Published online Sep 26, 2023. doi: 10.12998/wjcc.v11.i27.6558
Peer-review started: June 6, 2023
First decision: August 8, 2023
Revised: August 22, 2023
Accepted: August 31, 2023
Article in press: August 31, 2023
Published online: September 26, 2023
Processing time: 106 Days and 4.6 Hours
Portal vein tumor thrombus (PVTT) is a common complication, accounting for 44%-62.2% of Hepatocellular carcinoma (HCC), and often indicates the poor prognosis. There is no global consensus for the treatment of unresectable HCC with PVTT. In the present case, we reported a novel strategy of radiotherapy-antiangiogenesis-immune checkpoint blockade combination, which showed better response and prolonged survival.
A 51-year-old male diagnosed with HCC (Child-Pugh class A), chronic hepatitis B virus infection and Cheng’s type III PVTT, was given radiotherapy to part of the lesion plus targeted therapy as the first-line therapy, and achieved partial remission. After radiotherapy, lenvatinib plus pembrolizumab was used as maintenance therapy, and complete remission was achieved. The patient remains alive 46 months after the diagnosis of the HCC with PVTT.
This case of unresectable HCC patient with PVTT treated by radiation-lenvatinib-pembrolizumab combination therapy shows apparent clinical efficacy, which demonstrates that local radiotherapy plus antiangiogenesis and immune checkpoint blockad could also benefit patients with advanced HCC.
Core Tip: Primary hepatoma patients with inferior vena cava tumor thrombus and portal vein tumor thrombus are rare, and both are inferior prognostic factors for such patients. There are no worldwide consensuses or guidelines on the diagnosis and treatment of hepatocellular carcinoma patients with macrovascular invasion. We used a new treatment model for this patient: radiotherapy combined with target therapy followed by immune maintenance therapy; and achieved extended survival.
- Citation: Zhao Y, He GS, Li G. Triplet regimen as a novel modality for advanced unresectable hepatocellular carcinoma: A case report and review of literature. World J Clin Cases 2023; 11(27): 6558-6564
- URL: https://www.wjgnet.com/2307-8960/full/v11/i27/6558.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i27.6558
The incidence of portal vein tumor thrombus (PVTT) among patients with hepatocellular carcinoma (HCC) has been reported to be 44%-62.2%[1], which is well-known poor prognostic factor for HCC[2-6]. For patients with tumor thrombus involving only the segmental branches of portal vein, or left/right portal vein (Cheng’s type I/II), surgical resection provides the only chance for radical treatment if the primary tumor can be resected. However, for patients whose tumor thrombus has invaded the main portal vein trunk and above (Cheng’s type III/IV), surgery is not possible and the median overall survival (OS) is usually only 6-8 mo[7].
There is no global consensus or guideline for the treatment of HCC patients with PVTT. European and American guidelines followed the Barcelona Clinic Liver Cancer Staging (BCLC), regarded HCC with macroscopic vascular invasion at BCLC Stage C, and recommended systemic therapy as the therapeutic strategy, including atezolizumab plus bevacizumab, sorafenib or lenvatinib[6-10]. On the contrary, experts from Asian countries (e.g., China) recommended multidisciplinary therapies, including surgery, transcatheter arterial chemoembolization (TACE), radiotherapy (RT), and molecular targeted therapy, which may achieve satisfactory outcomes[11-14].
However, the best comprehensive treatment is still inconclusive and the clinical needs of such patients need to be met urgently. In the present case report, we found that radiotherapy-antiangiogenesis-immune checkpoint blockade combination therapy showed a marked response and prolonged survival, contributing to promising outcomes.
A 51-year-old male was admitted to our hospital with chief complaints of anorexia and fatigue of 2 mo duration.
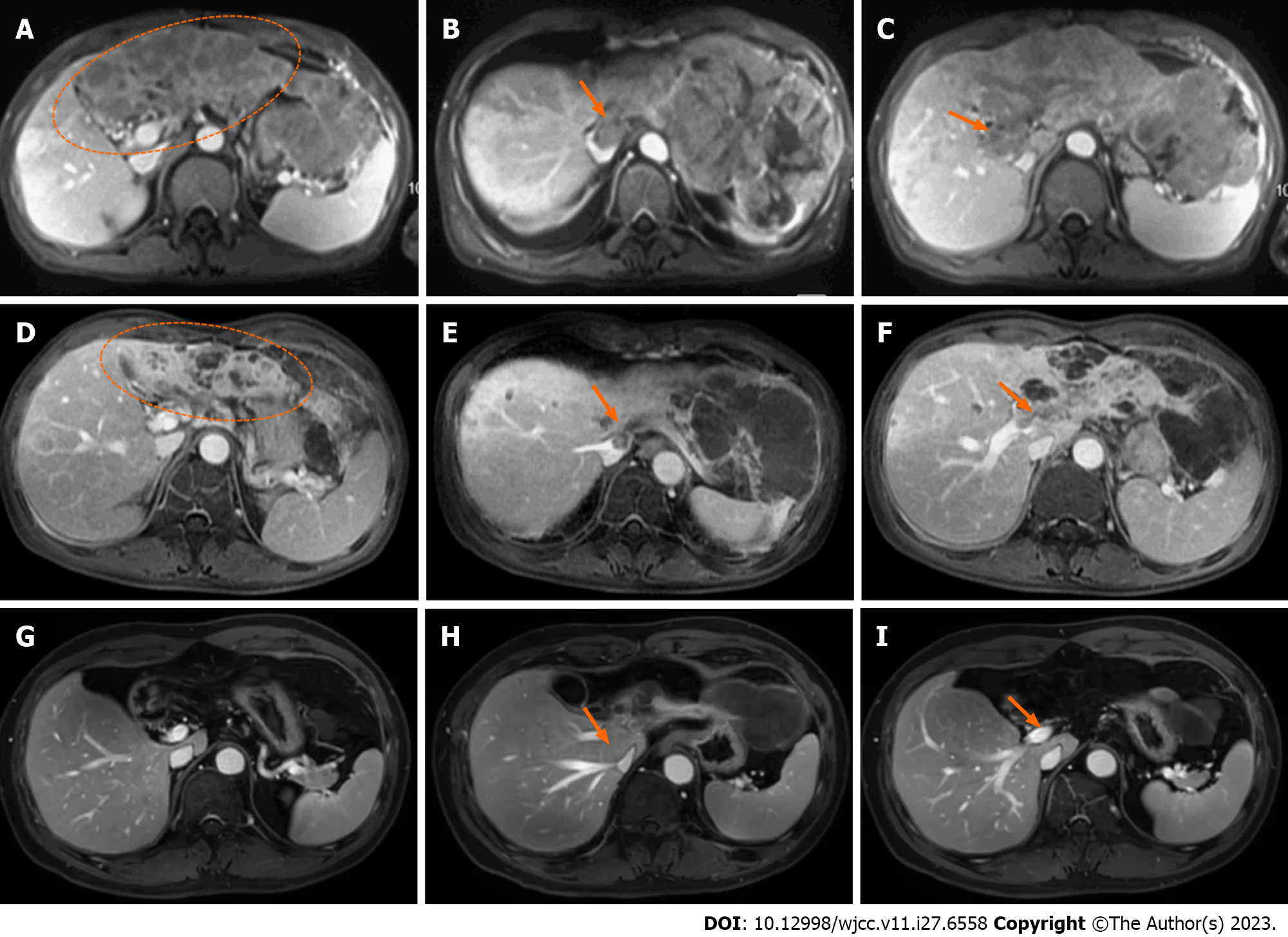
In February 2018, the patient felt anorexia and fatigue without any apparent inducements, accompanied by a weight loss of 5 kg during 2 mo. Routine blood tests showed no abnormalities, while biochemical tests showed albumin (ALB) level of 32 g/L, total bilirubin (TBIL) level of 23.3 µmol/L, and prothrombin time (PT) of 13.9 s, which were all abnormal. Besides, alpha-fetoprotein (AFP) level was significantly higher than the expected value (8875 ng/mL). Ultrasonography showed multiple lesions in the liver, of which the largest one was about 10 cm in the left lobe of the liver, and tumor thrombus was expanded into the portal vein. The dynamic contrast enhanced-magnetic resonance imaging (DCE-MRI) revealed a 10.6 cm × 8.3 cm mass in the left lobe of the liver (Figure 1A, ellipse), and multiple metastases were found in the right lobe of the liver. Tumor thrombus was detected in the inferior vena cava and major portal vein (Figure 1B and C).
The patient had a history of chronic hepatitis B virus (HBV) infection for 20 years, and underwent regular antiviral therapy with Entecavir that reduced HBV-DNA level to the normal range.
No special notes.
The abdominal examination revealed epigastric tenderness. The patient’s body temperature was 38.3 °C, with blood pressure of 130/85 mmHg, heart rate of 93 beats per min, respiratory rate of 18 breaths per min, and oxygen saturation on room air was 99%.
Routine blood tests showed no noticeable abnormalities, and biochemical tests showed ALB of 32 g/L, TBIL of 23.3 µmol/L, and PT of 13.9 s, which were all abnormal.
The DCE-MRI revealed a 10.6 cm × 8.3 cm mass in the left lobe of the liver, and multiple metastases were found in the right lobe of the liver. Tumor thrombus was detected in the inferior vena cava and major portal vein.
The final diagnosis of the present case was hepatocellular carcinoma (Child-Pugh class A), chronic hepatitis B virus infection and Cheng’s type III PVTT.
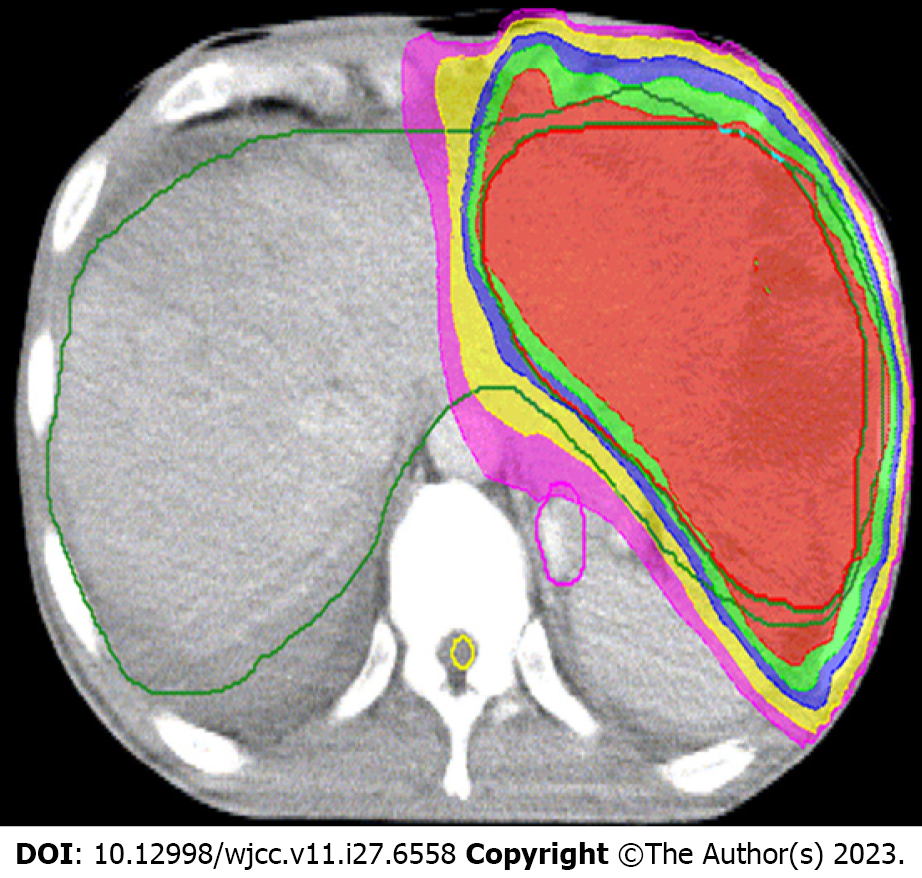
The patient underwent four-dimensional computed tomography simulations. The respiratory cycle was divided into 0%-90% respiratory phase images based on the respiratory signal, which were reconstructed by the system (Elekat, Sweden) for treatment planning. Thermoplastic shell was used as custom immobilization device in the simulation. RT was delivered to lesion using the volumetric modulated arc therapy technique. Cone-beam computed tomography (CT) images matching the target volume were used for daily imaging guidance.
The gross tumor volume (GTV) of liver lesion was defined as a visible tumor on fusion images of CT with magnetic resonance imaging (MRI). The internal target volume (ITV) was the envelope of all GTVs from the ten respiratory phases. The clinical tumour volume (CTV) of the primary tumour was generated by adding 5 mm to the ITV in all directions. The PTV of the primary tumour was expanded to include a 5 mm margin from the CTV. At least 95% of the PTV was covered by the prescribed dose, which was prescribed to the periphery of the PTV.
As PVTT can cause HCC cells to be disseminated, and considering the dose constraints of normal liver and neighboring organs at risk, meanwhile reducing disease burden to the greatest possible extent for helping to optimize responses to immune checkpoint inhibitors, we had partial irradiation. RT, which irradiated only on the whole PVTT and left part of the tumor (Figure 2), was started on March 15, 2018, with a prescription dose of 300 cGY×12F and oral administration of lenvatinib (12 mg, QD). Lenvatinib was used not only during radiotherapy, but also continuously for 2 mo after radiotherapy. In June 2018, 2 mo after RT, the DCE-MRI scan showed that the size of lesions in the left lobe of the liver was significantly reduced (Figure 1D, ellipse), a part of the primary tumor had become cystic lesions and necrotic, the metastatic lesions were decreased and narrowed, and the tumor thrombus was shrunk (Figure 1E and F, arrow). No side effect of grade III or above was observed during treatment. The patient achieved partial remission according to the Response Evaluation Criteria in Solid Tumors (RECIST, Ver. 1.1). From June 2018, maintenance therapy with pembrolizumab (200 mg IV, 1/3W) and lenvatinib (12 mg, QD) was performed for 41 months, and no severe side effect was recorded in this period.
Two months after RT, the size of the liver tumor was significantly reduced, and the tumor thrombus was shrunk. All symptoms were relieved after radiotherapy. During the follow-up (until December 2021), the tumor was well controlled (complete remission) without recurrence (Figure 1G-I), and the AFP level was reduced to 1.92 ng/mL. The patient has been survived for 46 months since the diagnosis of advanced HCC with PVTT.
Surgical resection is the radical treatment method for initially resectable HCC and good survival can be achieved in R0 patients. However, unresectable advanced stage HCC accounts for more than two-thirds of newly diagnosed HCC. In this case, the patient was unsuitable for radical resection because of the tumor thrombus in the main vessels. European and American guidelines followed the BCLC, regarded HCC with PVTT at BCLC Stage C, and recommended systemic treatments based on the positive outcomes of phase III randomized trials[15].
However, radiotherapy is an effective treatment for HCC with PVTT. Patients with type III/IV PVTT receiving radiotherapy alone could achieve an objective response rate (ORR) of 40%-57.7% and a median survival of 9.2-9.6 mo, which is the best level achievable with all single treatments[16]. The partial or complete disappearance of tumor thrombus after radiotherapy can effectively improve intrahepatic blood supply and facilitate the efficacy of drug therapy. A randomized controlled study conducted at the Shanghai Oriental Hepatobiliary Hospital compared the efficacy of neoadjuvant radiotherapy followed by surgery with direct surgery in patients with hepatocellular carcinoma combined with PVTT. Of the 164 patients enrolled, half were randomized to receive neoadjuvant radiotherapy. The results showed that the neoadjuvant radiotherapy group had significantly lower liver cancer-related mortality (HR 0.35, 95%CI 0.23-0.54) and recurrence rates (HR 0.45, 95%CI 0.31-0.64) than the surgical group[17]. Kamiyama et al[18] compared preoperative RT to PVTT in the main trunk or first branch in patients who underwent hepatectomy with those without preoperative RT, the total dose was 30–36 Gy in 10–12 fractions, and found that preoperative RT improved the prognosis of patients, and complete necrosis of PVTT was 53.3%, which showed that radiation is sensitive to PVTT.
In basic research, anti-VEGF therapy improves local intra-tumor oxygenation by improving vascular endothelial status, which can have a synergistic effect with radiation therapy. A phase II study enrolled 40 patients with unresectable locally advanced hepatocellular carcinoma who were not suitable for TACE treatment and were given conventional radiotherapy to the liver lesion in combination with sorafenib in parallel and sequential administration of sorafenib until disease progression. The 1 mo ORR after radiotherapy for the entire group was 55%, and the 2-year OS and PFS in the irradiated field were 32% and 39%, respectively[19]. Considering that complete resection was extremely difficult in this patient, to further reduce tumor loading, we combined RT with lenvatinib. Unexpectedly, the therapeutic response was significantly improved, and the majority of lesions achieved remission. The existing evidence suggested a positive correlation between total radiation dose and tumor response[20]. The effects of RT on the survival of cancer patients are generally interpreted as the consequence of improving local control of the tumor, directly attenuating systemic spread. Experimental data from multiple cancer models have provided sufficient evidence to propose a paradigm shift, whereby some of the effects of radiation contribute to systemic antitumor immunity[21,22]. We used fractionated RT, because it has been reported that fractionated RT could more noticeably enhance immunomodulation[23]. Meanwhile, considering the radiation sensitivity of PVTT, the dose constraints of normal liver and neighboring organs at risk, and radiation immunogenicity, we have chosen a total radiation dose of 36 Gy delivered in 12 fractions.
Multiple studies have confirmed that RT has a modulatory effect on the immune system. First, RT mediates immunogenic cell death, which releases tumor-specific antigens in the form of apoptotic vesicles, along with damage-associated molecular patterns[24,25]. It promotes tumor recruitment of dendritic cells (DCs) and the capture of tumor cell antigens by DCs, activating tumor-specific T cells[26]. Second, DNA damage caused by RT activates the cytoplasmic DNA-sensing cyclic GMP-AMP synthase-stimulator of interferon genes (STING) pathway[27], which induces the production of type I interferons (IFN-α and IFN-β). In addition, RT can reprogram the immune microenvironment, which ultimately makes tumor-infiltrating lymphocytes (TILs) to increase and activate cytotoxic T lymphocytes[28,29].
Mounting evidence suggests that radiation stimulates the immune system and this contributes to the abscopal effect, which is defined as “response at a distance from the irradiated volume.” Although this phenomenon was first described by Mole in 1953, it only gained widespread clinical attention recently[30]. Preclinical trials support the phenomenon of the abscopal effect, but there are few clinical reports, which suggesting that the immune system activated to translate into clinically visible therapeutic responses that still need to be further explored. Dagoglu et al[31] reported 94 cases in 52 articles between 1960 and November 2018 and found a total of 24 cases in 15 reports that demonstrated an abscopal effect finally. In our case, the therapeutic strategy of radiation-immunotherapy combination showed remarkable response-complete remission, including the disappeared right part of the lesion which was not in the irradiation area.
However, the optimal combinations to generate an abscopal effect are unclear. Fortunately, many clinical trials are reported as ongoing or planned investigating the use of RT with immunotherapy. The PACIFIC study concentrated on patients with stage III unresectable non-small cell lung cancer, in which maintenance therapy combined with durvalumab after chemoradiotherapy was associated with a significantly improved survival compared with placebo as the maintenance therapy (4-year survival rates of 49.6% and 38.3%, respectively)[32]. To date, few studies have concentrated on maintenance treatment for HCC, as RT could induce upregulation of immune checkpoint gene expression, elevate mutational load and neoantigen load, and increase the number of CD8+ TILs in the microenvironment. Pembrolizumab was used as maintenance treatment in our case report. In addition, lenvatinib was continued during maintenance therapy, which referred to another combination therapy for advanced HCC with promising early-phase data (KEYNOTE-524)[33], and reported an ORR of 46.0% (95%CI, 36.0% to 56.3%) and a median during of response of 8.6 mo (95%CI, 6.9 mo to not estimable). The median OS was 22 mo, and this study included portal vein invasion with Vp1-3. To our knowledge, this is the first report on immunotherapy combined with maintenance therapy after RT of HCC patients.
Based on the characteristics of this case and other reports in the literature for advanced HCC with PVTT, radiation plus antiangiogenesis and immunotherapy combination can achieve good local control and prolonged survival, which was markedly greater than our expectation. To our knowledge, this is the first patient who was treated by the mentioned therapy, and our findings may provide new insights into the therapy of such patients.
The authors appreciate Dr. Chen Wenting from MSD China for his significant suggestions and proofreading of the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Masaki N, Japan; Yeo SG, South Korea S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Zhang ZM, Lai EC, Zhang C, Yu HW, Liu Z, Wan BJ, Liu LM, Tian ZH, Deng H, Sun QH, Chen XP. The strategies for treating primary hepatocellular carcinoma with portal vein tumor thrombus. Int J Surg. 2015;20:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 2. | Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, Kudo M, Ku Y, Sakamoto M, Nakashima O, Kaneko S, Kokudo N; Liver Cancer Study Group of Japan. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 365] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 3. | Pawarode A, Voravud N, Sriuranpong V, Kullavanijaya P, Patt YZ. Natural history of untreated primary hepatocellular carcinoma: a retrospective study of 157 patients. Am J Clin Oncol. 1998;21:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 133] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Sekino Y, Okumura T, Fukumitsu N, Iizumi T, Numajiri H, Mizumoto M, Nakai K, Nonaka T, Ishikawa H, Sakurai H. Proton beam therapy for hepatocellular carcinoma associated with inferior vena cava tumor thrombus. J Cancer Res Clin Oncol. 2020;146:711-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Cheng S, Chen M, Cai J, Sun J, Guo R, Bi X, Lau WY, Wu M. Chinese Expert Consensus on Multidisciplinary Diagnosis and Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus (2018 Edition). Liver Cancer. 2020;9:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 6. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3887] [Article Influence: 971.8] [Reference Citation Analysis (3)] |
| 7. | Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, Lee HC, Lim YS. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 2018;4:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 320] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 8. | European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6064] [Article Influence: 866.3] [Reference Citation Analysis (3)] |
| 9. | Luo XY, Wu KM, He XX. Advances in drug development for hepatocellular carcinoma: clinical trials and potential therapeutic targets. J Exp Clin Cancer Res. 2021;40:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 10. | Rimassa L, Personeni N, Czauderna C, Foerster F, Galle P. Systemic treatment of HCC in special populations. J Hepatol. 2021;74:931-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 11. | Lu J, Zhang XP, Zhong BY, Lau WY, Madoff DC, Davidson JC, Qi X, Cheng SQ, Teng GJ. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. 2019;4:721-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 12. | Yarchoan M, Agarwal P, Villanueva A, Rao S, Dawson LA, Llovet JM, Finn RS, Groopman JD, El-Serag HB, Monga SP, Wang XW, Karin M, Schwartz RE, Tanabe KK, Roberts LR, Gunaratne PH, Tsung A, Brown KA, Lawrence TS, Salem R, Singal AG, Kim AK, Rabiee A, Resar L, Hoshida Y, He AR, Ghoshal K, Ryan PB, Jaffee EM, Guha C, Mishra L, Coleman CN, Ahmed MM. Recent Developments and Therapeutic Strategies against Hepatocellular Carcinoma. Cancer Res. 2019;79:4326-4330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 13. | Cheng S, Wei X, Shi J, Guo W, Feng S, Zhai J, Huang B. A Multidisciplinary Team Approach to the Management of Patients with Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. Oncologist. 2020;25:e998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | De Mattia E, Cecchin E, Guardascione M, Foltran L, Di Raimo T, Angelini F, D'Andrea M, Toffoli G. Pharmacogenetics of the systemic treatment in advanced hepatocellular carcinoma. World J Gastroenterol. 2019;25:3870-3896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 15. | Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, Fujishiro M. Sorafenib vs. Lenvatinib as First-line Therapy for Advanced Hepatocellular Carcinoma With Portal Vein Tumor Thrombosis. Anticancer Res. 2020;40:2283-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Kim JY, Chung SM, Choi BO, Kay CS. Hepatocellular carcinoma with portal vein tumor thrombosis: Improved treatment outcomes with external beam radiation therapy. Hepatol Res. 2011;41:813-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, Xing H, Xu Y, Shi J, Guo W, Zhou D, Zhang H, Sun H, Huang C, Lu C, Zheng Y, Meng Y, Huang B, Cong W, Lau WY, Cheng S. Neoadjuvant Three-Dimensional Conformal Radiotherapy for Resectable Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Randomized, Open-Label, Multicenter Controlled Study. J Clin Oncol. 2019;37:2141-2151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 18. | Kamiyama T, Nakanishi K, Yokoo H, Tahara M, Nakagawa T, Kamachi H, Taguchi H, Shirato H, Matsushita M, Todo S. Efficacy of preoperative radiotherapy to portal vein tumor thrombus in the main trunk or first branch in patients with hepatocellular carcinoma. Int J Clin Oncol. 2007;12:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Chen SW, Lin LC, Kuo YC, Liang JA, Kuo CC, Chiou JF. Phase 2 study of combined sorafenib and radiation therapy in patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2014;88:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 20. | Huang BS, Tsang NM, Lin SM, Lin DY, Lien JM, Lin CC, Chen WT, Chen WY, Hong JH. High-dose hypofractionated X-ray radiotherapy for hepatocellular carcinoma: Tumor responses and toxicities. Oncol Lett. 2013;6:1514-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer. 2016;4:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 302] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 22. | Lee YH, Tai D, Yip C, Choo SP, Chew V. Combinational Immunotherapy for Hepatocellular Carcinoma: Radiotherapy, Immune Checkpoint Blockade and Beyond. Front Immunol. 2020;11:568759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 23. | Savage T, Pandey S, Guha C. Postablation Modulation after Single High-Dose Radiation Therapy Improves Tumor Control via Enhanced Immunomodulation. Clin Cancer Res. 2020;26:910-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1206] [Cited by in RCA: 1236] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 25. | Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tör M, Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 460] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 26. | Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Métivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1986] [Cited by in RCA: 2416] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 27. | Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1055] [Cited by in RCA: 1564] [Article Influence: 142.2] [Reference Citation Analysis (0)] |
| 28. | Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180:3132-3139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 382] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 29. | Chen B, Alvarado DM, Iticovici M, Kau NS, Park H, Parikh PJ, Thotala D, Ciorba MA. Interferon-Induced IDO1 Mediates Radiation Resistance and Is a Therapeutic Target in Colorectal Cancer. Cancer Immunol Res. 2020;8:451-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 30. | Salama AK, Postow MA, Salama JK. Irradiation and immunotherapy: From concept to the clinic. Cancer. 2016;122:1659-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Dagoglu N, Karaman S, Caglar HB, Oral EN. Abscopal Effect of Radiotherapy in the Immunotherapy Era: Systematic Review of Reported Cases. Cureus. 2019;11:e4103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Hui R, Özgüroğlu M, Villegas A, Daniel D, Vicente D, Murakami S, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Gray JE, Rydén A, Viviers L, Poole L, Zhang Y, Dennis PA, Antonia SJ. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1670-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 33. | Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 876] [Article Influence: 175.2] [Reference Citation Analysis (0)] |