Published online Sep 26, 2023. doi: 10.12998/wjcc.v11.i27.6505
Peer-review started: May 7, 2023
First decision: July 3, 2023
Revised: August 8, 2023
Accepted: August 29, 2023
Article in press: August 29, 2023
Published online: September 26, 2023
Processing time: 136 Days and 3.1 Hours
Chromosomal Xq28 region duplication encompassing methyl-CpG-binding protein 2 (MECP2) results in an identifiable phenotype and global developmental delay known as MECP2 duplication syndrome (MDS). This syndrome has a wide range of clinical manifestations, including abnormalities in appearance, neurodevelopment, and gastrointestinal motility; recurrent infections; and spasticity. Here, we report a case of confirmed MDS at our institution.
A 12-year-old Chinese boy presented with intellectual disability (poor intellectual [reasoning, judgment, abstract thinking, and learning] and adaptive [lack of communication and absent social skills, apraxia, and ataxia] functioning) and dysmorphism. He had no history of recurrent infections, seizures, or bowel dysfunction, which is different from that in reported cases. Microarray comparative genomic hybridization confirmed MECP2 duplication in the patient and his mother who is a carrier. The duplication size was the same in the patient and his mother. No prophylactic antibiotic or anti-seizure therapy was offered to the patient or his mother before or after the consultation.
MDS is rare and has various clinical presentations. Clinical suspicion is critical in patients presenting with developmental delays.
Core Tip: Methyl-CpG-binding protein 2 (MECP2) duplication syndrome is a rare X-linked neurodevelopmental disorder. There is a paucity of data describing typical MECP2 syndrome cases in the literature. We describe the case of a Chinese boy with no history of seizure, recurrent infections, or bowel impairment from birth, who inherited duplication of the Xq28 region, including MECP2, from his mother who is a carrier, and discussed the clinical and genetic characteristics of this family. A lack of microduplication of genes in the Xq28 region may explain the absence of typical symptoms, which adds to previous reports of a rare genetic mutation in China.
- Citation: Xing XH, Takam R, Bao XY, Ba-alwi NA, Ji H. Methyl-CpG-Binding protein 2 duplication syndrome in a Chinese patient: A case report and review of the literature. World J Clin Cases 2023; 11(27): 6505-6514
- URL: https://www.wjgnet.com/2307-8960/full/v11/i27/6505.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i27.6505
Methyl-CpG-binding protein 2 (MECP2) duplication syndrome (MDS) is a rare X-linked neurodevelopmental disorder caused by chromosomal duplication of the Xq28 region, including MECP2[1,2]. MECP2 is an epigenetic modulator of gene function and comprises 486 amino acids[3]. This modulation is important in neurodevelopment, and loss of function results in Rett syndrome, which is most commonly observed in female patients. In contrast, MECP2 duplication results in MDS, which is 100% penetrant in males. As expected, Rett syndrome and MDS share some overlapping features[1,4]. MDS displays a wide array of phenotypic features, including infantile hypotonia, global severe-to-profound developmental delay, poor-to-absent speech ability, mostly lower-limb-predominant progressive spasticity, choreiform movements, various seizure types, unsteady gait, recurrent respiratory tract infections, gastrointestinal disorders, genitourinary disorders, and craniofacial dysmorphism[1-4]. The severity of the clinical manifestations varies according to the size of the Xq28 duplication; the larger the duplication, the more severe the symptoms[5,6]. In addition, triplication of MECP2 has been associated with severe symptomatology and macrocephaly[7]. Overexpression of MECP2 results in progressive neurological disorders, impairments in learning and coordination, delays in acquiring new motor skills, and seizures[8,9]. Of note, half of patients with MDS have a shortened lifespan, with most dying before the age of 25 years[1-3]. Some studies have suggested that MDS causes neuronal cell death as a result of astrocytic dysfunction, leading to a homeostatic imbalance in the central nervous system[10]. Furthermore, MECP2 duplication may affect neural tube formation and cause premature differentiation of neural precursors, ultimately leading to cell death and neuronal loss[11]. Therefore, antenatal ultrasonography has been suggested as a screening modality for MDS. Features observed on sonography include prenasal and prefrontal skin thickening in the second trimester, hydrocephalus, ventriculomegaly, corpus callosum agenesis, choroid plexus cyst, growth restriction, and hydronephrosis, which may be observed during antenatal visits[12].
MECP2 syndrome primarily affects males, and duplication is often inherited from mothers who are carriers because of nearly complete skewing of X-chromosomal inactivation[13,14]. Notably, some female carriers may present with depression, anxiety, and autistic features with a maintained intellect[4,15]. Despite the paucisymptomatic forms, severe or profound abnormal phenotypes in females are rare and are generally due to de novo duplications, unlike in males, where severe phenotype duplications are inherited[16,17]. Xq28 microduplication involves several genes, including interleukin-1 receptor-associated kinase 1 (IRAK1), L1 cell adhesion molecule (L1CAM), filamin A (FLNA), and RAB39B, which are responsible for immune functions, cognitive functions, bowel motility, and intellectual impairments, respectively[1-3,18,19]. In addition, affected male patients do not respond to polysaccharide-containing vaccines, such as those against Streptococcus pneumoniae, and may require booster shots[20,21]. Antisense oligonucleotide (ASO) therapy has been successfully used to reverse symptoms in mice; however, in vivo research is still in the trial phase[22]. Further research is needed to shed more light on this syndrome because of its rarity and phenotypic heterogeneity. There is a paucity of data in the literature describing MECP2 syndrome; only 545 cases with confirmed genotype have been published (504 males and 41 symptomatic females) from 1987 to date[23].
In this report, we describe the case of a Chinese boy who inherited duplication of the Xq28 region, including MECP2, from his mother who is a carrier (confirmed). Compared with that of patients reported earlier in the literature, he had no history of pulmonary infections, intestinal motility disorders, or seizures, compounded by a lack of awareness, which may have prevented an earlier diagnosis of his condition.
A 12-year-old boy presented with intellectual disability, poor speech, axial hypotonia, and dysmorphic features.
The patient reported a one-month bloody urethral discharge. He had no flank pain or urethral pain.
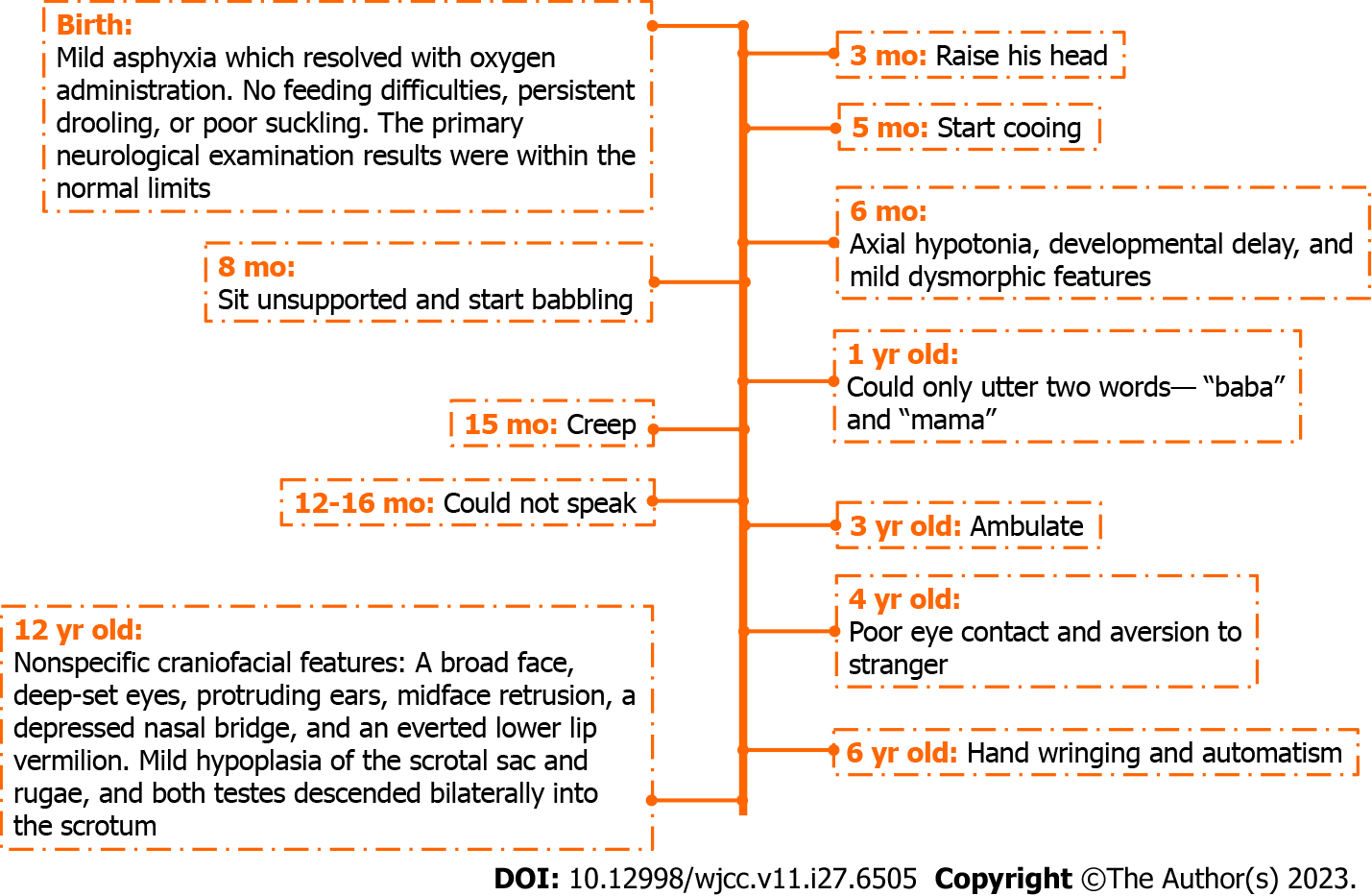
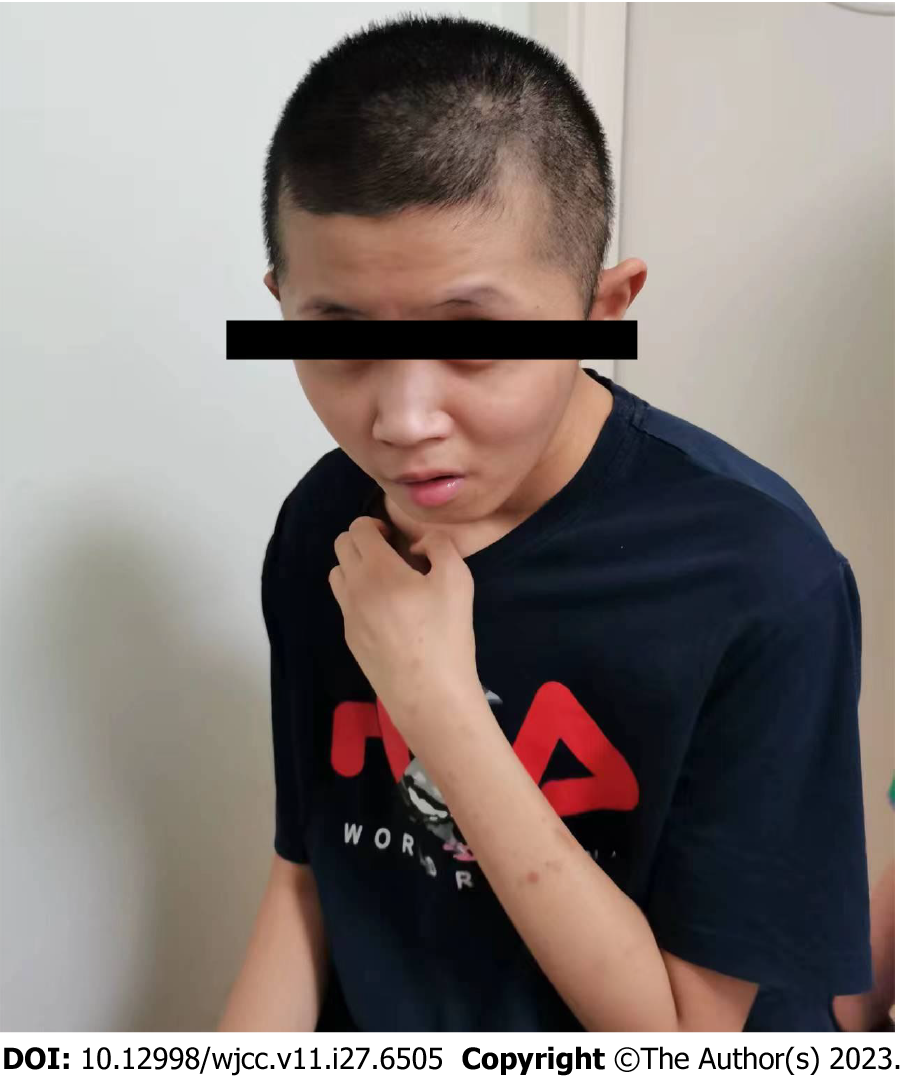
He was the second of two children, with an older sister who was otherwise healthy. Following an uneventful pregnancy, he was born via vaginal delivery at 37 wk of gestation with a birth weight of 3.8 kg, measuring 51 cm with a head circumference of 34 cm. He developed mild asphyxia at birth, which resolved with oxygen administration. He showed no feeding difficulties, persistent drooling, or poor suckling at birth, and the primary neurological examination results were within the normal limits. He was able to raise his head at 3 mo, sit unsupported at 8 mo, creep at 15 mo, and ambulate at 3 years of age. At 6 mo of age, he had axial hypotonia, developmental delay, and mild dysmorphic features. He started cooing and babbling at 5 and 8 mo of age, respectively, and at 1 year of age, he could utter only two words: “baba” and “mama.” He could not speak 6 mo later. At 4 years of age, poor eye contact and aversion to strangers were predominant. Hand wringing and automatism were occasionally noticed at 6 years of age (Figure 1). He had no history of hospitalization, recurrent respiratory tract infections, constipation, or seizures. Magnetic resonance imaging was not performed, and he was not on any chronic therapy or medication.
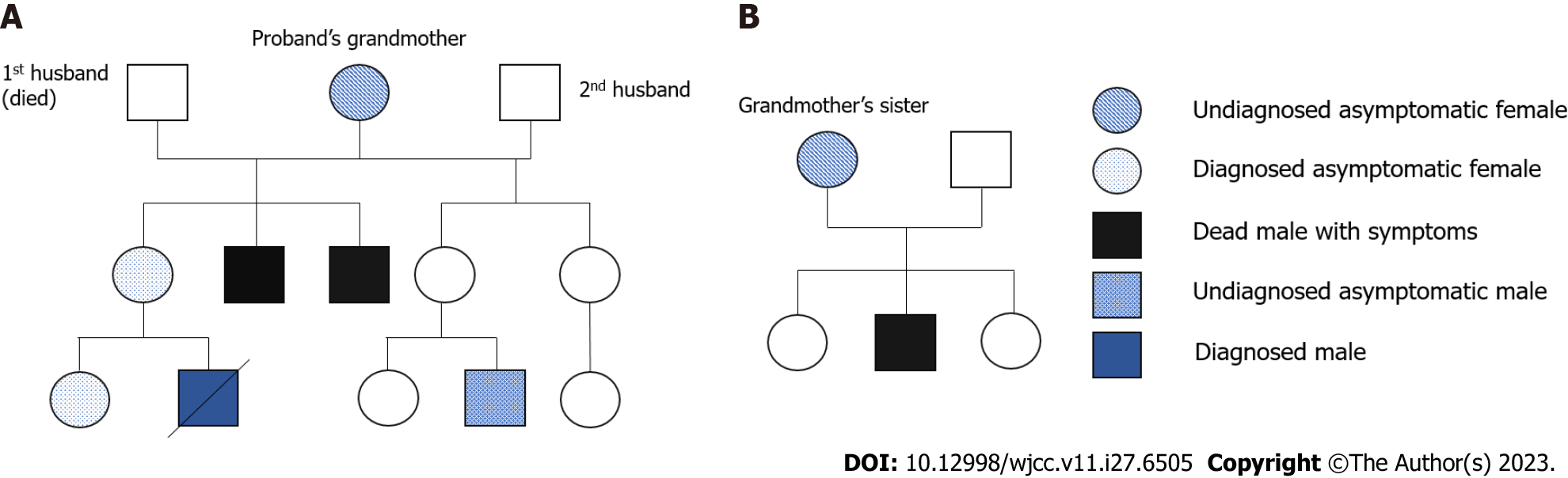
The boy’s mother and sister had a confirmed diagnosis of MECP2 duplication. The mother had two younger brothers with developmental delays, both of whom died at the age of 8 years. The mother recalled that her brothers could not walk or speak at the time of their death. The boy’s mother and grandmother had no symptoms of depression, anxiety, or autism. The grandmother’s first husband died of gastric cancer, and she remarried. The grandmother has two daughters with her new husband. One of her daughters (the boy’s aunt) has two children: a girl who is asymptomatic and a boy aged 8 years who has a similar developmental delay and is awaiting genetic confirmation. In addition, the boy’s grandaunt (grandmother’s sister) had three children: two girls and a boy with cognitive and developmental delays who died at the age of 5 years. Figure 2 shows the family pedigree with the proband indicated by an arrow.
On admission, the patient needed support while standing and moving and needed feeding assistance. The craniofacial features observed were nonspecific and included a broad face, deep-set eyes, protruding ears, midface retrusion, a depressed nasal bridge, and an everted lower lip vermilion (Figure 3). Mild hypoplasia of the scrotal sac and rugae were observed, and both testes descended bilaterally into the scrotum. In view of the patient's special condition, we assessed his intelligence using the Peabody Picture Vocabulary Test. He was considered to have severe mental retardation because of his inability to learn and understand complex words and because he could recognize only a few pictures and words.
Genome extraction: The precise size and breakpoints of the duplicates were determined by array comparative genomic hybridization (aCGH) of the proband and his family’s deoxyribonucleic acid (DNA) using the MagPure Buffy Coat DNA Midi KF Kit. A DNA segment with a band of 150–200 bp was randomly ligated using T4 DNA ligase after terminal repair. Axygen beads were used to amplify DNA fragments with splices at both ends using PCR. Up to 160 ng of DNA was isolated and cycled to obtain single-stranded circular DNA, according to the amount of data needed to select 14–48 PCR products for the same amount of substances to be mixed. Finally, the Huada self-sequencing platform BGISEQ-500 was used for 35 sequencing cycles, and the data were processed using the Zebracall software.
Sequence analysis: The original data (raw reads) were sequenced and evaluated to remove contaminated and poor-quality reads. The SOAP and Hg19 genome sequences were compared to exclude repeats. The results were used to divide the observed region into windows, and the number of reads in each window was calculated. After standardization, the corresponding depth value was used to evaluate the fluctuation of the window, and the data were corrected according to different GC contents. The candidate copy number variation (CNV) segments were then identified by binomial fragmentation, and the CNV results were obtained by threshold filtering through the set threshold.
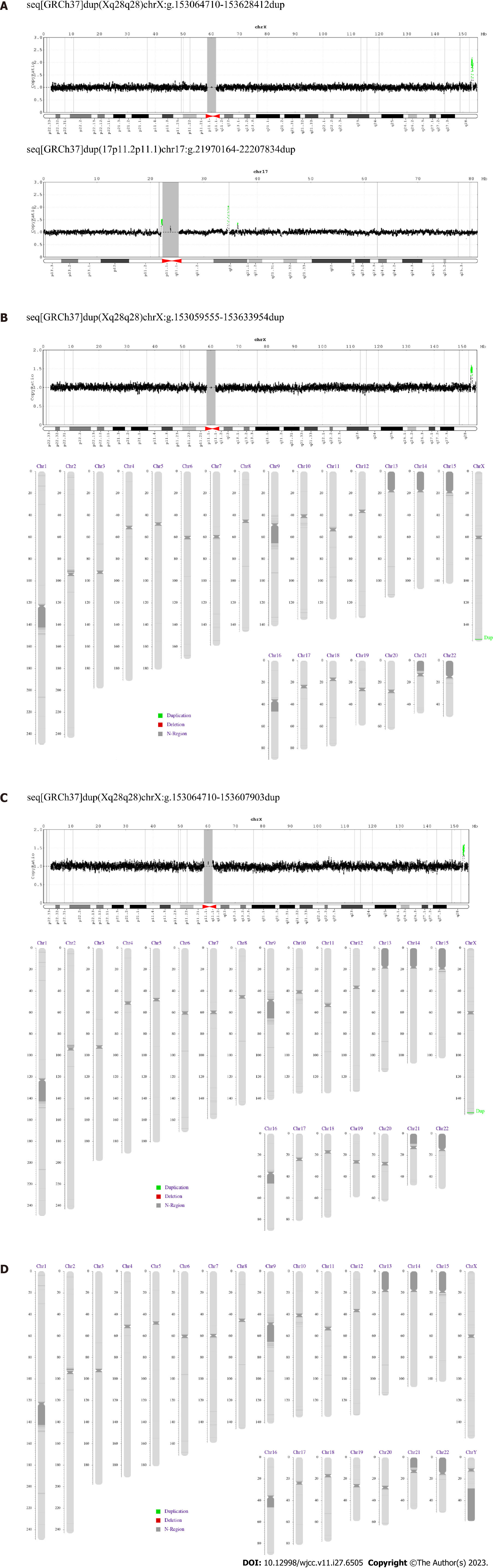
Results: The aCGH performed on the patient’s genomic DNA showed Xq28 duplication with breakpoints located between positions 153273980 and 153375749 bp with a size of 563.70 kb, a ClinGen CNV score of ≥ 0.99 (Figure 4A), and duplication of chromosome 17 (17p11.2p11.1) with a size of 237.67 kb. Size variations were also observed. In addition, analysis of the proband’s clinically normal sister revealed a duplication of the same region (153273980–153375749 bp), albeit with a different size, 574.40 kb (Figure 4B). The mother showed Xq28 duplication at the same breakpoint, with a size of 543.49 kb (Figure 4C), whereas the father’s DNA showed no duplication (Figure 4D). No microduplication of genes, such as IRAK1, L1CAM, FLNA, or RAB39B, was observed in the Xq28 region.
There was no imaging examination.
Hong Ye, PhD, Chief Doctor, Department of Gynecology. An expert in prenatal diagnosis and genetic counseling, major in improving prenatal and postnatal care, ultrasound screening for fetal and genetic counseling. In addition, the expert is experienced in the field of recurrent spontaneous abortion, peripartum medical care, and the diagnosis and treatment of pregnancy complications.
The patient was diagnosed with MDS.
Prior to consultation, the patient had two episodes of upper respiratory tract infection but no history of seizures, epilepsy, or bowel impairment. No prophylactic therapy was initiated, and no records of any previous surgeries were found. After the diagnosis was established, the patient was followed up by an interdisciplinary group of specialists, including but not limited to genetic counselors, physiotherapists, nutritionists, and experts from other specialties, to provide personalized treatment plans and suitable follow-up. No pharmacological treatments were administered.
Follow-up was not possible because the patient did not return to the hospital.
Here, we report a case of MDS in a 12-year-old Asian proband. The diagnosis was established during genome investigation based on the patient’s poor milestone attainment, regression of attained developmental skills, dysmorphism, and clinical presentation upon consultation. MECP2, which is located on chromosome Xq28, is a regulator of postnatal development. MDS was first described in a family of five males with neurodevelopmental delay[24]. Previous studies involving Asian patients with MDS have shown that the clinical manifestations are similar to those in Western patients with MDS; however, most studies described a single-family member[1,4-6,25], with only one study examining all members of the proband’s pedigree: Five affected males and four asymptomatic female carriers[26].
Contrary to patients in the previously reported cases[4-6,25,26], our patient had no history of seizures, recurrent infections, gastrointestinal abnormalities, or vasomotor instability. He experienced loss of language skills at 18 months of age and could not utter any words or maintain eye contact upon consultation.
Studies have shown that the regression in attained skills is not correlated with the size of MECP2 protein duplication[6,27,28]. However, seizure onset generally occurs around the time of regression[29,30]. Although this was not observed in our patient. The patient had no history of seizures, recurrent infections, or bowel impairment since birth. No microduplication of genes, such as IRAK1, L1CAM, FLNA, or RAB39B, was observed in the Xq28 region. This may explain the absence of the typical symptoms of MDS in our patient. We compared core MDS symptoms from previous case reports with those of our proband, as shown in Table 1.
| Clinical features of the proband | Presence/absence | Cases in the literature[6], % |
| Age at testing | 12 yr old | < 16 yr old |
| Developmental delay | Present | 100 |
| Hypotonia in infancy | Present | 100 |
| Spasticity | Present | 59[9] |
| Seizures | Absent | 56 |
| IPO or constipation | Absent | 76[9] |
| Speech delay and regression | Present | 84 |
| Genital abnormality | Absent | 68 |
| History of recurrent infections | Absent | 83 |
| Neuroimaging, electroencephalography | Not performed | Available |
| Duplicate region and size | 563.70 kb | 200 kb to 2.2 Mb |
| Inheritance | Maternal | Maternal, few de novo |
| Family history | Present | Present |
Microarray comparative genomic hybridization confirmed MECP2 duplication in the patient and his mother who is a carrier. The duplication size was the same in the patient and his parent, confirming that the condition was inherited and not de novo. Our patient lost two uncles with symptoms suggestive of MDS due to recurrent respiratory tract infections; however, MECP2 was never administered to confirm MDS. In addition, the proband had a friend aged 5 years with global developmental delay, hypotonia, and cognitive delay; however, genetic analysis was not performed. It is highly possible that they all had MDS. This is a major limitation of our study, as proper follow-up of the patient’s condition and that of possibly affected family members was not performed or initiated early enough, and no past imaging was performed or recorded.
Consistent symptomatology affecting male family members, including but not limited to severe intellectual impairment, delayed attainment of milestones, and regression in motor and other skills, may help clinicians recognize this rare disorder and distinguish it from other chromosomal disorders. Our patient had no typical history of recurrent infections, bowel disorders, seizures, or vasomotor instability as reported in the literature. This report adds to the growing number of cases observed in China and may aid future studies to evaluate the similarities and differences observed between the Asian, African, and Caucasian populations and to better understand the course of the disease. The outlook is poor, as patients with this syndrome cannot live independent lives; hence, proper communication is imperative with the families of those affected. This study surmised that the decreased prevalence of seizures, recurrent respiratory tract infections, and bowel dysfunction observed in patients with MDS may be related to the absence of duplicate genes within the Xq28 region. MDS diagnosis is important for genetic counseling, and there is a high possibility of reversing some of the debilitating symptoms observed in MDS in ongoing clinical trials using ASO therapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Havali C, Turkey; Liehr T, Germany; Pitton Rissardo J, Brazil S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | Van Esch H, Bauters M, Ignatius J, Jansen M, Raynaud M, Hollanders K, Lugtenberg D, Bienvenu T, Jensen LR, Gecz J, Moraine C, Marynen P, Fryns JP, Froyen G. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am J Hum Genet. 2005;77:442-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 480] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 2. | Klose RJ, Bird AP. MeCP2 behaves as an elongated monomer that does not stably associate with the Sin3a chromatin remodeling complex. J Biol Chem. 2004;279:46490-46496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Della Ragione F, Vacca M, Fioriniello S, Pepe G, D'Esposito M. MECP2, a multi-talented modulator of chromatin architecture. Brief Funct Genomics. 2016;15:420-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Ramocki MB, Tavyev YJ, Peters SU. The MECP2 duplication syndrome. Am J Med Genet A. 2010;152A:1079-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 5. | Peters SU, Fu C, Suter B, Marsh E, Benke TA, Skinner SA, Lieberman DN, Standridge S, Jones M, Beisang A, Feyma T, Heydeman P, Ryther R, Kaufmann WE, Glaze DG, Neul JL, Percy AK. Characterizing the phenotypic effect of Xq28 duplication size in MECP2 duplication syndrome. Clin Genet. 2019;95:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | del Gaudio D, Fang P, Scaglia F, Ward PA, Craigen WJ, Glaze DG, Neul JL, Patel A, Lee JA, Irons M, Berry SA, Pursley AA, Grebe TA, Freedenberg D, Martin RA, Hsich GE, Khera JR, Friedman NR, Zoghbi HY, Eng CM, Lupski JR, Beaudet AL, Cheung SW, Roa BB. Increased MECP2 gene copy number as the result of genomic duplication in neurodevelopmentally delayed males. Genet Med. 2006;8:784-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Tang SS, Fernandez D, Lazarou LP, Singh R, Fallon P. MECP2 triplication in 3 brothers - a rarely described cause of familial neurological regression in boys. Eur J Paediatr Neurol. 2012;16:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, David Sweatt J, Zoghbi HY. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet. 2004;13:2679-2689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 471] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 9. | Ramocki MB, Zoghbi HY. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455:912-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 304] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 10. | Russell JC, Blue ME, Johnston MV, Naidu S, Hossain MA. Enhanced cell death in MeCP2 null cerebellar granule neurons exposed to excitotoxicity and hypoxia. Neuroscience. 2007;150:563-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Petazzi P, Akizu N, García A, Estarás C, Martínez de Paz A, Rodríguez-Paredes M, Martínez-Balbás MA, Huertas D, Esteller M. An increase in MECP2 dosage impairs neural tube formation. Neurobiol Dis. 2014;67:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Fu F, Liu HL, Li R, Han J, Yang X, Min P, Zhen L, Zhang YL, Xie GE, Lei TY, Li Y, Li J, Li DZ, Liao C. Prenatal diagnosis of foetuses with congenital abnormalities and duplication of the MECP2 region. Gene. 2014;546:222-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Auber B, Burfeind P, Thiels C, Alsat EA, Shoukier M, Liehr T, Nelle H, Bartels I, Salinas-Riester G, Laccone F. An unbalanced translocation resulting in a duplication of Xq28 causes a Rett syndrome-like phenotype in a female patient. Clin Genet. 2010;77:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Grasshoff U, Bonin M, Goehring I, Ekici A, Dufke A, Cremer K, Wagner N, Rossier E, Jauch A, Walter M, Bauer C, Bauer P, Horber K, Beck-Woedl S, Wieczorek D. De novo MECP2 duplication in two females with random X-inactivation and moderate mental retardation. Eur J Hum Genet. 2011;19:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Shimada S, Okamoto N, Ito M, Arai Y, Momosaki K, Togawa M, Maegaki Y, Sugawara M, Shimojima K, Osawa M, Yamamoto T. MECP2 duplication syndrome in both genders. Brain Dev. 2013;35:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Fieremans N, Bauters M, Belet S, Verbeeck J, Jansen AC, Seneca S, Roelens F, De Baere E, Marynen P, Froyen G. De novo MECP2 duplications in two females with intellectual disability and unfavorable complete skewed X-inactivation. Hum Genet. 2014;133:1359-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | El Chehadeh S, Touraine R, Prieur F, Reardon W, Bienvenu T, Chantot-Bastaraud S, Doco-Fenzy M, Landais E, Philippe C, Marle N, Callier P, Mosca-Boidron AL, Mugneret F, Le Meur N, Goldenberg A, Guerrot AM, Chambon P, Satre V, Coutton C, Jouk PS, Devillard F, Dieterich K, Afenjar A, Burglen L, Moutard ML, Addor MC, Lebon S, Martinet D, Alessandri JL, Doray B, Miguet M, Devys D, Saugier-Veber P, Drunat S, Aral B, Kremer V, Rondeau S, Tabet AC, Thevenon J, Thauvin-Robinet C, Perreton N, Des Portes V, Faivre L. Xq28 duplication including MECP2 in six unreported affected females: what can we learn for diagnosis and genetic counselling? Clin Genet. 2017;91:576-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Vanmarsenille L, Giannandrea M, Fieremans N, Verbeeck J, Belet S, Raynaud M, Vogels A, Männik K, Õunap K, Jacqueline V, Briault S, Van Esch H, D'Adamo P, Froyen G. Increased dosage of RAB39B affects neuronal development and could explain the cognitive impairment in male patients with distal Xq28 copy number gains. Hum Mutat. 2014;35:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Clayton-Smith J, Walters S, Hobson E, Burkitt-Wright E, Smith R, Toutain A, Amiel J, Lyonnet S, Mansour S, Fitzpatrick D, Ciccone R, Ricca I, Zuffardi O, Donnai D. Xq28 duplication presenting with intestinal and bladder dysfunction and a distinctive facial appearance. Eur J Hum Genet. 2009;17:434-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Friez MJ, Jones JR, Clarkson K, Lubs H, Abuelo D, Bier JA, Pai S, Simensen R, Williams C, Giampietro PF, Schwartz CE, Stevenson RE. Recurrent infections, hypotonia, and mental retardation caused by duplication of MECP2 and adjacent region in Xq28. Pediatrics. 2006;118:e1687-e1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Prescott TE, Rødningen OK, Bjørnstad A, Stray-Pedersen A. Two brothers with a microduplication including the MECP2 gene: rapid head growth in infancy and resolution of susceptibility to infection. Clin Dysmorphol. 2009;18:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Sztainberg Y, Chen HM, Swann JW, Hao S, Tang B, Wu Z, Tang J, Wan YW, Liu Z, Rigo F, Zoghbi HY. Reversal of phenotypes in MECP2 duplication mice using genetic rescue or antisense oligonucleotides. Nature. 2015;528:123-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 23. | Gottschalk I, Kölsch U, Wagner DL, Kath J, Martini S, Krüger R, Puel A, Casanova JL, Jezela-Stanek A, Rossi R, Chehadeh SE, Van Esch H, von Bernuth H. IRAK1 Duplication in MECP2 Duplication Syndrome Does Not Increase Canonical NF-κB-Induced Inflammation. J Clin Immunol. 2023;43:421-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 24. | Pai GS, Hane B, Joseph M, Nelson R, Hammond LS, Arena JF, Lubs HA, Stevenson RE, Schwartz CE. A new X linked recessive syndrome of mental retardation and mild dysmorphism maps to Xq28. J Med Genet. 1997;34:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Xu X, Xu Q, Zhang Y, Zhang X, Cheng T, Wu B, Ding Y, Lu P, Zheng J, Zhang M, Qiu Z, Yu X. A case report of Chinese brothers with inherited MECP2-containing duplication: autism and intellectual disability, but not seizures or respiratory infections. BMC Med Genet. 2012;13:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Li X, Xie H, Chen Q, Yu X, Yi Z, Li E, Zhang T, Wang J, Zhong J, Chen X. Clinical and molecular genetic characterization of familial MECP2 duplication syndrome in a Chinese family. BMC Med Genet. 2017;18:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Peters SU, Hundley RJ, Wilson AK, Carvalho CM, Lupski JR, Ramocki MB. Brief report: regression timing and associated features in MECP2 duplication syndrome. J Autism Dev Disord. 2013;43:2484-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Ramocki MB, Peters SU, Tavyev YJ, Zhang F, Carvalho CM, Schaaf CP, Richman R, Fang P, Glaze DG, Lupski JR, Zoghbi HY. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann Neurol. 2009;66:771-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 29. | Barger BD, Campbell JM, McDonough JD. Prevalence and onset of regression within autism spectrum disorders: a meta-analytic review. J Autism Dev Disord. 2013;43:817-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Neul JL, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, Bahi-Buisson N, Leonard H, Bailey ME, Schanen NC, Zappella M, Renieri A, Huppke P, Percy AK; RettSearch Consortium. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68:944-950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1076] [Cited by in RCA: 975] [Article Influence: 65.0] [Reference Citation Analysis (0)] |