Published online Sep 26, 2023. doi: 10.12998/wjcc.v11.i27.6579
Peer-review started: June 13, 2023
First decision: August 8, 2023
Revised: August 17, 2023
Accepted: August 23, 2023
Article in press: August 23, 2023
Published online: September 26, 2023
Processing time: 98 Days and 22.3 Hours
Toripalimab and anlotinib have shown good response in esophageal cancer, with high objective response rate and progression free survival. Thus, they have been approved as second-line or above-line therapy for advanced or unresectable esophageal carcinoma. Combination of these two drugs may have synergistic effects, but evidence of which is lacking.
Here, we report on a 73-year-old male, newly diagnosed with advanced esop
The combination therapy of toripalimab and anlotinib is a promising treatment for unresectable ESCC and related clinical trials are warranted.
Core Tip: Toripalimab and anlotinib have emerged as second-line or above-line treatment options for advanced esophageal cancer. However, their objective remission rates and progression-free survival are low when used alone. We report a case of complete response after combination therapy of toripalimab and anlotinib in a patient newly diagnosed with advanced esophageal squamous cell carcinoma. The patient was in remission for 14 mo. Hence, toripalimab combined with anlotinib may be a new and effective treatment for advanced unresectable esophageal squamous cell cancer.
- Citation: Chen SC, Ma DH, Zhong JJ. Combination therapy with toripalimab and anlotinib in advanced esophageal squamous cell carcinoma: A case report. World J Clin Cases 2023; 11(27): 6579-6586
- URL: https://www.wjgnet.com/2307-8960/full/v11/i27/6579.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i27.6579
Esophageal cancers are diagnosed at an advanced stage in several cases, and most patients with radical operations are prone to relapse and metastasis[1]. For unresectable, advanced, or metastatic esophageal cancer, platinum-based chemotherapy regimens have been recommended globally for decades. The median survival duration of patients on chemotherapy is less than 1 year[1]. Compared with the poor survival outcomes of chemotherapy, immune checkpoint inhibitors and tyrosine kinase inhibitors have shown promising result. These newer therapies are now being used widely and have resulted in longer overall survival, longer progression-free survival (PFS), and lasting objective response rates[2,3].
Toripalimab exhibited remarkable anti-tumor activity in a variety of tumor types such as nasopharyngeal carcinoma, malignant melanoma, esophageal squamous cell carcinoma (ESCC), and other tumors by inhibiting the programmed cell death protein 1 (PD-1) /programmed death ligand-1 (PD-L1) pathway and, thus, promoting apoptosis[2]. Anlotinib is a new, oral, small molecule and multi-targeting tyrosine kinase inhibitor. It targets vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), and platelet-derived growth factor receptors (PDGFR), thereby inhibiting tumor angiogenesis and cell proliferation[3].
The relevant studies on combined therapy with toripalimab and anlotinib in advanced ESCC are limited[4-6]. Here we report a patient newly diagnosed ESCC, who achieved complete response (CR) after 5 cycles of combination therapy with toripalimab and anlotinib and was in remission for up to 14 mo.
A 72-year-old man complaining of pain in the upper abdomen for a period of 1 mo was admitted to the hospital on February 11, 2022.
The clinical symptoms of vague upper abdominal pain started 1 mo ago with no apparent cause.
The patient was previously in good health.
The patient had no personal or family history of cancer or liver diseases.
The abdomen was flat and tender, with no pressure or rebound pain throughout the abdomen. No abdominal masses were detected through palpation.
Tumor markers suggested increased levels of cytokeratin-19-fragment (42.3 ng/mL), squamous cell carcinoma antigen (4.6 ng/mL), carbohydrate antigen 125 (168.3 U/mL), and carbohydrate antigen 199 (44.4 U/mL).
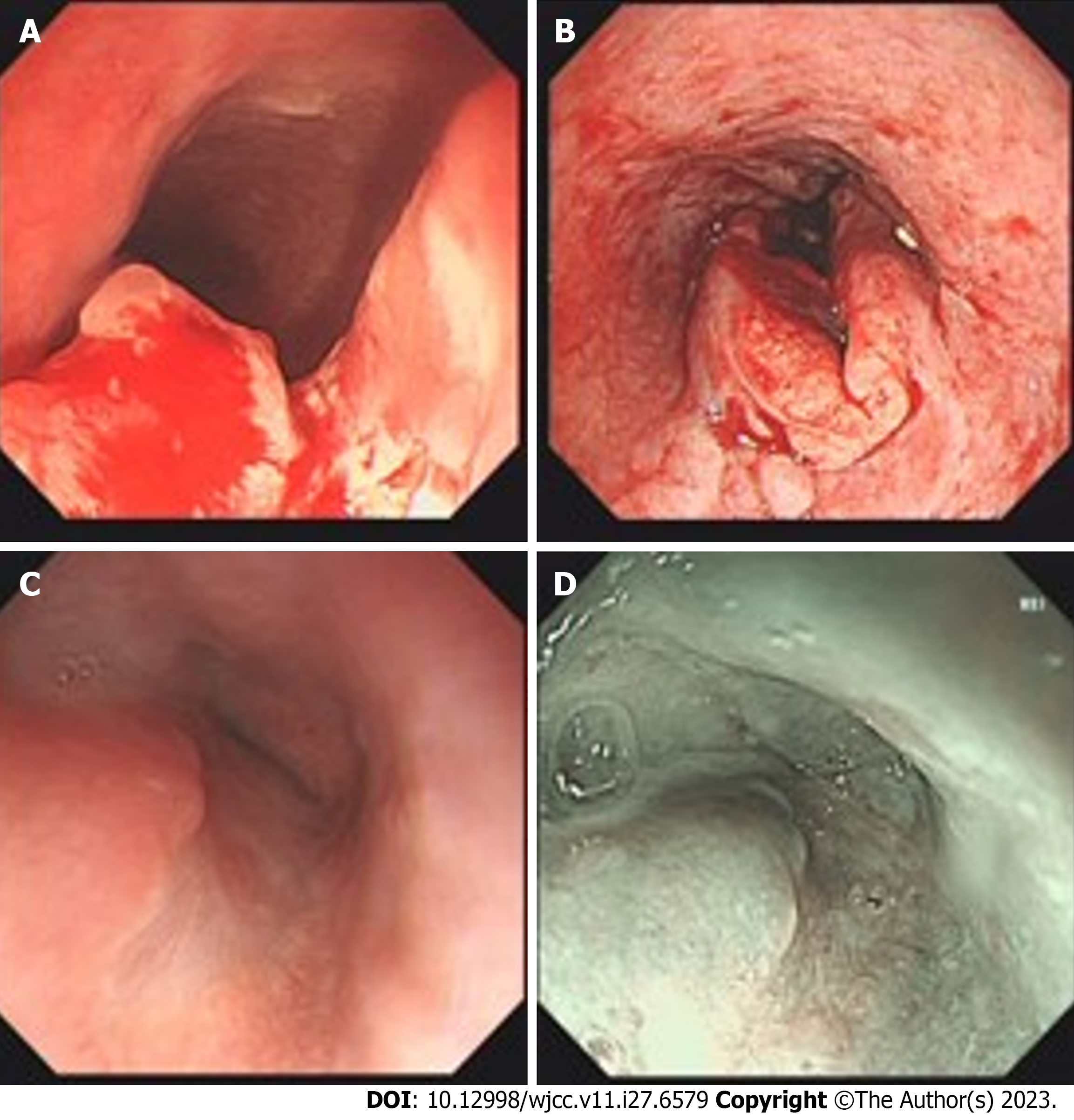
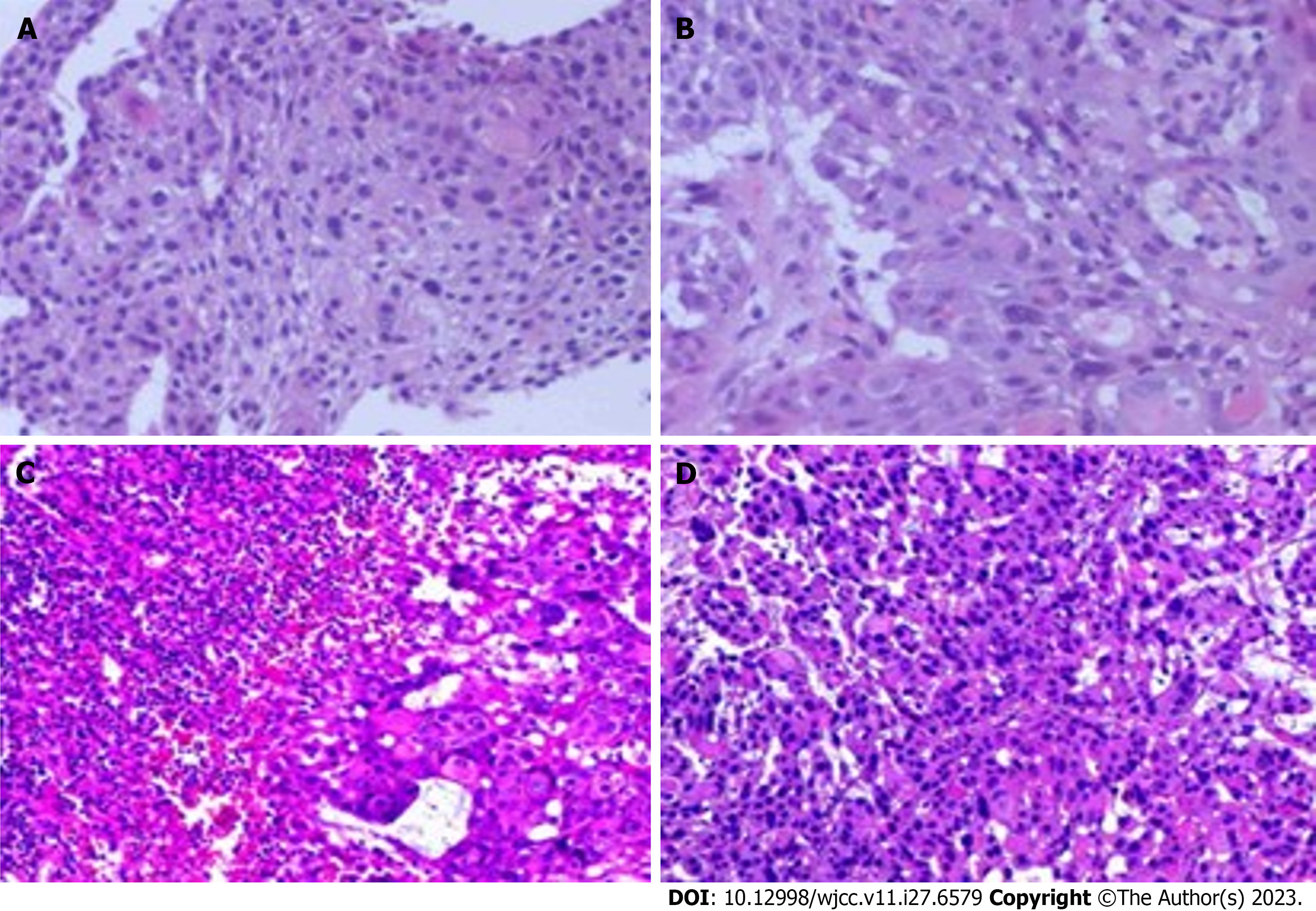
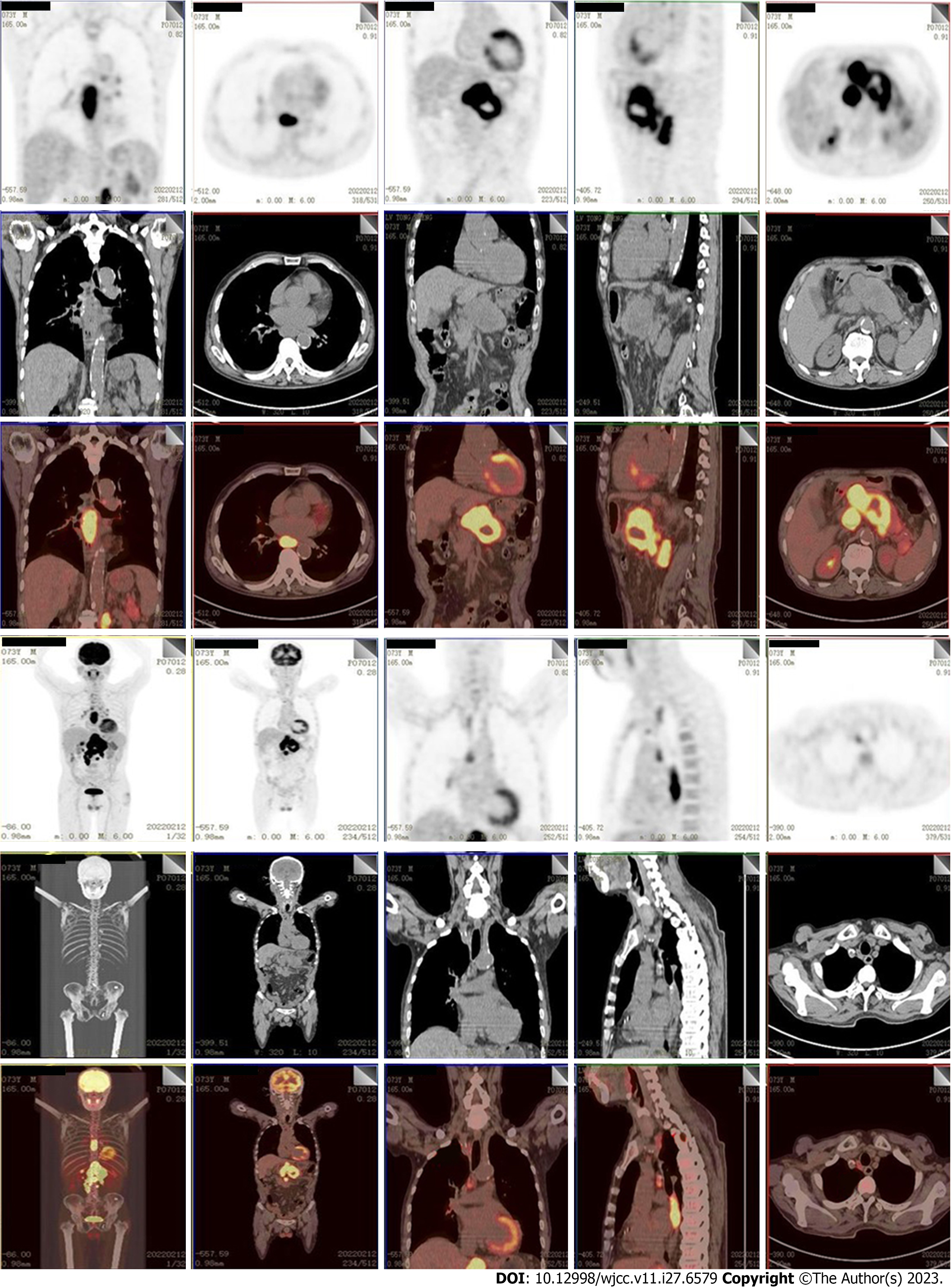
The gastroscope showed a protruded lesion with an ulcer located at the middle and distal esophagus (Figure 1). Subsequently, the presence of infiltrative squamous cell carcinoma was confirmed through endoscopic biopsy (Figure 2). Positron emission tomography/computed tomography (CT) showed a 5.2 cm × 4.4 cm × 5.4 cm hypermetabolic mass located in the body of pancreas, and thickened middle and lower segments of the esophagus. Additionally, hyper
Laparoscopic biopsy was performed to identify the primary tumor and the combined pancreatic metastases. During the operation, a hardened texture was noted on the body of the pancreas, therefore, few lymph nodes near the common hepatic artery were resected and sent for pathological examination. The pathologic findings revealed lymphatic metastasis of esophageal carcinoma (Figure 2C and D). This patient was diagnosed with ESCC based on gastroscopy and pathological examination findings. Imaging findings suggested multiple lymph node metastases in the abdomen and distal areas. Consequently, he was diagnosed with advanced and unresectable ESCC.
According to the patient’s condition and wishes, he was administered toripalimab intravenously (240 mg administered on day 1 of every 3-wk cycle) combined with anlotinib orally (12 mg administered on days 1-14 of every 3-wk cycle) post-operatively.
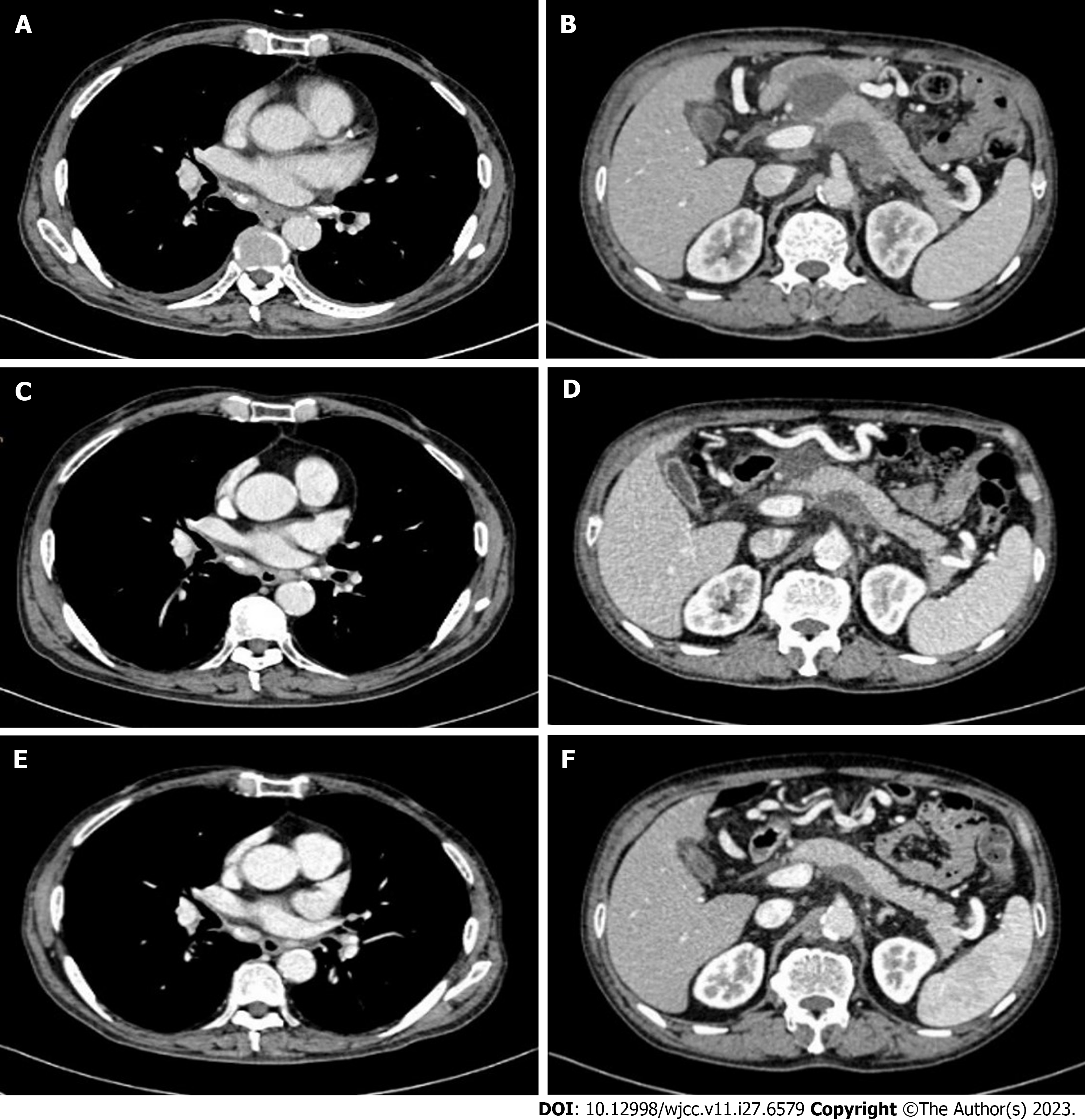
Within 1 mo of starting the combination therapy, tumor markers decreased to normal levels and CT reexamination showed a shrinkage in tumor mass and lymph nodes (Figure 4A and B). After 3 mo of treatment, repeated endoscopy showed that the esophageal tumor had diminished or disappeared, and repeated CT also revealed the disappearance of the tumor and metastatic lymph nodes (Figure 4C and D). CR was achieved according to response evaluation criteria in solid tumors criteria 1.1[7]. Based on CT reexamination, the patient was in remission for up to 14 mo since the initiation of combination therapy (Figure 4E and F).
PD-1, an inhibitory transmembrane protein, is widely expressed on T, B, natural killer, and myeloid-derived suppressor cells. By binding to its receptors PD-L1/L2, PD-1 can inhibit T cell activation or proliferation, which leads to tumor immune escape[8]. Nivolumab and pembrolizumab bind to the N-terminal loop and the C‘D loop of PD-1 respectively[2]. Comparatively, toripalimab binds to the FG loop of PD-1 and blocks the PD-1 pathway, ultimately promoting apoptosis of tumor cells[2]. NCT02915432 concluded that toripalimab displayed potential anticancer activity with a manageable safety profile in chemotherapy drug resistant ESCC[9]. JUPITER-06, a double-blind and multicenter phase III clinical trial, involving 514 advanced ESCC patients revealed that compared to a simple chemotherapy (paclitaxel plus cisplatin) regimen, toripalimab combined with chemotherapy had higher overall response rate (ORR) (69.3% vs. 52.1%) and PFS (5.7 vs. 5.5 mo)[10]. The studies of NCT03985670[11] further support the synergistic effectiveness of toripalimab plus chemotherapy in the treatment of advanced esophageal cancer.
Anlotinib inhibits tumor angiogenesis and cancer cell proliferation by inhibiting VEGFR2, PDGFRβ, FGFR1, and downstream extracellular signal-regulated kinase signal transduction[3]. In a double-blind randomized phase II clinical trial (NCT02649361) involving 165 patients with recurrent or metastatic ESCC who were previously treated with chemotherapy, anlotinib was used to treat 110 patients, and the remaining 55 patients were given a placebo. The trial reported a prolonged PFS (3.02 vs. 1.41 mo) in patients who received anlotinib[12]. A multicenter, single-arm, phase II clinical trial (NCT04063683), involving 47 patients with previously untreated metastatic or unresectable locally advanced ESCC is currently underway, wherein anlotinib in combination with a chemotherapy regimen (paclitaxel and cisplatin) is being used for treatment. The preliminary results of this trial have shown a high ORR (74.1%) with the most significant adverse reactions being myelosuppression (18.5%) and hypertension (7.4%)[13]. These studies support the use of anlotinib as second-line treatment for advanced ESCC, either as monotherapy or in combination with chemotherapy.
Previous studies have demonstrated the potential synergistic anti-tumor effects of immunotherapy and targeted therapies, which were closely linked to the tumor microenvironment[14]. Intratumoral angiogenesis is essential for tumors to survive and grow. Dilated and fragile neovascularization predisposes the tumor tissue to hypoperfusion, thus leading to intratumoral hypoxia and acidosis[15]. Hypoxia and acidosis suppress anti-tumor immunity by the aggregation of immune suppressor cells (Treg and regulatory B cells) and by inhibiting the activation of immune effector cells (activated CD8+ T and NK cells)[15]. In addition, increased vascular permeability and lymphatic drainage disorders increase the interstitial pressure around the tumor, which inhibits the delivery of drugs and immune effector cells to tumor[16]. The combination of amlotinib and teraplizumab exerted a synergistic anti-tumor effect. Anlotinib normalizes the tumor vasculature, upregulates the adhesion molecules in the tumor endothelium and induces a relatively immune-supportive tumor microenvironment by inhibiting VEGFR. Furthermore, anlotinib promotes the differentiation and maturation of dendritic cells, enhancing their ability to present tumor antigens to T cells. As a consequence, the activation and penetration of the effector T cells in tumor cells are enhanced, which improves the efficacy of toripalimab[17].
Combination anti-PD-1 and targeted therapy is increasingly widely used for treatment of several malignancies such as gastric cancer, non-small cell lung cancer, and liver cancer, but its use is uncommon in ESCC[18-20]. Liu et al[5] retrospectively reviewed and analyzed 98 patients with advanced ESCC, wherein 48 patients were administered anlotinib plus an anti-PD-1 and remaining 50 patients received anlotinib monotherapy. Overall, patients receiving the combination therapy showed a longer PFS (5.4 vs. 3.0 mo) with a higher ORR (23.9% vs. 10.4%) than the patients receiving anlotinib monotherapy[5]. Additionally, Tang et al[6] reported that a postoperative recurrent ESCC patient receiving a combination therapy of nivolumab and anlotinib showed CR. Similarly, Jiang and Zhang[4] reported that a patient with chemo-resistant small-cell carcinoma of the esophagus achieved CR after 3 mo of toripalimab plus anlotinib. Our study is the first report where toripalimab in combination with anlotinib is being used as the first-line treatment for advanced or unresectable ESCC. The patient showed CR in 3 mo and has been in remission for up to 14 mo of follow-up with no treatment-related adverse events.
The potential for drug resistance and side effects associated with long-term drug use is a concern, although this did not occur in our case. In addition, since medical insurance can only reimburse a percentage of the cost, the long-term use of such high-priced drugs can further increase the financial burden of patients.
Our case report suggests that the combination of toripalimab and anlotinib may be a potential treatment option for advanced or unresectable ESCC. Clinical trials studying the use of this combination for treating ESCC should be conducted in the future to further verify these findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Christodoulidis G, Greece; Nagahara H, Japan S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | He S, Xu J, Liu X, Zhen Y. Advances and challenges in the treatment of esophageal cancer. Acta Pharm Sin B. 2021;11:3379-3392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 169] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 2. | Zhang L, Hao B, Geng Z, Geng Q. Toripalimab: the First Domestic Anti-Tumor PD-1 Antibody in China. Front Immunol. 2021;12:730666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 3. | Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, Zhao F, Ahmad R, Zhao J. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 439] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 4. | Jiang M, Zhang X. Antiangiogenesis Combined with Immunotherapy to Treat Advanced Small-Cell Carcinoma of the Esophagus Resistant to Chemotherapy: According to the Guidance of Next-Generation Sequencing. Onco Targets Ther. 2021;14:1613-1621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Liu Y, Ge Q, Xu S, Li K, Liu Y. Efficacy and safety of anlotinib plus programmed death-1 blockade verus anlotinib monotherapy as second or further-line treatment in advanced esophageal squamous cell carcinoma: A retrospective study. Front Oncol. 2022;12:942678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Tang Y, Ou Z, Yao Z, Qiao G. A case report of immune checkpoint inhibitor nivolumab combined with anti-angiogenesis agent anlotinib for advanced esophageal squamous cell carcinoma. Medicine (Baltimore). 2019;98:e17164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, Hayes W, Hodi FS, Hoekstra OS, Huang EP, Lin N, Liu Y, Therasse P, Wolchok JD, Seymour L. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer. 2016;62:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 1328] [Article Influence: 147.6] [Reference Citation Analysis (0)] |
| 8. | Thuss-Patience P, Stein A. Immunotherapy in Squamous Cell Cancer of the Esophagus. Curr Oncol. 2022;29:2461-2471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Wang F, Ren C, Zhao Q, Xu N, Shen L, Dai G, Yuan X, Chen Y, Yang S, Shi J, Hu X, Lin X, Zhang Q, Feng J, Ba Y, Liu Y, Li W, Shu Y, Wang F and Xu R-h. Association of frequent amplification of chromosome 11q13 in esophageal squamous cell cancer with clinical benefit to immune check point blockade. Journal of Clinical Oncolog. 37:4036-4036. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Yamamoto S, Kato K. JUPITER-06 establishes immune checkpoint inhibitors as essential first-line drugs for the treatment of advanced esophageal squamous cell carcinoma. Cancer Cell. 2022;40:238-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Xing W, Zhao L, Zheng Y, Liu B, Liu X, Li T, Zhang Y, Ma B, Yang Y, Shang Y, Fu X, Liang G, Yuan D, Qu J, Chai X, Zhang H, Wang Z, Lin H, Liu L, Ren X, Zhang J, Gao Q. The Sequence of Chemotherapy and Toripalimab Might Influence the Efficacy of Neoadjuvant Chemoimmunotherapy in Locally Advanced Esophageal Squamous Cell Cancer-A Phase II Study. Front Immunol. 2021;12:772450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 12. | Huang J, Xiao J, Fang W, Lu P, Fan Q, Shu Y, Feng J, Zhang S, Ba Y, Zhao Y, Liu Y, Bai C, Bai Y, Tang Y, Song Y, He J. Anlotinib for previously treated advanced or metastatic esophageal squamous cell carcinoma: A double-blind randomized phase 2 trial. Cancer Med. 2021;10:1681-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Wang J, Luo S, Li N, Wu T, Hong Y, Guo Y, Cheng Y, Li B and Tan B. Update results of paclitaxel and cisplatin in combination with anlotinib as first-line regimen for advanced esophageal squamous cell carcinoma (ESCC): A multicenter, single-arm, open-label phase Ⅱ clinical trial. Journal of Clinical Oncology. 2021;39:181-181. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Guo CX, Huang X, Xu J, Zhang XZ, Shen YN, Liang TB, Bai XL. Combined targeted therapy and immunotherapy for cancer treatment. World J Clin Cases. 2021;9:7643-7652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 15. | Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20:409-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 1016] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 16. | Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 1396] [Article Influence: 199.4] [Reference Citation Analysis (0)] |
| 17. | Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis. 2017;20:185-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 506] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 18. | Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Lee A, Coleman S, Deng Y, Kowanetz M, Shankar G, Lin W, Socinski MA; IMpower150 Study Group. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7:387-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 714] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 19. | He W, Leng X, Mao T, Luo X, Zhou L, Yan J, Peng L, Fang Q, Liu G, Wei X, Wang K, Wang C, Zhang S, Zhang X, Shen X, Huang D, Yi H, Bei T, She X, Xiao W, Han Y. Toripalimab Plus Paclitaxel and Carboplatin as Neoadjuvant Therapy in Locally Advanced Resectable Esophageal Squamous Cell Carcinoma. Oncologist. 2022;27:e18-e28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 20. | Han C, Ye S, Hu C, Shen L, Qin Q, Bai Y, Yang S, Bai C, Zang A, Jiao S, Bai L. Clinical Activity and Safety of Penpulimab (Anti-PD-1) With Anlotinib as First-Line Therapy for Unresectable Hepatocellular Carcinoma: An Open-Label, Multicenter, Phase Ib/II Trial (AK105-203). Front Oncol. 2021;11:684867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |