Published online Sep 26, 2023. doi: 10.12998/wjcc.v11.i27.6543
Peer-review started: June 1, 2023
First decision: July 17, 2023
Revised: July 30, 2023
Accepted: August 18, 2023
Article in press: August 18, 2023
Published online: September 26, 2023
Processing time: 111 Days and 2.3 Hours
Stroke is the second and third leading cause of death and disability, respectively. To date, no definitive treatment can repair lost brain function. Recently, various preclinical studies have been reported on mesenchymal stromal cells (MSCs) and their derivatives and their potential as alternative therapies for stroke.
A 45-year-old female suffered an acute stroke, which led to paralysis in the left upper and lower limbs. The amniotic membrane MSC-derived secretome (MSC-secretome) was intravenously transplanted once a week for 4 wk. MSC-secretome-regulated regulatory T cells were investigated for the beneficial effects. The clinical improvement of this patient was accompanied by an increased frequency of regulatory T cells after transplantation.
Intravenous administration of MSC-secretome can potentially treat patients who suffer from acute ischemic stroke.
Core Tip: Mesenchymal stromal cells (MSCs) and their derivatives have been studied as a therapeutic alternative for stroke. A 45-year-old female suffered an acute stroke, causing paralysis in the left upper and lower limbs. We transplanted the amniotic membrane MSC-derived secretome once a week for 4 wk. The clinical improvement of this patient was accompanied by an increased frequency of regulatory T cells after transplantation. This study demonstrated that intravenous transplantation of the MSC-secretome can potentially treat patients who suffer from acute ischemic stroke.
- Citation: Lin FH, Yang YX, Wang YJ, Subbiah SK, Wu XY. Amniotic membrane mesenchymal stromal cell-derived secretome in the treatment of acute ischemic stroke: A case report. World J Clin Cases 2023; 11(27): 6543-6550
- URL: https://www.wjgnet.com/2307-8960/full/v11/i27/6543.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i27.6543
Acute stroke is the second and third leading cause of death and disability, respectively, worldwide[1]. Although thrombolytic therapy is the only stroke treatment available, it can only be applied to a certain group of individuals. Additionally, dead neurons are not capable of regeneration. There is no long-term solution for recovering lost brain function[2]. Mesenchymal stromal cell (MSC) therapy has emerged as a promising therapeutic option for stroke as MSCs can migrate to lesions, secrete neurotrophic factors, reduce inflammation, and promote rehabilitation, thereby minimizing damage[3].
Neonatal birth-associated tissues, such as the umbilical cord (UC) and amniotic membrane (AM), are advantageous sources of MSCs because of their abundant availability, ease of collection, and lack of ethical restrictions[4]. We have developed a serum-free defined medium by replacing all serum components with synthetic alternatives for deriving clinical-grade UC-MSCs[5]. We found that this serum-free medium enhanced the immunosuppressive effects of UC-MSCs[6]. Preliminary clinical studies using UC-MSCs to treat stroke have verified their beneficial effects and safety[2]. Our findings further demonstrated that AM-MSCs in a serum-free medium are more potent immunosuppressors than UC-MSCs[7], suggesting the usefulness of AM-MSCs for the treatment of stroke.
It has been found that the beneficial effects of MSCs are mediated primarily by the components of their secretome, and the MSC-secretome is currently being studied in several clinical contexts, using either MSC-conditioned medium or purified MSC-derived extracellular vesicles to modulate tissue responses to a wide array of injuries[8]. There are significant benefits to using the MSC-secretome over MSCs in handling, safety, and the potential for standardization[9,10]. Our previous research confirmed that the immunomodulatory function of MSCs and the MSC-secretome are similar[11]. These positive outcomes have led to clinical trials assessing the safety of the MSC-secretome for applications in the treatment of stroke as well as the emergence of multiple commercial MSC-secretome sources marketed for topical application in cosmetic medicine[12,13]. In this article, we reported the first case of a patient suffering from stroke who was treated with four infusions of allogeneic MSC-secretome.
On March 13, 2021, a 45-year-old female patient afflicted by stroke sought consultation at our medical facility. She presented with hemiplegia affecting the musculature of the upper and lower left extremities.
While moving around at home, the patient acquired slurred speech, numbness, weakness in the left limb, impairment in the left hand, and inability to walk independently.
The patient had a documented medical background of hypertension spanning a duration of 9 years, which was treated with oral nifedipine. However, the blood pressure was not monitored regularly. The patient also denied a history of diabetes, cerebral hemorrhage, and coronary atherosclerosis.
No special notes.
The patient exhibited paralysis in the upper and lower left extremities, resulting in ambulatory incapacitation and compromised speech function. The severity of stroke was evaluated based on the National Institute of Health Stroke Scale (NIHSS). At her first visit, the score was 12. The activities of daily living scale was evaluated based on the Barthel index (BI). The score was 30.
The patient’s complete blood count, coagulation, liver and kidney function, and electrolytes were generally normal following admission. The erythrocyte sedimentation rate was 9 mm/h, glycosylated hemoglobin was 5.50%, triglycerides were 1.10 mmol/L, total cholesterol was 5.07 mmol/L, lipoprotein (a) was 69.40 nmol/L, homocysteine was 18.30 µmol/L, high-density lipoprotein cholesterol was 1.18 mmol/L, LDL cholesterol was 3.69 mmol/L, and glucose was 7.09 mmol/L.
Computed tomography of the patient’s head showed multiple lacunar cerebral infarctions with ischemic changes in the white matter. Diffusion-weighted imaging showed a new infarct focus in the paraventricular nucleus on the right side. Carotid ultrasound revealed several carotid plaques, whereas vertebral artery ultrasound indicated no abnormalities. The electrocardiogram showed normal sinus rhythm.
The diagnosis of acute ischemic stroke was established by analyzing the patient’s history of symptoms, blood tests, and brain computed tomography images. The categorization of stroke severity was determined as moderate to severe, as inferred from the NIHSS scoring.
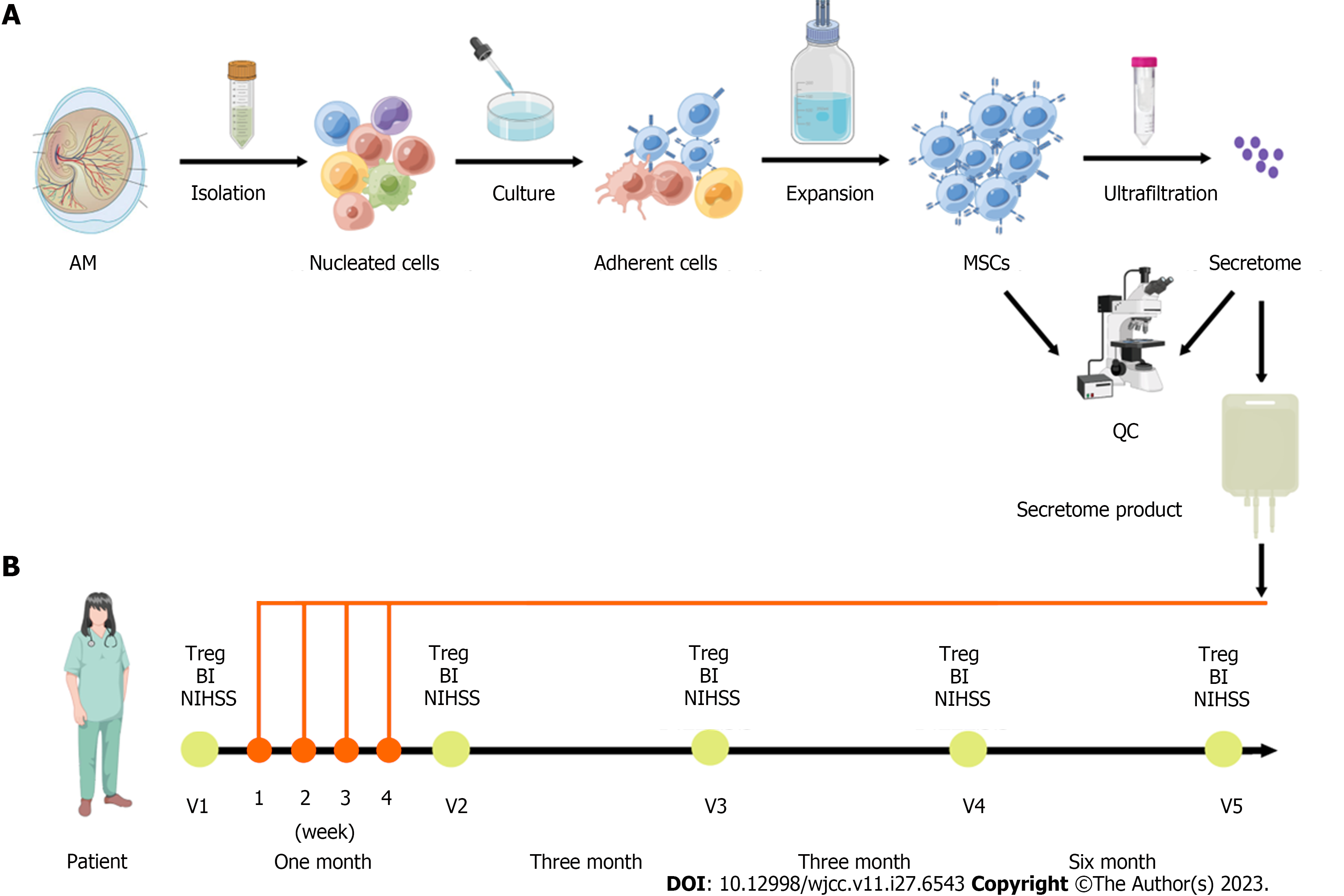
All human placental samples were collected from healthy, full-term, uncomplicated pregnancies. Prior to participation, all subjects provided informed consent, and the research protocol received formal approval from the Ethics Committee of the Affiliated Hospital of Chifeng College (No: 2021-033). Human AM-MSCs were collected as previously described[7]. In brief, the AM was mechanically peeled off from the placenta, washed with phosphate-buffered saline, and minced into approximately 1 mm3 × 1 mm3 pieces. The AM was incubated in 0.25% trypsin solution for 60 min at 37 °C, followed by incubation in 200 U/mL collagenase solution for 60 min at 37 °C. The released cells were plated for 5 d in a serum-free defined medium developed in our laboratory and removed to unattached cells. The formulation of serum-free defined medium including basal medium and xeno-free defined supplement was described in our previous articles[14], and serum-free defined medium was prepared in our laboratory.
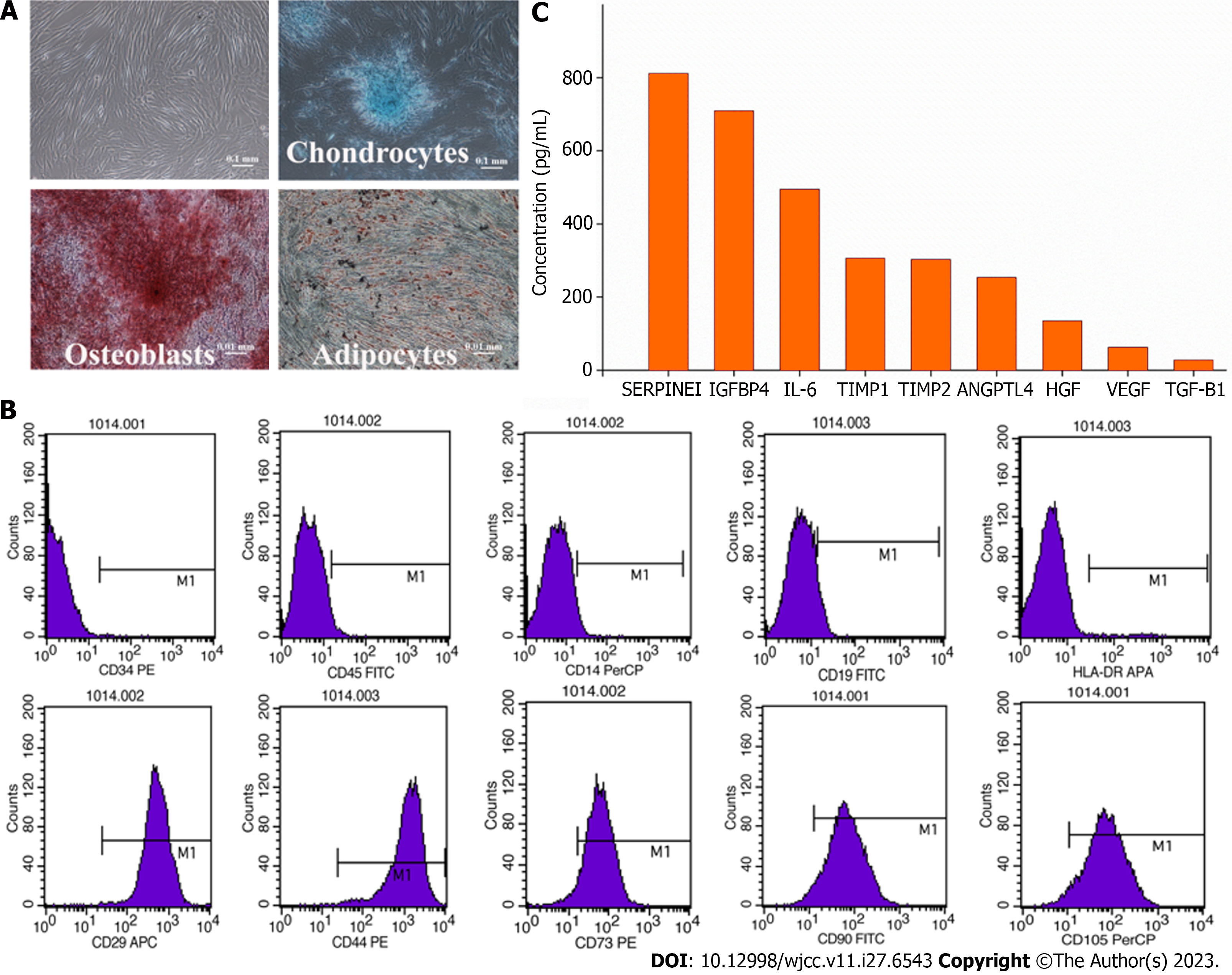
The adherent cells were washed and cultured at 37 °C in 5% CO2. The medium was changed twice per week. At 80% confluence, the cells were passaged at 3000 cells/cm2 (Figure 1). The AM-MSCs were characterized by their fibroblast-like morphology, the presence of distinct surface markers (CD29+, CD44+, CD73+, CD90+, CD105+, CD14-, CD19-, CD34-, CD45-, and HLA-DR-), and their potency to differentiate into osteoblasts, chondrocytes, or adipocytes in specific induction media (Figure 2A and B). The absence of contaminating pathogenic microorganisms and endotoxin release (< 0.5 EU/mL) from the cells was confirmed as described previously[5].
The MSC-secretome was prepared from Beijing Protercell Biotechnology Co., Ltd, and the detailed process of manufacture and quality control were followed. Briefly, AM-MSCs at the fifth passage were cultured until 80% confluent, carefully cleaned, and seeded in serum-free Iscove’s Modified Dulbecco’s Medium to prepare the MSC-secretome. The conditioned medium was collected after 48 h, centrifuged at 5000 × g for 15 min to remove cells and cell debris, and passed through a 0.22-µm filter. The filtered media were concentrated by centrifugation using ultrafiltration units (Millipore, Bedford, MA, United States) with a 3-kDa cutoff. The protein concentration in the concentrated medium was measured using a BCA Protein Assay Kit (Neobioscience, China), and enzyme-linked immunosorbent assay kits (Neobioscience) were used to measure the contents of secreted cytokines, including serine protease inhibitor clade E member 1 (SERPINE1), insulin-like growth factor binding protein 4 (IGFBP4), interleukin-6 (IL-6), tissue inhibitors of metalloproteinase (TIMP)-1, TIMP-2, angiopoietin-like 4 (ANGPTL4), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and transforming growth factor-beta 1 (TGF-β1). Media were stored at -80 °C until use. The average protein concentration for the entire secretome was 2.5 mg/mL. Nine molecules were detected in the secretome samples (Figure 2C), of which both SERPINE1 and IGFBP4 were secreted at amounts higher than 500 pg/mL (SERPINE1, 812 pg/mL; IGFBP4, 710 pg/mL). Five proteins were present at concentrations between 100 and 500 pg/mL (IL-6, 495 pg/mL; TIMP-1, 307 pg/mL; TIMP-2, 303 pg/mL; ANGPTL4, 254 pg/mL; HGF, 135 pg/mL), while two were between 10-100 pg/mL (VEGF, 63 pg/mL; TGF-β1, 28 pg/mL).
One week after the stroke onset, the MSC-secretome (50 mg of protein in 100 mL of 0.9% sodium chloride injection) were administered intravenously to the patient. The same process was performed four times total over a 4-wk interval. The patient was discharged 2 d after the transfusion, with follow-up visits at 1 mo, 3 mo, 6 mo, and 12 mo to assess the NIHSS and BI scores and the proportion of peripheral regulatory T (Treg) cells (Figure 1). No other drugs or physiotherapy were used during treatment and follow-up monitoring.
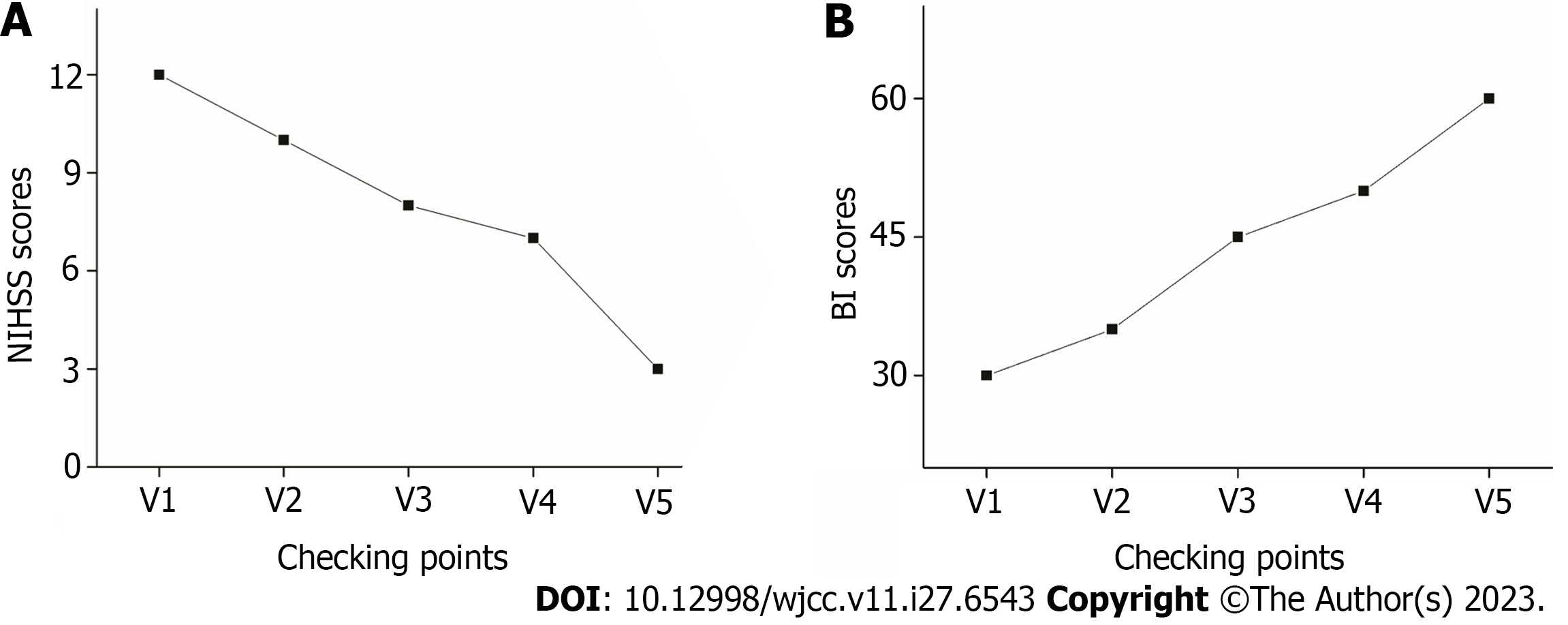
The patient’s neurological function improved gradually after MSC-secretome transfusion with significant improvement of the weakness in the left limbs. Moreover, the patient’s mobility exhibited significant enhancement, encompassing not only basic ambulation and limb motility but also proficiency in intricate activities and prolonged periods of upright stance. The NIHSS score decreased from 12 at baseline (before transplantation) to 3 at 12 mo after transplantation (Figure 3A). Moreover, the BI score increased from 30 to 60 (Figure 3B).
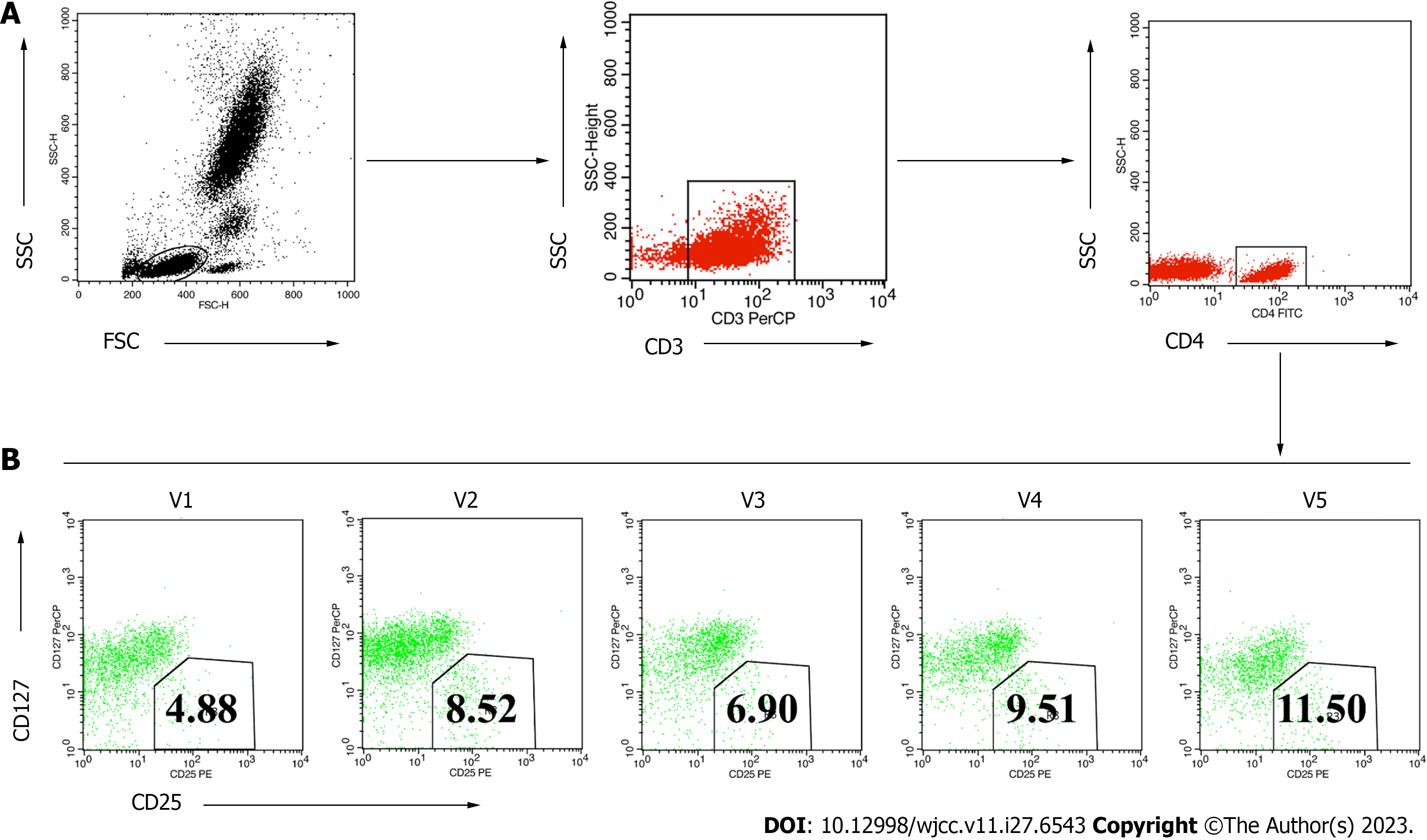
During treatment and follow-up monitoring, the patient observed or reported no adverse reactions related to mobility. In addition, the patient manifested an absence of symptoms such as fever, chills, or nausea that frequently occur in patients who receive MSC-secretome transplantation. The patient’s peripheral Treg levels were analyzed before and after the treatments. This showed that the proportion of CD4+CD25+127low/- Tregs in the CD4+ T cell population increased gradually over the 12 mo after transplantation (Figure 4). These findings suggested that the secretome transplantation changed Treg levels, producing advantageous benefits. After receiving the MSC-secretome therapy, the patient’s clinical condition improved along with an increase in the number of Tregs.
AM-MSCs possess strong immunomodulatory functions and promote tissue repair and regeneration through paracrine-soluble factors[15]. There are many advantages to using the MSC-secretome for stroke recovery compared to employing the MSCs alone[13]. First, treatment with the MSC-secretome avoids the safety concerns associated with cellular transplantation, such as uncontrolled cell proliferation, differentiation, and division. Second, the MSC-secretome does not have the problems of immune rejection and tumor promotion. Third, the MSC-secretome is easier to store and transport than cells. In addition, the MSC-secretome has an inherent ability to target tissue injury sites due to the small size of the vesicles and their lipid bilayer membrane structure, and in particular can cross the blood-brain barrier[16].
In this study, we described a case of stroke treated with cell-free MSC-derived therapy using the MSC-secretome. The patient received four transfusions of the MSC secretome at 1-wk intervals. The patient had not received any other form of therapy during or after the MSC-secretome treatment. At 12 mo following treatment, the patient’s NIHSS score dropped from 12 to 3, suggesting the return of motor function, while her BI score increased from 30 to 60, confirming significant improvements in her quality of life.
A previous study identified 200 factors released by AM-MSCs[15]. The present study analyzed the AM-MSC secretome to determine the presence of trophic, chemotactic, and immunomodulatory factors. The results revealed an abundance of cytokines in the secretome, including SERPINE1, IGFBP4, IL-6, TIMP-1, TIMP-2, ANGPTL4, VEGF, HGF, and TGF-β1. The vascular trophic factors (VEGF, HGF, IGFBP4, and ANGPTL4) are important effector molecules that promote tissue repair, not only restoring the blood supply in ischemic tissues but also providing neuroprotective effects in acute stroke[17,18].
Recent studies have confirmed that the chemotactic factors SERPINE1, TIMP-1, and TIMP-2 play crucial roles in peripheral neutrophil infiltration, which may reduce the effects of neuronal injury induced by ischemia-reperfusion[19]. This finding showed that SERPINE1, abundant in the MSC-secretome, may play an important role in the acute stage of ischemic stroke. More importantly, IL-6, HGF, and TGF-β1 are molecules associated with immune function and could contribute to immunomodulatory properties. It has been confirmed that these molecules either reduce inflammation[20] or have a pivotal role in the positive regulation of the immune system[21]. Further investigations into the specific cytokine(s) responsible for the most significant improvements in stroke are required.
As an important subpopulation of immunosuppressive T cells, Treg cells are involved in maintaining immune homeostasis and regulating immunomodulatory responses in the pathological process of ischemic stroke[22,23]. The proportion of Tregs in this patient was found to be below normal values, consistent with previous findings[24]. Immunosuppression by the MSC-secretome has been revealed to contribute substantially to the efficacy of stroke treatment. This is associated with an upregulation of anti-inflammatory Treg cells[25], which is consistent with the present patient’s follow-up results. Recent studies have also shown that Treg cells may participate in the recovery of ischemic stroke patients[26,27], and ex vivo expansion of Treg cells and their products could suggest therapeutic prospects aimed at safeguarding neurons against stroke and mitigating neuroinflammatory disorders[28,29]. These findings support the possibility that increased peripheral Treg cell levels may be associated with the therapeutic benefits observed in this patient.
In conclusion, our study indicated the feasibility of using cell-free MSC-secretome therapy for stroke. This is the first human case treated with intravenous transplantation of the MSC-secretome. The beneficial effects may be associated with increased peripheral Treg cell levels. Additional studies might eventually result in the development of novel secretome-based treatments for stroke alongside the implementation of the currently used MSCs. However, more research involving a larger sample size of patients is required.
We thank all medical personnel and technicians at the dialysis centers who graciously consented to partake in this investigative endeavor.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maurya DK, India; Shamseldeen AM, Egypt S-Editor: Liu JH L-Editor: Filipodia P-Editor: Cai YX
| 1. | Zhang Y, Dong N, Hong H, Qi J, Zhang S, Wang J. Mesenchymal Stem Cells: Therapeutic Mechanisms for Stroke. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Jingli Y, Jing W, Saeed Y. Ischemic Brain Stroke and Mesenchymal Stem Cells: An Overview of Molecular Mechanisms and Therapeutic Potential. Stem Cells Int. 2022;2022:5930244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Rust R, Tackenberg C. Stem Cell Therapy for Repair of the Injured Brain: Five Principles. Neuroscientist. 2022;10738584221110100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Magatti M, Stefani FR, Papait A, Cargnoni A, Masserdotti A, Silini AR, Parolini O. Perinatal Mesenchymal Stromal Cells and Their Possible Contribution to Fetal-Maternal Tolerance. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Wu X, Ma Z, Wu D. Derivation of clinical-grade mesenchymal stromal cells from umbilical cord under chemically defined culture condition - platform for future clinical application. Cytotherapy. 2020;22:377-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Wu X, Wu D, Mu Y, Zhao Y, Ma Z. Serum-Free Medium Enhances the Therapeutic Effects of Umbilical Cord Mesenchymal Stromal Cells on a Murine Model for Acute Colitis. Front Bioeng Biotechnol. 2020;8:586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Oikarinen A, Palatsi R, Linna SL, Peltonen L. Types I and III collagens and the activities of prolyl hydroxylase and galactosylhydroxylysyl glucosyltransferase in skin lesions of tuberous sclerosis. Br J Dermatol. 1982;107:659-664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Karagyaur M, Dzhauari S, Basalova N, Aleksandrushkina N, Sagaradze G, Danilova N, Malkov P, Popov V, Skryabina M, Efimenko A, Tkachuk V. MSC Secretome as a Promising Tool for Neuroprotection and Neuroregeneration in a Model of Intracerebral Hemorrhage. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Pischiutta F, Caruso E, Cavaleiro H, Salgado AJ, Loane DJ, Zanier ER. Mesenchymal stromal cell secretome for traumatic brain injury: Focus on immunomodulatory action. Exp Neurol. 2022;357:114199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Zriek F, Di Battista JA, Alaaeddine N. Mesenchymal Stromal Cell Secretome: Immunomodulation, Tissue Repair and Effects on Neurodegenerative Conditions. Curr Stem Cell Res Ther. 2021;16:656-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Ma ZJ, Wang YH, Li ZG, Wang Y, Li BY, Kang HY, Wu XY. Immunosuppressive Effect of Exosomes from Mesenchymal Stromal Cells in Defined Medium on Experimental Colitis. Int J Stem Cells. 2019;12:440-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Muhammad SA. Mesenchymal stromal cell secretome as a therapeutic strategy for traumatic brain injury. Biofactors. 2019;45:880-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Harman RM, Marx C, Van de Walle GR. Translational Animal Models Provide Insight Into Mesenchymal Stromal Cell (MSC) Secretome Therapy. Front Cell Dev Biol. 2021;9:654885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Wu X, Kang H, Liu X, Gao J, Zhao K, Ma Z. Serum and xeno-free, chemically defined, no-plate-coating-based culture system for mesenchymal stromal cells from the umbilical cord. Cell Prolif. 2016;49:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Ragni E, Papait A, Perucca Orfei C, Silini AR, Colombini A, Viganò M, Libonati F, Parolini O, de Girolamo L. Amniotic membrane-mesenchymal stromal cells secreted factors and extracellular vesicle-miRNAs: Anti-inflammatory and regenerative features for musculoskeletal tissues. Stem Cells Transl Med. 2021;10:1044-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | Shetgaonkar GG, Marques SM, DCruz CEM, Vibhavari RJA, Kumar L, Shirodkar RK. Exosomes as cell-derivative carriers in the diagnosis and treatment of central nervous system diseases. Drug Deliv Transl Res. 2022;12:1047-1079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Han Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, Hua D, Shao C, Shi Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Ther. 2022;7:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 337] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 18. | Qiu Z, Yang J, Deng G, Li D, Zhang S. Angiopoietin-like 4 promotes angiogenesis and neurogenesis in a mouse model of acute ischemic stroke. Brain Res Bull. 2021;168:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Pu Z, Bao X, Xia S, Shao P, Xu Y. Serpine1 Regulates Peripheral Neutrophil Recruitment and Acts as Potential Target in Ischemic Stroke. J Inflamm Res. 2022;15:2649-2663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 20. | Toh WS, Zhang B, Lai RC, Lim SK. Immune regulatory targets of mesenchymal stromal cell exosomes/small extracellular vesicles in tissue regeneration. Cytotherapy. 2018;20:1419-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Ragni E, Perucca Orfei C, De Luca P, Mondadori C, Viganò M, Colombini A, de Girolamo L. Inflammatory priming enhances mesenchymal stromal cell secretome potential as a clinical product for regenerative medicine approaches through secreted factors and EV-miRNAs: the example of joint disease. Stem Cell Res Ther. 2020;11:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 22. | Wang HY, Ye JR, Cui LY, Chu SF, Chen NH. Regulatory T cells in ischemic stroke. Acta Pharmacol Sin. 2022;43:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 23. | Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, Sakai R, Matsuo K, Nakayama T, Yoshie O, Nakatsukasa H, Chikuma S, Shichita T, Yoshimura A. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565:246-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 528] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 24. | Ruhnau J, Schulze J, von Sarnowski B, Heinrich M, Langner S, Pötschke C, Wilden A, Kessler C, Bröker BM, Vogelgesang A, Dressel A. Reduced Numbers and Impaired Function of Regulatory T Cells in Peripheral Blood of Ischemic Stroke Patients. Mediators Inflamm. 2016;2016:2974605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Xia Y, Hu G, Chen Y, Yuan J, Zhang J, Wang S, Li Q, Wang Y, Deng Z. Embryonic Stem Cell Derived Small Extracellular Vesicles Modulate Regulatory T Cells to Protect against Ischemic Stroke. ACS Nano. 2021;15:7370-7385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 26. | Santamaría-Cadavid M, Rodríguez-Castro E, Rodríguez-Yáñez M, Arias-Rivas S, López-Dequidt I, Pérez-Mato M, Rodríguez-Pérez M, López-Loureiro I, Hervella P, Campos F, Castillo J, Iglesias-Rey R, Sobrino T. Regulatory T cells participate in the recovery of ischemic stroke patients. BMC Neurol. 2020;20:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Noh MY, Lee WM, Lee SJ, Kim HY, Kim SH, Kim YS. Regulatory T cells increase after treatment with poly (ADP-ribose) polymerase-1 inhibitor in ischemic stroke patients. Int Immunopharmacol. 2018;60:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Na SY, Mracsko E, Liesz A, Hünig T, Veltkamp R. Amplification of regulatory T cells using a CD28 superagonist reduces brain damage after ischemic stroke in mice. Stroke. 2015;46:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Zhang H, Xia Y, Ye Q, Yu F, Zhu W, Li P, Wei Z, Yang Y, Shi Y, Thomson AW, Chen J, Hu X. In Vivo Expansion of Regulatory T Cells with IL-2/IL-2 Antibody Complex Protects against Transient Ischemic Stroke. J Neurosci. 2018;38:10168-10179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |