Published online Sep 26, 2023. doi: 10.12998/wjcc.v11.i27.6565
Peer-review started: June 6, 2023
First decision: August 4, 2023
Revised: August 11, 2023
Accepted: August 25, 2023
Article in press: August 25, 2023
Published online: September 26, 2023
Processing time: 103 Days and 7.7 Hours
With the withdrawal of paraquat from the market, diquat is widely used, so the treatment of diquat poisoning has become one of the focuses of emergency poisoning diagnosis and treatment.
We studied the case of a 17-year-old male patient who drank 200 mL (20 g/100 mL) of diquat solution two hours before arriving at the hospital. Despite the use of treatments such as gastric lavage, hemoperfusion, continuous hemodialysis, glucocorticoids, and organ support, the patient’s condition rapidly progressed to multiorgan failure, and he died 23.5 h after admission.
We summarized the clinical characteristics and treatment strategies of diquat poisoning through this case and performed a literature review to provide a basis and direction for clinical treatment.
Core Tip: The clinical manifestations of diquat poisoning are mainly local mucosal injury and multiple organ damage. The earlier organ function damage occurs, and the more systems are involved, the poorer the prognosis is. In addition, the prognosis of patients is significantly correlated with the poisoning dose, and fulminant poisoning has a high mortality rate. In the treatment of patients, early and adequate removal of toxins is the focus of treatment, and comprehensive and systematic organ function evaluation and support is also an important part of treatment.
- Citation: Fan CY, Zhang CG, Zhang PS, Chen Y, He JQ, Yin H, Gong XJ. Acute diquat poisoning case with multiorgan failure and a literature review: A case report. World J Clin Cases 2023; 11(27): 6565-6572
- URL: https://www.wjgnet.com/2307-8960/full/v11/i27/6565.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i27.6565
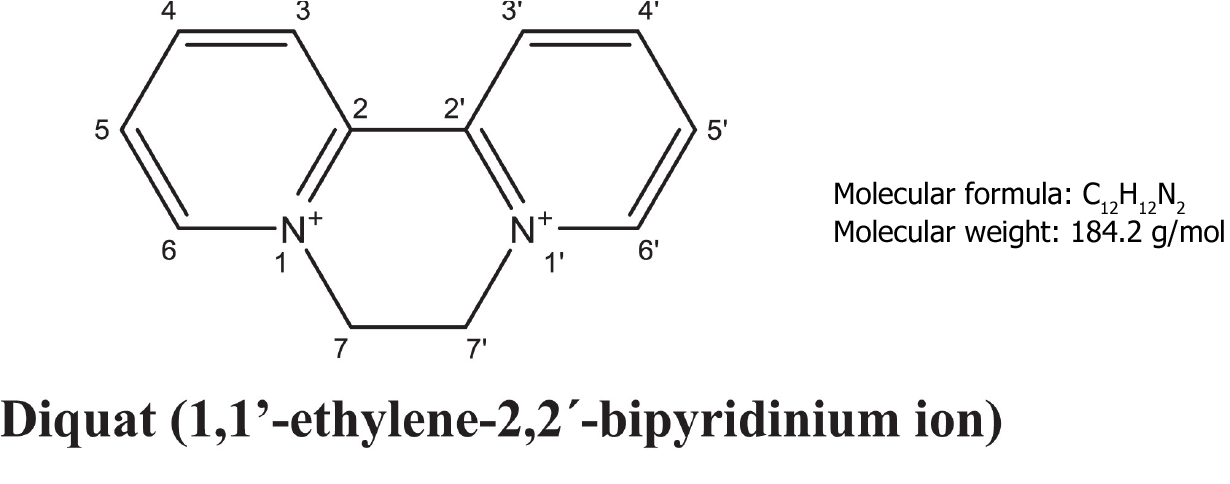
Diquat (1,1’-ethylene-2,2’-bipyridinium ion; DQ; Figure 1) is a nonselective and fast-acting herbicide that belongs to the bipyridine compound family with paraquat[1]. At present, 20% diquat aqueous solution is mainly used in agriculture. With the phasing out of the sale and use of paraquat in our country, the use of diquat has gradually increased. Even though, diquat is less toxic than paraquat, the mechanism of diquat poisoning is not clear, and there is no specific antidote for the treatment of poisoning. Therefore, the disposal of diquat poisoning remains a challenge in emergency departments[2].
A 17-year-old previously healthy male patient was admitted to the emergency department due to intentional ingestion of diquat on August 11, 2021.
Two hours earlier, the patient was found by a friend after taking 200 mL of diquat solution (20 g/100 mL) orally. Later, the patient started to experience nausea, vomiting, and abdominal pain. The patient’s vomit was gastric contents with bloody components. The patient was sent to another hospital for gastric lavage one hour prior and then was sent to our department.
He was healthy before.
The patient denied any family history.
The patient’s vital signs when he first visited our hospital were as follows: Temperature 37.2 °C, blood pressure 19.7/15.6 kPa, heart rate 104 beats/min, respiratory rate 24 breaths/min, and arterial oxygen saturation (breathing room air) 100%. Physical examination showed that he had tachycardia, and his bowel sounds were weak. All other physical examination results were normal.
Arterial blood gas analysis revealed the following: pH (7.49), PCO2 (13 mmHg), PO2 (157 mmHg), HCO3- (9.9 mmol/L), lactic acid (4.0 mmol/L), and anion gap (19 mmol/L). A complete blood count revealed the following: White blood cell count (23.67 × 109/L), hemoglobin (181.00 g/L), platelet count (316.00 × 109/L), and neutrophil % count (90.10%). The biochemical data were as follows: Blood urea nitrogen (5.4 mmol/L), creatinine (92.0 mmol/L), and myoglobin (77.47 ng/mL). The alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, direct bilirubin, creatine kinase, and cardiac troponin I concentrations were within their normal ranges. The coagulation function test data were as follows: Prothrombin time (10.7 s), prothrombin activation (115.1%), international normalized ratio (0.89), activated partial thromboplastin time (23.5 s), and D-dimer (0.5 mg/LFEU).
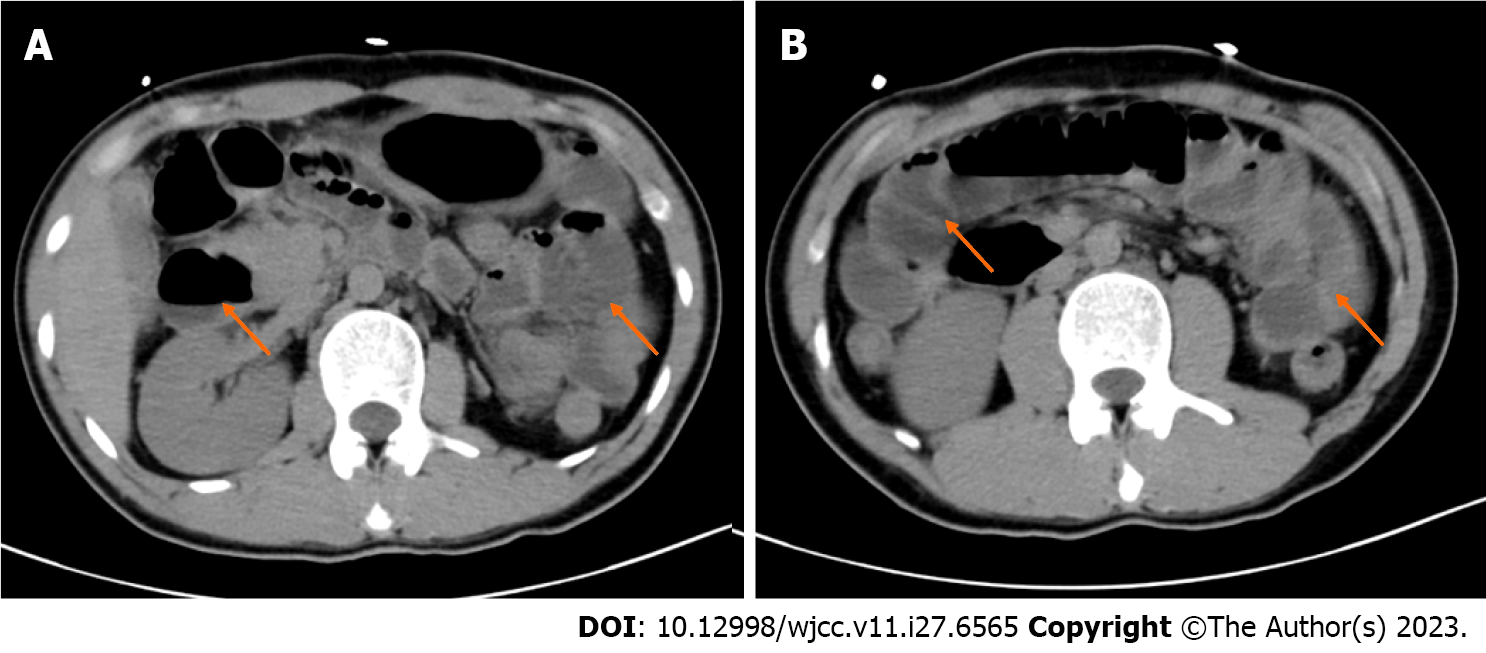
The results of imaging examinations were as follows: Abdominal computed tomography (CT) showed small intestinal effusion and gas accumulation (Figure 2). Brain and chest CT scans were normal.
The patient was diagnosed as diquat poisoning.
The patient ingested 40 g of diquat. Although the patient had been treated with gastric lavage in other hospitals, the blood concentration of diquat was still high (51 mg/L). We used gastrointestinal decompression, montmorillonite powder adsorption, glycerin enema catharsis, intravenous fluid infusion, furosemide diuresis and other methods to promote the elimination of toxic substances. Hemoperfusion was performed 6 h after exposure (total 4 h). Hemodialysis was performed after hemoperfusion. Intravenous vitamin C, glutathione, and methylprednisolone were administered as well. Another important aspect of treatment is organ function assessment and supportive care. About 6.5 h the admission, the patient had seizures and loss of consciousness, along with hypoxemia, so we administered sedation and mechanical ventilation. Twenty hours after the patient’s admission, the laboratory test results markedly deteriorated, as shown in Table 1.
| Item | On admission | 20 h post-admission |
| WBC count (4-10 × 109/L) | 23.67 | 24.2 |
| Urea (2.6-7.5 mmol/L) | 5.4 | 5.7 |
| Creatinine (57-97 μmol/L) | 92 | 140.3 |
| ALT (7-40 U/L) | 11.7 | 2084.9 |
| AST (13-35 U/L) | 10.1 | 2633 |
| Lac (0.5-1.7 mmol/L) | 4.0 | 9.4 |
| PTA (70%-130%) | 115.1 | 18.9 |
| INR (0.8-1.2) | 0.89 | 3.63 |
| Myoglobin (28-72 ng/mL) | 77.47 | 9703 |
| Creatine kinase (50-310 U/L) | 604 | > 22000 |
Despite the above active treatment, the patient’s condition deteriorated progressively, and the patient was declared clinically dead 23.5 h after admission.
CNKI and Wanfang data were searched with “Diquat” as the key words, and PubMed, Cochrane Library, and Embasewere searched with “Diquat” and “case” as the key words. The date of literature retrieval was from the database founding to May 1, 2023. The inclusion criteria were as follows: Acute poisoning, the poison was not mixed with any other poison, and the patient was exposed to the poison orally. The exclusion criteria were as follows: End-stage nonchronic diseases; age > 14 years old; and relatively complete clinical data. The exclusion criteria were as follows: Studies with incomplete data, abstracts, reviews, systematic reviews, experience summaries, theoretical discussions, etc. Animal experiments, in vitro experiments, etc. (Table 2)[3-13].
| Case | Gender | Age | Dose (g) | Organ function damage | Measures of treatment | Prognosis | |
| Outcome | Duration | ||||||
| 1[3] | Male | 21 | 20 | AKI, liver dysfunction, epilepsy, brain stem infarction, leukocytosis | Gastric lavage, HP + CVVH, MV, glucocorticoids | Death | 96.0 h |
| 2[3] | Female | 26 | 10 | AKI, liver dysfunction, epilepsy, mental and behavioral abnormalities, leukocytosis | Gastric lavage, HP + CVVH, glucocorticoids | Alive | - |
| 3[4] | Female | 24 | 60 | AKI, liver dysfunction, epilepsy, rhabdomyolysis, leukocytosis, myocardial injury | Gastric lavage, HP + CVVH | Death | 17.8 h |
| 4[5] | Female | 27 | 4 | Gastrointestinal Symptoms | Gastric lavage, HP + CVVH | Alive | - |
| 5[5] | Female | 30 | 4 | Gastrointestinal symptoms, leukocytosis | Gastric lavage, HP | Alive | - |
| 6[5] | Female | 68 | 10 | AKI, liver dysfunction, toxic encephalopathy, leukocytosis, lung injury | Gastric lavage, HP | Death | Unknown |
| 7[5] | Male | 24 | 10 | AKI, leukocytosis, myocardial Injury | Gastric lavage, HP + CVVH | Alive | - |
| 8[5] | Male | 33 | 20 | AKI, liver dysfunction, leukocytosis, lung injury, myocardial injury | Gastric lavage, HP + CVVH | Death | Unknown |
| 9[5] | Male | 62 | 40 | AKI, toxic encephalopathy, lung injury, myocardial injury, leukocytosis | Gastric lavage, HP + CVVH | Death | Unknown |
| 10[6] | Female | 32 | 8 | AKI, liver dysfunction, epilepsy, leukocytosis, shock | Gastric lavage, HP + CVVH, MV, glucocorticoids | Death | 68.5 h |
| 11[7] | Male | 29 | 50 | AKI, liver dysfunction, leukocytosis | Gastric lavage, HP + CVVH, MV, glucocorticoids | Death | 480.0 h |
| 12[8] | Female | 41 | 40 | AKI, liver dysfunction, leukocytosis, rhabdomyolysis | Gastric lavage, HP, glucocorticoids | Death | 46.0 h |
| 13[9] | Male | 17 | 40 | AKI, liver dysfunction, paralytic ileus, coma, myocardial injury, leukocytosis, rhabdomyolysis, shock | Gastric lavage, HP + CVVH, MV, glucocorticoids | Death | 40.5 h |
| 14[9] | Female | 18 | 40 | AKI, liver dysfunction, paralytic ileus, coma, leukocytosis, rhabdomyolysis, arrhythmia | Gastric lavage, HP + CVVH, glucocorticoids | Death | 29.0 h |
| 15[10] | Male | 21 | 20 | AKI, liver dysfunction, Pontine demyelination, Lung injury, leukocytosis | Gastric lavage, HP + CVVH, glucocorticoids | Death | 360.0 h |
| 16[11] | Female | 36 | 6 | AKI, liver dysfunction, leukocytosis, lung injury, rhabdomyolysis | Gastric lavage, HP + CVVH, glucocorticoids | Alive | - |
| 17[12] | Male | 30 | 32 | AKI, liver dysfunction, respiratory failure, leukocytosis | Gastric lavage, HP + CVVH, MV | Death | 312.0 h |
| 18[13] | Male | 20 | 32 | AKI, liver dysfunction, central pontine myelinolysis, leukocytosis | Gastric lavage, HP + CVVH, MV, glucocorticoids | Alive | - |
A total of 63 Chinese articles and 76 English articles were retrieved. According to the inclusion and exclusion criteria, 127 of them were excluded, and 11 of them were finally analyzed.
General clinical data: A total of 18 patients were enrolled, including 9 males and 9 females, with an average age of 31.1 years (range: 17-68 years). The average oral dose of diquat was 24.8 g (range, 4-60 g), and the median time from ingestion to gastric lavage was 3.5 h (range, 0.7-19.0 h).
Clinical features: Clinical manifestations: The clinical manifestations of diquat poisoning are mainly local mucosal injury and multiple organ damage. The patients had different degrees of local mucosal injury manifestations, such as sore throat, nausea, vomiting, and abdominal pain, minutes to hours after taking the poison. Among them, 2 patients had paralytic intestinal obstruction, and 14 patients had liver dysfunction (ALT: 70-1222 U/L, AST: 65-2116 U/L). Sixteen patients had acute kidney injury (Cre 124.0-1006.6 μmol/L), 10 patients had toxic encephalopathy (manifested as seizures, coma, mental and behavior abnormalities, acute demyelination changes and brain edema on CT, etc.), 17 patients had increased white blood cell counts (13.1-41.6 × 109/L), and 5 patients had rhabdomyolysis.
Seventeen patients received hemoperfusion therapy, and 14 patients received hemodialysis. Ten patients received glucocorticoid therapy, and 12 patients died.
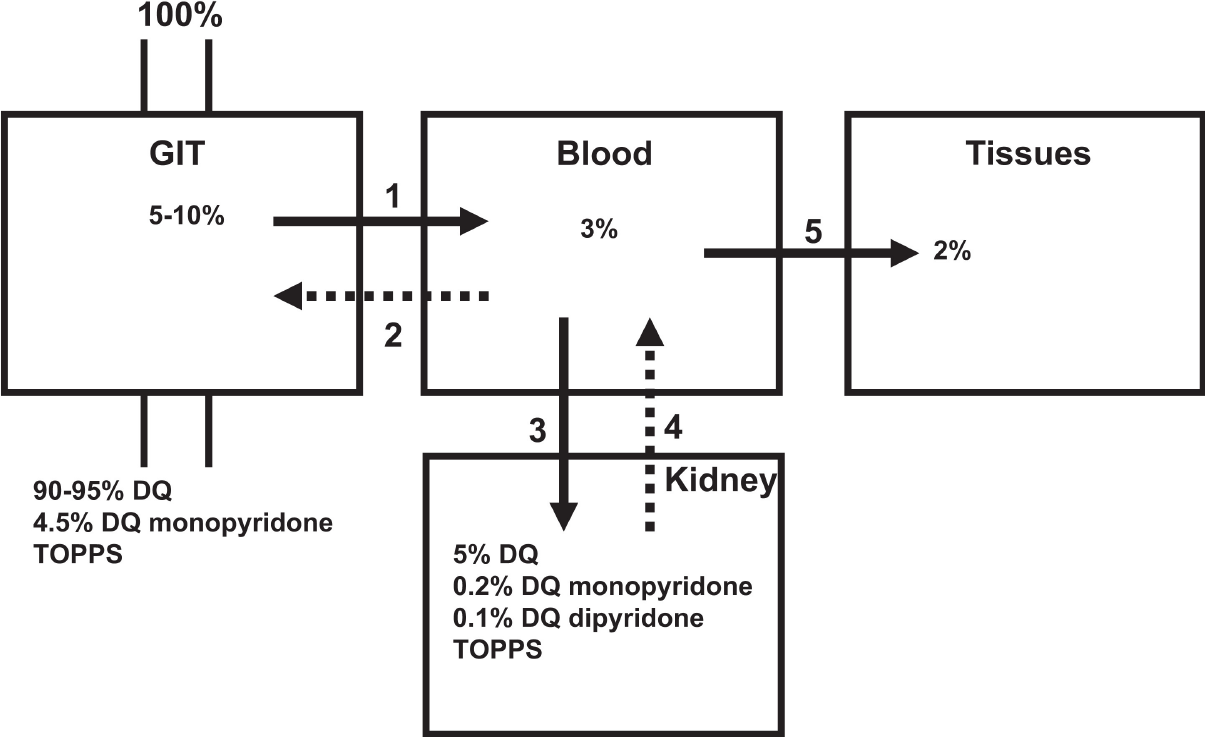
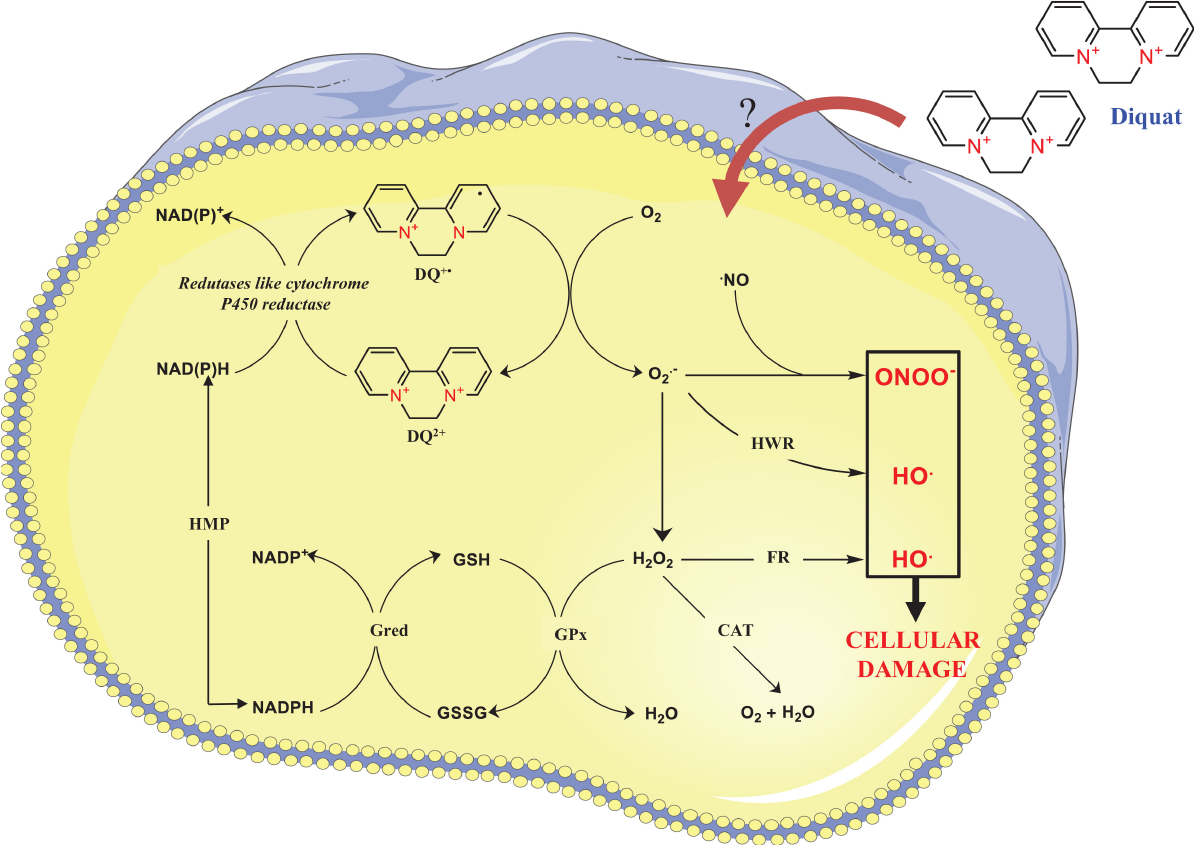
Diquat can be absorbed through multiple pathways (Figure 3)[2]. Most patients with diquat poisoning deliberately ingest it orally, and it is absorbed through the digestive tract. Diquat can also produce toxic reactions through exposure to the lung, eye, or skin, but due to its hydrophilic nature, the absorption ratio in the lung and skin is not high[14]. Most of the diquat ingested through the digestive tract is excreted in feces within 24 h, while the diquat absorbed into the blood circulation mainly accumulates in the kidney and liver and is excreted mainly by the kidney[1]. The mechanism of DQ poisoning is believed to be mainly related to oxidative stress. DQ may lead to oxidative stress due to the ability of DQ to generate reactive oxygen species (ROS) intracellularly through reduction-oxidation (redox) cycling processes (Figure 4)[1]. At the same time, diquat causes neurodegeneration and reproductive and developmental toxicity[15,16]. The lethal dose to humans is approximately 6-12 g. Ingestion of > 12 g of diquat cation (20% aqueous solution of diquat > 112.2 mL, Diquat dibromide monohydrate 22.4 g) can rapidly progress to multiple organ failure, and most patients die within 24-48 h, which is also called fulminant poisoning[2].
The clinical features of oral diquat poisoning are mainly local damage and multiple organ damage. Digestive system: Patients may have gastrointestinal mucosal damage, such as sore throat, nausea, vomiting, liver damage, pancreatitis and other manifestations. In this review, a total of 14 patients (78.8%) had liver function damage, and some patients were complicated with acute liver failure. One patient developed acute pancreatitis. In severe cases, paralytic ileus can occur within 1-4 d after exposure to the poison, leading to the accumulation of a large amount of fluid in the intestine, resulting in hypovolemic shock and multiple organ damage. Therefore, some scholars believe that paralytic intestinal obstruction often indicates a poor prognosis in patients with diquat poisoning[17]. In this review, 2 patients (11.1%) had paralytic ileus, and the clinical outcome was death. Acute kidney injury: Renal function damage can occur in the early stage of diquat poisoning. In this review, 16 patients (88.9%) had acute kidney injury, and 14 of them received renal replacement therapy. The clinical manifestations of renal injury are related to the severity of renal injury. Renal tubular dysfunction is the initial manifestation of renal injury caused by diquat poisoning. The main pathogenesis of renal injury caused by diquat poisoning is acute tubular necrosis after toxicant exposure, blockage of renal tubules by exfoliated renal tubular epithelial cells, and reabsorption of original urine into blood through the exposed renal tubular wall through the “reflux” mechanism, leading to a significant decrease in glomerular filtration rate and delayed recovery of renal function[18]. Toxic encephalopathy: Patients may manifest symptoms such as coma, epileptic seizures or mental and behavioral abnormalities, and imaging examination can show cerebral infarction, brain edema, and acute demyelination changes. In this review, 10 patients (55.6%) had central nervous system injuries. Studies have confirmed that diquat can penetrate the blood-brain barrier, which is also thought to be one of the reasons why diquat is more neurotoxic than paraquat. Lung injury: Studies have shown that diquat poisoning causes fewer pulmonary exudative lesions than paraquat poisoning. The hydrophilic property of diquat leads to a low lung absorption rate. Also, diquat does not meet the substrate structure requirements of the pulmonary polyamine uptake system, so lung tissue cannot take up diquat[19,20]. Even so, patients with fulminant diquat poisoning may still present with pulmonary edema and respiratory failure. Because oxygen free radicals can increase toxicity, the Chinese expert consensus does not recommend active oxygen therapy, and oxygen therapy is only recommended when the patient has hypoxemia. In this review, 6 patients (33.3%) required mechanical ventilation, but some patients were intubated for respiratory support due to seizures and other conditions, which were not caused by lung lesions. Therefore, the data from this review could not accurately evaluate the real situation of respiratory system involvement. In addition to the above conditions, clinical manifestations of myocardial damage, rhabdomyolysis and blood system involvement can also be seen. The earlier organ function damage occurs, the worse the prognosis[21].
There is no specific antidote for diquat poisoning. The main treatment methods include the following: Termination of toxicant exposure, mainly including gastric lavage and blood purification. Gastric lavage performed as early as possible, especially within one hour, has the best effect of removing the poison. One patient[13] in our review who ingested 32 g of diquat survived with treatment. Analysis of the treatment process of this patient shows that the blood drug concentration of this patient was only 0.93 mg/L, which was inseparable from the patient’s adequate gastric lavage treatment after 40 min of toxicant exposure.Although hemoperfusion is superior in the removal of toxic substances, hemodialysis can better maintain the stability of the internal environment, remove some inflammatory factors, and replace renal function. Therefore, hemoperfusion combined with hemodialysis is recommended in clinical practice[15]. The study[22] have shown that the peak time of DQ concentration is within 3 h of ingestion, and the excretion of DQ is complete 48-72 h after ingestion. Therefore, delayed hemoperfusion is not beneficial to the prognosis of patients. However, the actual start time of hemoperfusion is affected by many factors, such as the time of patients’ arrival at the hospital, whether the first hospital has the qualification of hemoperfusion treatment, the time for patients and their families to consider, and the economic situation. At present, the commonly used antioxidation and oxygen free radical scavenging drugs are mainly N-acetylcysteine and reduced glutathione. As mentioned above, the lung involvement in diquat poisoning is less severe than that in paraquat poisoning. Therefore, the efficacy of routine use of high-dose glucocorticoids and immunosuppressants on diquat poisoning is not clear. A total of 8 patients (44.4%) received glucocorticoid therapy, but due to the poor consistency of other clinical treatments (including the time and adequacy of gastric lavage, the time and method of starting hemoperfusion, etc.), the effect of glucocorticoids on prognosis cannot be analyzed according to the existing data, which is also one of the limitations of this paper. Organ function support and symptomatic treatment mainly included hemodialysis, mechanical ventilation, extracorporeal membrane oxygenation, nutritional support, etc.
The clinical manifestations of diquat poisoning are mainly local mucosal injury and multiple organ damage. The earlier organ function damage occurs, and the more systems are involved, the poorer the prognosis is. In addition, the prognosis of patients is significantly correlated with the poisoning dose, and fulminant poisoning has a high mortality rate. In the treatment of patients, early and adequate removal of toxins is the focus of treatment, and comprehensive and systematic organ function evaluation and support is also an important part of treatment. At present, the specific scheme and evaluation methods of hemoperfusion for patients with diquat poisoning and the benefits of glucocorticoid treatment need to be further explored and evaluated.
We would like to thank the patient and his family. We extend our thanks to the, laboratory, radiology, and ICU of the Beijing Tsinghua Changgung Hospital for facilitating the acquisition of the relevant materials.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Emergency medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Juneja D, India S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Magalhães N, Carvalho F, Dinis-Oliveira RJ. Human and experimental toxicology of diquat poisoning: Toxicokinetics, mechanisms of toxicity, clinical features, and treatment. Hum Exp Toxicol. 2018;37:1131-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 2. | Zhang JS. Expert consensus on diagnosis and treatment of acute dimethylene poisoning. Zhonghua Jizhen Yixue Zazhi. 2020;29:1282-1289. [DOI] [Full Text] |
| 3. | Wang JJ, Tong S, Zhang TJ. Seven cases of toxic encephalopathy associated with diquat poisoning. Zhonghua Jizhen Yixue Zazhi. 2022;31:1648-1653. [DOI] [Full Text] |
| 4. | Chen YQ, Chen K. Analysis of 1 case of convulsion death caused by large dose of diquat poisoning. Zhongguo Gongyeweisheng Yu Zhiyebing Zazhi. 2022;40:75-77. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 5. | Zhao XM, Zhu JJ. A new understanding of acute poisoning by ingestion of diquat:paraquat′s twin brother-a summary of 6 cases and a review of the literature. Zhongguo Zhongzhengyixue Zazhi. 2018;38:493-496. [DOI] [Full Text] |
| 6. | Cai XL, Teng L. Four cases of acute diquat poisoning with prominent epileptoid seizure and literature review. Zhongguo Gongyeweisheng Yu Zhiyebing Zazhi. 2021;39:359-362. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Yuan LL, Mai ZJ. Clinical analysis of 6 cases of acute dimethylene poisoning. Zhongguo Gongyeweisheng Yu Zhiyebing Zazhi. 2019;37:468-470. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Yang M, Xiong W. The effect of diagnosis and treatment of a patient with rhabdomyolysis caused by diachronium poisoning. Dangdai Yixue Luntan. 2020;18:148-150. [DOI] [Full Text] |
| 9. | Yu G, Wang J, Jian T, Shi L, Zhao L, Li Y, Gao Y, Kan B, Jian X. Case series: Diquat poisoning with acute kidney failure, myocardial damage, and rhabdomyolysis. Front Public Health. 2022;10:991587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 10. | Xing J, Chu Z, Han D, Jiang X, Zang X, Liu Y, Gao S, Sun L. Lethal diquat poisoning manifesting as central pontine myelinolysis and acute kidney injury: A case report and literature review. J Int Med Res. 2020;48:300060520943824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 11. | Feng D, Fu L, Du X, Yao L. Acute diquat poisoning causes rhabdomyolysis. Am J Med Sci. 2022;364:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Huang Y, Zhang R, Meng M, Chen D, Deng Y. High-dose diquat poisoning: a case report. J Int Med Res. 2021;49:3000605211026117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Yu G, Jian T, Cui S, Shi L, Kan B, Jian X. Acute diquat poisoning resulting in toxic encephalopathy: a report of three cases. Clin Toxicol (Phila). 2022;60:647-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Feldmann RJ, Maibach HI. Percutaneous penetration of some pesticides and herbicides in man. Toxicol Appl Pharmacol. 1974;28:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 198] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Wang WZ, Liu Q. Effects and significance of continuous hemoperfusion on patients with diquat poisoning. Zhongguo Zhongzheng Yixue. 2022;34:1320-1324. [DOI] [Full Text] |
| 16. | Circu ML, Maloney RE, Aw TY. Diquat-induced cellular pyridine nucleotide redox changes and alteration of metabolic enzyme activities in colonic carcinoma cells. Chem Biol Interact. 2017;264:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Vanholder R, Colardyn F, De Reuck J, Praet M, Lameire N, Ringoir S. Diquat intoxication: report of two cases and review of the literature. Am J Med. 1981;70:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Zhang HZ, Sun H. Renal biopsy of acute renal injury caused by diachronium poisoning: report of 2 cases. Zhonghua Jizhen Yixue Zazhi. 2022;31:1121-1123. [DOI] [Full Text] |
| 19. | Rose MS, Smith LL, Wyatt I. Evidence for energy-dependent accumulation of paraquat into rat lung. Nature. 1974;252:314-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 203] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Meng N, Sun YQ. Clinical features of 86 cases of acute diquat poisoning. Zhongguo Zhongzheng Yixue. 2022;34:301-305. [RCA] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 21. | Wang YW, Zhao M. Analysis of risk factors for death in 71 cases of diquat poisoning. Zhongguo Yike Daxue Xuebao. 2022;51:203-208. [DOI] [Full Text] |
| 22. | Meng N, Sun YQ. Human toxicokinetics and hemoperfusion efficacy evaluation of diquat. Zhonghua Jizhen Yixue Zazhi. 2020;29:1403-1410. [DOI] [Full Text] |