Published online Sep 26, 2023. doi: 10.12998/wjcc.v11.i27.6523
Peer-review started: May 17, 2023
First decision: August 8, 2023
Revised: August 18, 2023
Accepted: September 1, 2023
Article in press: September 1, 2023
Published online: September 26, 2023
Processing time: 126 Days and 4 Hours
Eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome, is a rare form of anti-neutrophil cytoplasmic antibody-associated vasculitis characterized by asthma, vasculitis, and eosinophilia.
We report an atypical case of EGPA in a 20-year-old female patient. Unlike previously reported cases of EGPA, this patient’s initial symptom was asthma associated with a respiratory infection. This was followed by Loeffler endocarditis and cardiac insufficiency. She received treatment with methylprednisolone sodium succinate, low molecular weight heparin, recombinant human brain natriuretic peptide, furosemide, cefoperazone sodium/sulbactam sodium, and acyclovir. Despite prophylactic anticoagulation, she developed a large right ventricular thrombus. EGPA diagnosis was confirmed based on ancillary test results and specialty consultations. Subsequent treatment included mycophenolate mofetil. Her overall condition improved significantly after treatment, as evidenced by decreased peripheral blood eosinophils and cardiac markers. She was discharged after 17 d. Her most recent follow-up showed normal peripheral blood eosinophil levels, restored cardiac function, and a reduced cardiac mural thrombus size.
This case illustrates the swift progression of EGPA and underscores the significance of early detection and immediate intervention to ensure a favorable prognosis.
Core Tip: Eosinophilic granulomatosis with polyangiitis (EGPA) is the rarest type of anti-neutrophil cytoplasmic antibody-associated vasculitis. The vast majority of patients present first with bronchial asthma and sinusitis, with a high risk of misdiagnosis. Clinicians should be alert to the possibility of EGPA when asthma and eosinophilia (> 10%) are found. Here, we report a 20-year-old female patient whose first symptom was asthma, followed by Loeffler endocarditis. She was finally diagnosed with EGPA, and after treatment with methylprednisolone sodium succinate, low molecular weight heparin, and mycophenolate mofetil, her condition improved and she was discharged. The patient’s condition was stable at follow-up.
- Citation: He JL, Liu XY, Zhang Y, Niu L, Li XL, Xie XY, Kang YT, Yang LQ, Cai ZY, Long H, Ye GF, Zou JX. Eosinophilic granulomatosis with polyangiitis, asthma as the first symptom, and subsequent Loeffler endocarditis: A case report. World J Clin Cases 2023; 11(27): 6523-6530
- URL: https://www.wjgnet.com/2307-8960/full/v11/i27/6523.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i27.6523
Eosinophilic granulomatosis with polyangiitis (EGPA) is an anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis characterized by asthma, infiltrative necrotizing vasculitis, extravascular granulomas, eosinophilia, and multi-organ infiltration[1]. Despite the association between EGPA and ANCA, only 30%-40% of patients with EGPA produce ANCAs[2,3]. Therefore, patients can be divided into two subtypes: ANCA-positive patients who typically experience vasculitis-related symptoms (purpura, neuropathy, pulmonary-renal syndrome), and ANCA-negative patients who are more likely to experience eosinophil-driven symptoms (pulmonary infiltrates, cardiomyopathy)[4,5].
Cardiac involvement is a major cause of poor prognosis and death in patients with EGPA[6]. Therefore, we share the following case report with the aim of increasing understanding and awareness of this condition; this is a rare case of ANCA-negative EGPA with cardiac and pulmonary involvement. In contrast to previous EGPA case reports in which cardiac involvement occurred several years following asthma, this patient developed cardiac insufficiency 15 mo after the onset of asthma.
A 20-year-old female patient presented with a cough and shortness of breath for over one year and chest pain that had been present for 16 d and was aggravated for 1 wk.
The patient had been diagnosed with bronchial asthma after presenting with coughing and dyspnea 15 mo previously and having been hospitalized several times without complete relief. These symptoms recurred with precordial chest pain 16 d prior to her presentation at our hospital.
On March 15, 2023, she had visited Dongguan Dalang Hospital where she had a reported blood pressure of 88/62 mmHg, white blood cell count of 21.19 × 109/L, eosinophil (E)% of 42.6%, and creatine kinase isoenzyme (CKMB) concentration of 46.9 U/L. She was diagnosed with suspected myocarditis and treated with traditional Chinese medicine. After some initial improvement, the patient developed a respiratory infection, worsened symptoms, and epistaxis.
On March 27, 2023, she had also visited the Qiannan People’s Hospital, where tests revealed considerably elevated E% as well as cardiac troponin T and B-type natriuretic peptide (BNP) concentrations. Chest computed tomography (CT) also showed patchy hyperdense infiltrates in both lungs, bronchial dilatation in the lower lobes of both lungs and the middle lobe of the right lung, enlarged mediastinal lymph nodes, and bilateral pleural thickening and adhesions. Cardiac ultrasound revealed a slightly enlarged right heart, hypertrophic right ventricular anterior and lateral apical segments, moderate tricuspid reflux and mild mitral and pulmonary reflux, reduced left ventricular ejection fraction (LVEF; 42%), and mild pericardial effusion. She was diagnosed with infectious myocarditis. After treatment with methylprednisolone sodium succinate (40 mg) and prophylactic anticoagulation, the patient’s symptoms did not resolve. Therefore, she was referred to our critical care unit (CCU) on March 30, 2023.
The patient had a history of allergic rhinitis and had been taking glucocorticoids intermittently.
Her grandmother suffered from asthma, the details of which are not clear. No other relevant family history is known.
Temperature: 36.3 °C, pulse: 78 beats per minute (bpm), blood pressure: 79/54 mmHg. She presented with a pale complexion, and a soft holosystolic murmur was auscultated at the left sternal border of the fourth and fifth intercostal spaces of the sternum with no significant enhancement during inspiration. The rest of the examination was unremarkable.
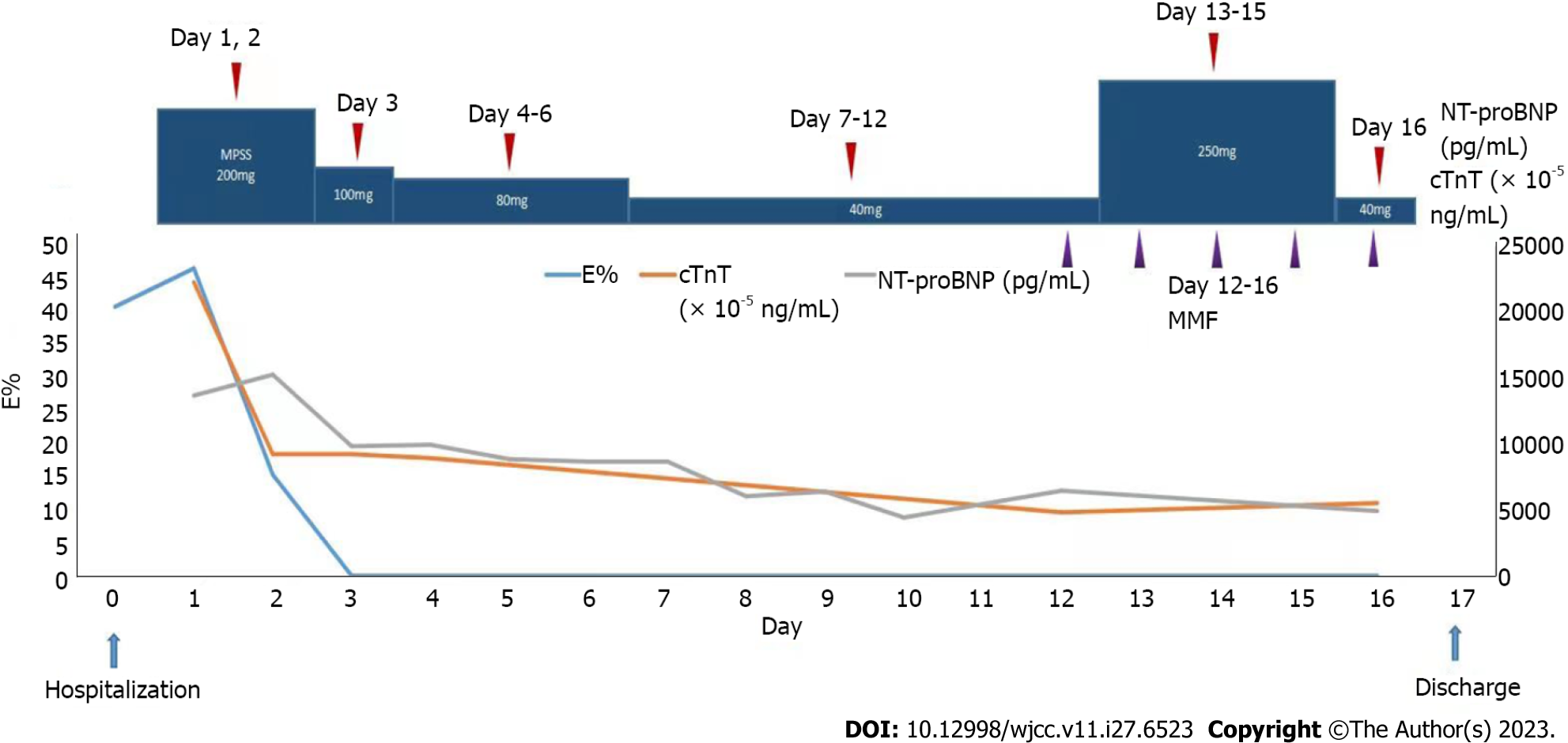
Serial complete blood count assays showed a significant increase in E% (with a peak of 46.5%) and a transient decrease in hemoglobin (Hb; a minimum of 84.00 g/L). Blood biochemical tests showed a transient decrease in albumin (ALB; a minimum of 27.96 g/L) concentrations. The urine dipstick test showed the presence of protein (+-), procalcitonin, an elevated erythrocyte sedimentation rate, and increased C-reactive proteins. A significant elevation of cardiac troponin T, CKMB, and N-terminal pro-BNP (NT-proBNP) concentrations (Figure 1) were found. The serum allergen-specific antibody total IgE concentration was significantly elevated, thyroid-stimulating hormone and free triiodothyronine concentrations were decreased, free thyroxine concentration was normal, and liver and kidney functions were not markedly abnormal. The patient’s autoimmune serology revealed that only anti-SS-A52 antibodies were somewhat positive, while the rest of the immune serologies, including ANCA, were negative. IgG antibody for Schistosoma hepaticum tested positive. No platelet-derived growth factor receptor A rearrangement or B translocation was observed via fluorescence in situ hybridization nor did we detect acute leukemia genetic markers, and acute leukemia immune electrophoresis test results were normal.
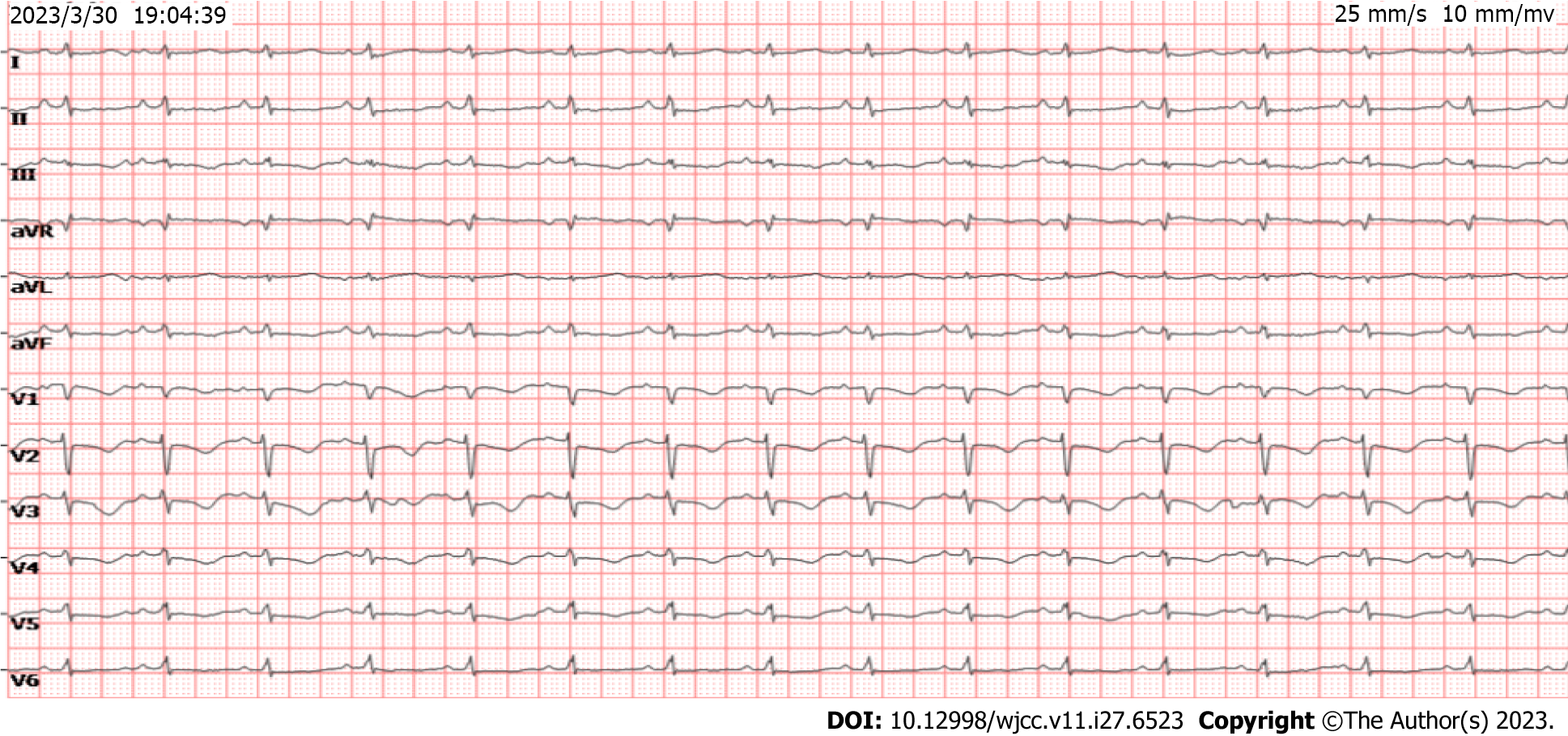
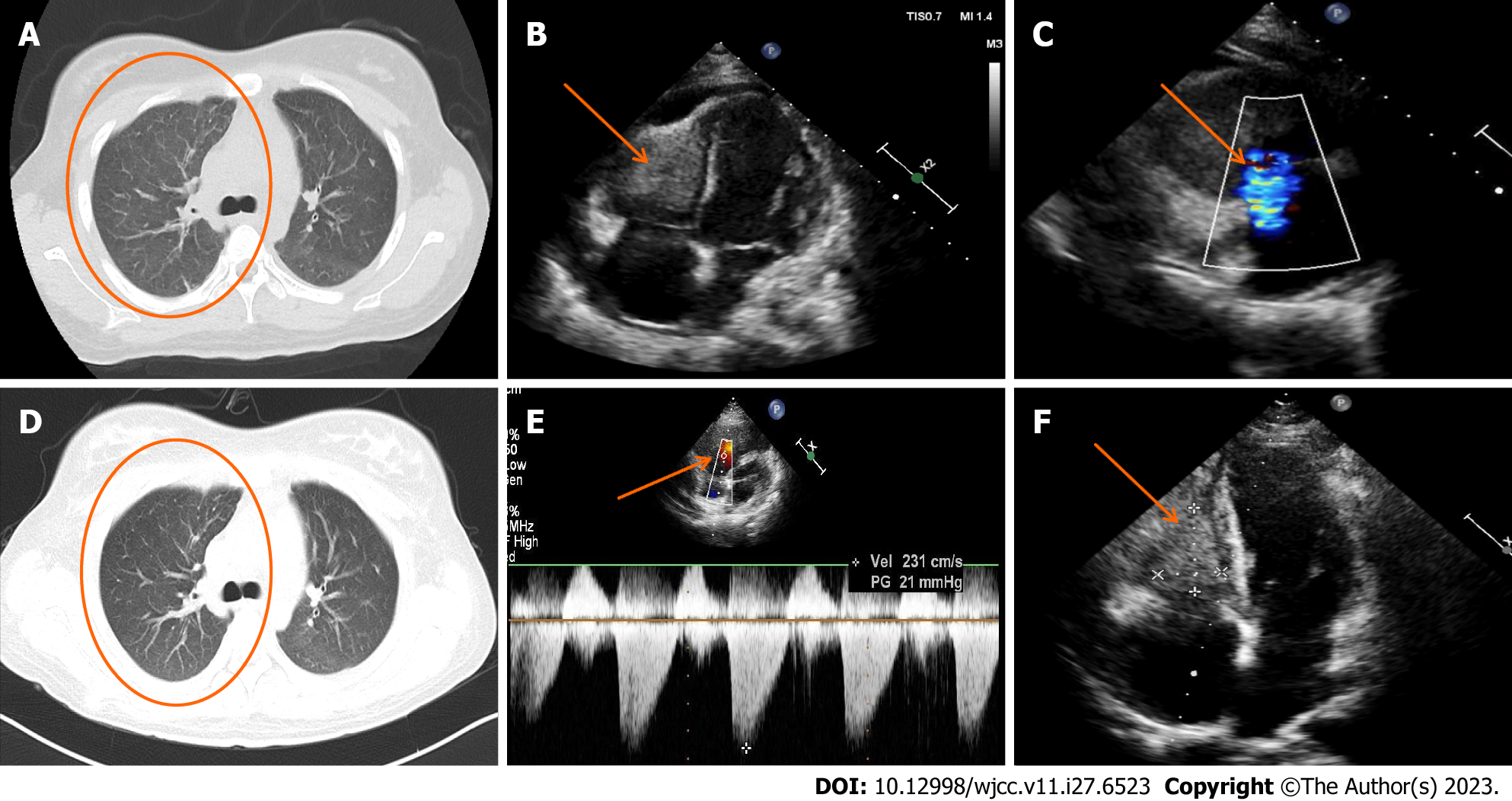
The basal electrocardiogram (Figure 2) was consistent with increased right heart load. Chest CT (Figure 3A) revealed diffuse exudative patches in both lungs, multiple bronchial dilatations and pleural thickening in the middle lobe of the right lung and lower lobe of both lungs, partial bronchial mucus plug formation, and obstructive emphysema in the distal lung tissue (observed in the middle and lower lobe of the right lung), bilateral pleural cavity microfluid and partial bilateral thickening of the pleura, and slightly enlarged mediastinal lymph nodes. Head CT showed paranasal sinusitis.
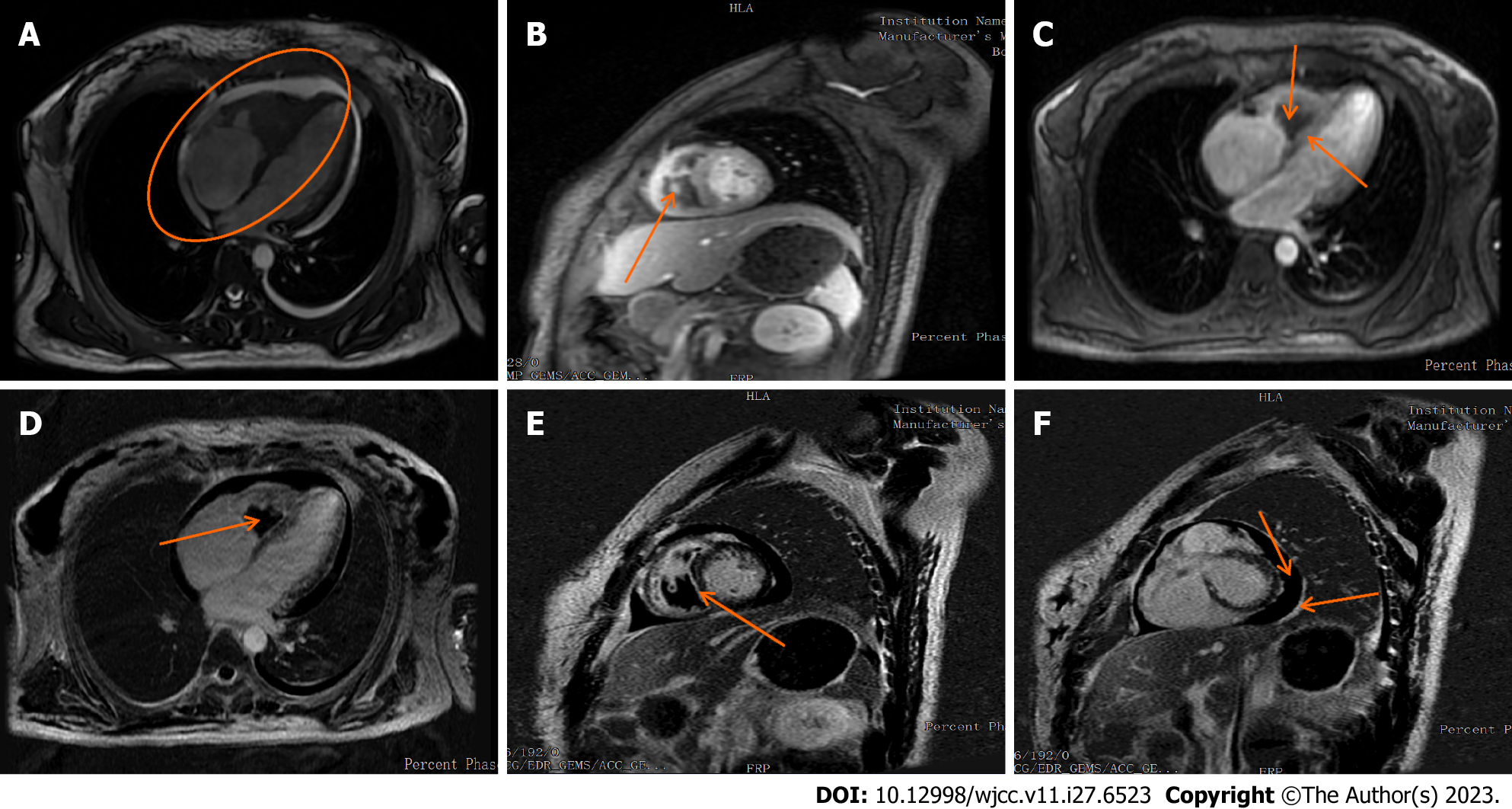
Cardiac ultrasound (Figure 3B and C) showed right ventricular hypertrophy and a vegetation on the right ventricular wall with hypoechoic characteristics (approximately 25 mm × 24 mm × 38 mm). At this point, in combination with the hematological findings, the presumed diagnosis was Loeffler syndrome. The right ventricular wall exhibited sonographic enhancement, and the right ventricular systolic function was reduced. The left ventricular wall motion and left ventricular systolic function were reduced. We observed severe reflux in the tricuspid valve, mild mitral reflux, and mild pericardial diffusion. No parasitic worm eggs were found in the stool after several attempts and abdominal CT features were unremarkable. Afterwards, we conducted a plain cardiovascular magnetic resonance (CMR) scan and enhancement imaging and further cardiac function tests on the patient (Figure 4). The findings were as follows: (1) reduced cardiac function (LVEF: 36.38%), right endocardial hypertrophy, and thrombosis, consistent with Loeffler endocarditis; (2) compensatory dilation of the right atrium, severe tricuspid reflux, and mild mitral reflux; and (3) signs of pericarditis (Supplementary material).
The patient was diagnosed as EGPA, Loeffler syndrome, and bronchial asthma.
During the patient’s CCU stay, the following treatment regimen was prescribed: intravenous methylprednisolone sodium succinate (200 mg once daily on days 1-2; 100 mg once daily on day 3; 80 mg once daily on days 4-6; and 40 mg once daily, on days 7-9); anti-infective therapy in the form of intravenous cefoperazone sodium/sulbactam sodium (3 g every 12 h for 9 d) and acyclovir (0.25 g every 8 h for 8 d); metaraminol bitartrate micro pumping to maintain blood pressure (3-6 mg/h); continuous recombinant human brain natriuretic peptide micro pumping to combat heart failure (0.0075 µg/kg/min for 3 d); subcutaneous enoxaparin sodium anticoagulation therapy (4000 AxaIU every 12 h on day 1; 5000 AxaIU every 12 h on days 2-9); intravenous furosemide diuretic (20 mg once daily for 8 days); oral rabeprazole sodium (20 mg once daily for 3 d) and intravenous drip esomeprazole sodium (40 mg once daily for 6 days) to protect the gastric mucosa; and oral calcium carbonate D3 supplementation (0.6 g once daily). On the seventh day of hospitalization, oral spironolactone (20 mg once daily) was added as anti-heart failure treatment, and on the ninth day of admission, enoxaparin sodium was switched to oral rivaroxaban (20 mg once daily) as anticoagulation treatment. Thereafter, the patient’s symptoms markedly resolved, while the E% and cardiac troponin T and NT-proBNP concentrations quickly decreased (Figure 1). A repeat chest CT (Figure 3D) showed a decrease in bilateral lung exudate compared with that in the previous chest CT. Although her blood pressure improved, it remained low and required maintenance therapy with metaraminol bitartrate (1.0-1.5 mg/h) micro pumping. After the patient’s condition stabilized slightly and the diagnosis of EGPA was established, she was transferred to the rheumatology department for further treatment. There, the CCU treatment protocol was continued for 3 d. On day 13 of the patient’s hospitalization, the rheumatology department adjusted her treatment regimen (Figure 1): The methylprednisolone sodium succinate dose was adjusted to 250 mg once daily for 3 d, followed by another adjustment to 40 mg once daily for 1 d on day 16. The patient declined cyclophosphamide treatment, and rituximab was not administered owing to the risk of pulmonary infection. The oral immunosuppressant mycophenolate mofetil was chosen as an alternative treatment (0.75 g twice daily) on day 12 and administered for 5 d.
The patient’s blood pressure returned to normal on day 13 of hospitalization, she no longer required maintenance treatment with metaraminol bitartrate, and her symptoms largely resolved. Therefore, the patient was discharged on day 17, after which she was prescribed oral prednisone acetate tablets (50 mg once daily for 13 d), mycophenolate mofetil dispersible tablets (0.75 g twice daily for 13 d), and rivaroxaban tablets (20 mg once daily 13 d). On the fourth day after discharge, the patient developed generalized edema, and when asked about medication, the patient was not taking spironolactone, so she was given oral furosemide (20 mg once daily for 9 d) combined with spironolactone (20 mg once daily for 9 d) as an anti-cardiac failure diuretic, and the edema subsided after approximately 2 d.
After 12 d, the patient presented to the outpatient clinic for follow-up. The patient complained of occasional palpitations, no other discomfort, and significant improvement in exercise tolerance. The follow-up report showed normal eosinophil count, normalization of ALB concentration, echocardiographic indication of smaller right ventricular thrombus echo (approximately 35 mm × 22 mm) than before, normal right ventricular systolic pressure, improvement of LVEF to 57%, and a small amount of mitral and tricuspid regurgitation (Figure 3E and F). The patient was scheduled for a lower dose of oral prednisone acetate tablets (45 mg once daily), with a dosage decrease by 5 mg every 10 d until the maintenance dose was reached; mycophenolate mofetil dispersible tablets (0.5 g twice daily for 30 d); and rivaroxaban tablets (10 mg once daily for 30 d), and the patient was informed to return to the hospital for a review in 1 mo.
EGPA is a rare autoimmune disease that affects multiple systems and organs throughout the body. It is characterized by eosinophilia, vascular infiltrates, and necrotizing granulomatous vasculitis of small- and medium-sized blood vessels[7,8]. The incidence of EGPA is approximately 0.5-4.2 per million in the population[1]. It mainly affects people aged 40-60 years[9], but this patient was only 20 years old, making this a rare case. The etiology of this condition is unknown, although it may include environmental, genetic, and immune factors. Although it is an ANCA-associated vasculitis, only 30%-40% of patients with EGPA have ANCA-positive disease[10]. In patients with ANCA positivity, vasculitis-related disease manifestations such as neuropathy, purpura, and glomerulonephritis are more likely to be seen. In patients with ANCA negativity, cardiac and pulmonary involvements are more common. In this case, an ANCA-negative patient had upper and lower respiratory tract involvement that was rapidly complicated by myocarditis, pericarditis, and Loeffler endocarditis, all of which are consistent with the ANCA-negative phenotype.
In 1990, the American College of Rheumatology established six diagnostic criteria (specificity of 99.7%): (1) History of asthma; (2) eosinophilia > 10%; (3) mono- or polyneuritis; (4) transient pulmonary infiltrates; (5) paranasal sinusitis; and (6) tissue biopsy confirming extravascular eosinophilic infiltrates[11]. EGPA can be diagnosed if four or more criteria are met and other granulomatous and vasculitic diseases are excluded. This patient met four criteria: history of asthma, eosinophilia > 10%, paranasal sinusitis, and transient pulmonary infiltrates. Although we did not obtain objective pathological results because the patient refused to undergo a biopsy, both cardiac ultrasound and CMR suggested Loeffler endocarditis. Therefore, extravascular eosinophilic infiltrates may have been present in this patient. After extensive consultations with the Department of Hematology, Respiratory, Infection and Rheumatology at our institution, as well as the Department of Cardiovascular Medicine at Peking Union Medical College Hospital, the predominant diagnosis leaned towards EGPA.
A vital differential diagnosis considered was clonal hypereosinophilic syndrome. This syndrome mimics the presentation of ANCA-negative EGPA patients, both characterized by organ tissue EOS infiltration and elevated peripheral blood EOS. However, it diverges in that patients usually do not exhibit preliminary asthma-like symptoms, vasculitic complications, and their biopsies typically do not show granuloma formation[1]. The diagnostic protocol for this condition combines bone marrow morphology, cytogenetics, immunophenotyping, and molecular analysis to identify any histopathological or clonal signs of an acute or chronic hematolymphoid neoplasm, with specific emphasis on mutations in the FIP1L1 and PDGFR-alpha gene rearrangements[12]. The comprehensive review of this patient’s blood and marrow, combined with standard cytogenetics, fluorescence in situ-hybridization, and flow immunophenotyping, did not point towards clonal hypereosinophilic syndromes. Interestingly, recent research suggests that patients with eosinophilia and asthma exhibiting low serum C-reactive protein levels at diagnosis might lean towards idiopathic hypereosinophilic syndrome rather than ANCA-negative EGPA[13]. Moreover, mediastinal lymphadenopathy has emerged as a hallmark of systemic inflammation in EGPA[3]. The presence of both these indicators, C-reactive protein levels and mediastinal lymphadenopathy, in our patient, further solidified the diagnosis of ANCA-negative EGPA. However, the medical community still grapples with the absence of consistent biological markers to definitively differentiate between the two conditions.
Regarding the prognosis of EGPA, the French Vasculitis Study Group established the Five-Factor Score (FFS) in 2009 to predict severity, with 1 point for each item: (1) Age > 65 years; (2) cardiac insufficiency; (3) gastrointestinal involvement; (4) renal insufficiency (serum creatinine concentration > 150 µmol/L); and (5) absence of otorhinolaryngological symptoms[14,15]. An FFS of 0-1 predicts 9%-20% 5-year mortality, whereas an FFS ≥ 2 predicts up to 40% 5-year mortality. Patients with an FFS of zero can be treated with glucocorticoid monotherapy. Immunosuppressants combined with glucocorticoids are used in patients with poor prognoses (FFS ≥ 1). In our patient (FFS = 1), chest pain and dyspnea significantly resolved after treatment with glucocorticoids and immunosuppressants. Moreover, E% and cardiac troponin T, CKMB, and NT-proBNP concentrations rapidly decreased. Both lungs had fewer exudates than that during the first chest CT. The follow-up report on day 12 after discharge from the hospital showed normal peripheral blood eosinophil levels and normalization of ALB concentration, and echocardiogram on day 19 after discharge from the hospital showed normal cardiac function and a smaller thrombus in the right ventricle.
This case highlights a few special considerations. First, our patient was 20 years old, younger than the typical age at which EGPA develops. Second, the disease progressed rapidly to Loeffler endocarditis and complete cardiac insufficiency shortly after upper and lower airway involvement. This has not been previously reported. Third, anticoagulants applied prophylactically did not prevent the formation of a right ventricular thrombus.
This study has several limitations. First, in reviewing the patient’s ancillary test results, we noted that she had a weakly positive urine protein test and a low serum ALB concentration. However, as we did not perform a 24 h urine micro-total protein test in this case, we could not exclude renal involvement. Second, our patient did not consent to a pathological tissue biopsy, preventing a definitive EGPA diagnosis. Third, as the patient did not initially receive anticoagulation therapy, a right ventricular thrombus formed, which contributed to the late diagnosis of the disease. Although we administered glucocorticosteroids, it was much lower than the recommended dose for EGPA, and the immunosuppressive agents were not administered promptly. Fourth, the follow-up time was insufficient because the patient had just been discharged from the hospital.
We recommend that clinicians should include EGPA as part of the differential diagnoses when asthma and eosinophilia (> 10%) are found. This would facilitate the early diagnosis and treatment of EGPA. When these patients present with cardiac involvement, it is important to emphasize the importance of early use of anticoagulants in addition to the use of glucocorticoids and immunosuppressive agents as part of treatment.
EGPA is a rare, complex, and diverse multisystemic disease with insidious and wide-ranging clinical manifestations, highly susceptible to misdiagnosis and late diagnosis in clinical practice. Therefore, early diagnosis and treatment are essential. This case emphasizes the effectiveness of glucocorticoids combined with immunosuppressive agents in treating EGPA. The timing and dosage of anticoagulant use should be further explored and standardized to minimize the risk of serious adverse events such as thromboembolism in patients with EGPA.
We thank our patient for allowing us to present this case and use the photographs. We also thank the Affiliated Hospital of Guizhou Medical University for providing the clinical data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barbosa OA, Brazil S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | White J, Dubey S. Eosinophilic granulomatosis with polyangiitis: A review. Autoimmun Rev. 2023;22:103219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 2. | Liu S, Han L, Liu Y, Yang J, Zhang Y, Li M, Tian X, Zeng X, Wang L, Zhang F. Clinical Significance of MPO-ANCA in Eosinophilic Granulomatosis With Polyangiitis: Experience From a Longitudinal Chinese Cohort. Front Immunol. 2022;13:885198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 3. | Sasaki H, Miyata J, Suematsu R, Kimizuka Y, Fujikura Y, Kichikawa Y, Sugiura H, Itoh K, Kawana A. Radiological significance of mediastinal lymphadenopathy in eosinophilic granulomatosis with polyangiitis. Allergol Int. 2022;71:536-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Trivioli G, Terrier B, Vaglio A. Eosinophilic granulomatosis with polyangiitis: understanding the disease and its management. Rheumatology (Oxford). 2020;59:iii84-iii94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 5. | Bettiol A, Urban ML, Bello F, Fiori D, Mattioli I, Lopalco G, Iannone F, Egan A, Dagna L, Caminati M, Negrini S, Bargagli E, Folci M, Franceschini F, Padoan R, Flossmann O, Solans R, Schroeder J, André M, Moi L, Parronchi P, Roccatello D, Sciascia S, Jayne D, Prisco D, Vaglio A, Emmi G; European EGPA Study Group. Sequential rituximab and mepolizumab in eosinophilic granulomatosis with polyangiitis (EGPA): a European multicentre observational study. Ann Rheum Dis. 2022;81:1769-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Li Y, Zhou H, Zhou Y, Tang H. Case Report: An Unusual Presentation of Cardiovascular Involvement in Eosinophilic Granulomatosis With Polyangiitis. Front Cardiovasc Med. 2022;9:928192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Lin L, Yu R, Zheng L, Gong S, Yang J. Eosinophilic Granulomatosis With Polyangiitis Presenting With Oral Granuloma as the Initial Symptom: A Case Report. Front Med (Lausanne). 2022;9:842137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 8. | Bello F, Bettiol A, Silvestri E, Mattioli I, Urban ML, Palermo A, Mazzetti M, Malandrino D, Calcaterra I, Vaglio A, Di Minno MND, Emmi G, Prisco D. Evidence of subclinical atherosclerosis in eosinophilic granulomatosis with polyangiitis. Rheumatology (Oxford). 2023;62:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Laorden D, Romero D, Domínguez-Ortega J. Benralizumab in eosinophilic granulomatosis with polyangiitis. Med Clin (Barc). 2022;158:441-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Takahashi H, Komai T, Setoguchi K, Shoda H, Fujio K. A diagnostic score for eosinophilic granulomatosis with polyangiitis among eosinophilic disorders. Allergol Int. 2023;72:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 11. | Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5950] [Cited by in RCA: 5745] [Article Influence: 164.1] [Reference Citation Analysis (0)] |
| 12. | Shomali W, Gotlib J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am J Hematol. 2019;94:1149-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 13. | Leurs A, Chenivesse C, Lopez B, Gibier JB, Clément G, Groh M, Copin MC, Staumont-Sallé D, Mortuaire G, Balquet MH, Dezoteux F, Bautin N, Buchdahl AL, Le Gouellec N, Etienne N, Terriou L, Dubucquoi S, Labalette M, Morell-Dubois S, Maillard-Lefebvre H, Lambert M, Hachulla E, Launay D, Kahn JE, Hatron PY, Lefèvre G. C-Reactive protein as a diagnostic tool in differential diagnosis of hypereosinophilic syndrome and antineutrophil cytoplasmic antibody-negative eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol Pract. 2019;7:1347-1351.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Comarmond C, Pagnoux C, Khellaf M, Cordier JF, Hamidou M, Viallard JF, Maurier F, Jouneau S, Bienvenu B, Puéchal X, Aumaître O, Le Guenno G, Le Quellec A, Cevallos R, Fain O, Godeau B, Seror R, Dunogué B, Mahr A, Guilpain P, Cohen P, Aouba A, Mouthon L, Guillevin L; French Vasculitis Study Group. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013;65:270-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 578] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 15. | Garcia-Vives E, Rodriguez-Palomares JF, Harty L, Solans-Laque R, Jayne D. Heart disease in eosinophilic granulomatosis with polyangiitis (EGPA) patients: a screening approach proposal. Rheumatology (Oxford). 2021;60:4538-4547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |