.C.) in postoperative infections after dorsal spine surgery. Eur Spine J. 2006;15:1388-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006;19:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 340] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 7. | Zimmerli W. Clinical presentation and treatment of orthopaedic implant-associated infection. J Intern Med. 2014;276:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 324] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 8. | Chue AL, Moran E, Atkins BL, Byren I. Orthopaedic device associated Candidal infection - a retrospective review of patient characteristics and outcome. J Infect. 2011;63:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Barrey C, Launay O, Freitas E, Michel F, Laurent F, Chidiac C, Perrin G, Ferry T. The follow-up of patients with postoperative infection of the spine. Eur J Orthop Surg Traumatol. 2013;23 Suppl 1:S29-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Stocks G, Janssen HF. Infection in patients after implantation of an orthopedic device. ASAIO J. 2000;46:S41-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Lee K, Yoon SS. Pseudomonas aeruginosa Biofilm, a Programmed Bacterial Life for Fitness. J Microbiol Biotechnol. 2017;27:1053-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 12. | Zimmerli W. Orthopaedic device-associated infection. Clin Microbiol Infect. 2012;18:1160-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Durgun M, Baş S, Aslan C, Canbaz Y, Işık D. Use of dorsal intercostal artery perforator flap in the repair of back defects. J Plast Surg Hand Surg. 2016;50:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Komagoe S, Watanabe T, Komatsu S, Kimata Y. Pedicled Flaps Versus Free Flaps for Back Reconstruction. Ann Plast Surg. 2018;81:702-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Kato H, Hasegawa M, Takada T, Torii S. The lumbar artery perforator based island flap: anatomical study and case reports. Br J Plast Surg. 1999;52:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Di Rienzo A, Colasanti R, Liverotti V, Benigni R, Paracino R, Bizzocchi G, Scerrati M, Iacoangeli M. On-ward surgical management of wound dehiscence: report of a single neurosurgical center experience and comparison of safety and effectiveness with conventional treatment. Neurosurg Rev. 2020;43:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |