Published online Sep 26, 2023. doi: 10.12998/wjcc.v11.i27.6483
Peer-review started: March 30, 2023
First decision: July 3, 2023
Revised: July 8, 2023
Accepted: July 21, 2023
Article in press: July 21, 2023
Published online: September 26, 2023
Processing time: 174 Days and 8.5 Hours
The pathological complete response (ypCR) rate following neoadjuvant chemotherapy for advanced gastric cancer remains low and lacks a universally accepted treatment protocol. Immunotherapy has achieved breakthrough progress.
We report two female patients with gastric cancer defined as clinical stage cT4N1-2M0. Detection of mismatch repair protein showed mismatch repair function defect, and perioperative treatment with programmed death protein 1 inhibitor combined with S-1+oxaliplatin achieved ypCR. Surprisingly, the patients underwent clinical observation after surgery but developed different degrees of metastasis at ~6 mo after surgery.
PD-1 inhibitor combined with chemotherapy provides a more strategic choice for comprehensive perioperative treatment of gastric cancer.
Core tip: The enhanced antitumor effect of synergistic chemotherapy and immunotherapy has achieved commendable remission rates and survival benefit. We reported two patients who achieved pathological complete remission (ypCR) with perioperative treatment with programmed death protein 1 inhibitor combined with S-1+oxaliplatin. Surprisingly, the patients underwent clinical observation after surgery but developed different degrees of metastasis at ~6 mo after surgery. Achieving ypCR reflects an excellent short-term treatment response but does not necessarily predict long-term survival.
- Citation: Xing Y, Zhang ZL, Ding ZY, Song WL, Li T. Tumor recurrence after pathological complete response in locally advanced gastric cancer after neoadjuvant therapy: Two case reports. World J Clin Cases 2023; 11(27): 6483-6490
- URL: https://www.wjgnet.com/2307-8960/full/v11/i27/6483.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i27.6483
We report two female patients with gastric cancer defined as clinical stage cT4N1-2M0. The detection of mismatch repair protein showed mismatch repair function defect (dMMR), and perioperative treatment with programmed death protein 1 (PD-1) inhibitor combined with SOX (S-1+oxaliplatin) achieved pathological complete response (ypCR). It is controversial for ypCR patients to continue comprehensive chemotherapy or clinical observation after surgery. Surprisingly, both our patients underwent clinical observation after surgery but developed different degrees of metastasis at ~6 mo after surgery. This unexpected outcome prompted a review of the comprehensive perioperative treatment for these two patients in an effort to provide valuable insights for clinical decision-making.
Case 1: The patient was an 81-year-old woman with upper abdominal pain, loss of appetite, belching, and acid reflux for 3 mo.
Case 2: A 69-year-old female patient presented with cryptic pain in the upper abdomen and loss of appetite for 5 mo.
Case 1: The patient complained of upper abdominal pain without obvious inducement 3 mo previously, accompanied by loss of appetite, belching, and gastric acid reflux. Before 1 mo, the above symptoms were aggravated, and eating difficulties and nausea occurred. Since the onset of the disease, her weight has dropped by 2 kg.
Case 2: The patient had upper abdominal pain for > 5 mo, and vomiting occurred intermittently after eating. Since the onset of the disease, her body weight decreased by 4.5 kg.
Case 1: The patient had a history of hypertension and type 2 diabetes, and the oral medication was well-controlled.
Case 2: Previous cholecystectomy due to gallstones.
Case 1: The patient had no relevant personal or family history.
Case 2: Drinking history of 40 years, no family history.
Case 1: Blood pressure 130/82 mmHg (normal range: 90–140/60–90 mmHg), pulse rate 72 beats/min (normal range: 60–90 beats/min), and respiratory rate 18 breaths/min (normal range: 16–20 breaths/min).
Case 2: Blood pressure 120/70 mmHg, pulse rate of 62 beats/min, and respiratory rate 17 breaths/min. Normal ranges as for Case 1.
Case 1: Blood tests revealed abnormal results: Carbohydrate antigen (CA)19-9 378.0 U/mL (normal range: 0–39.0 U/mL); CA72-4 220 U/mL (normal range: 0–8.20 U/mL); carcinoembryonic antigen (CEA) 478.0 ng/mL (normal range: 0–5.0 ng/mL); and hemoglobin 111 g/L (normal range: 115–150 g/L).
Case 2: Blood tests revealed abnormal results: CA19-9 285.0 U/mL; CA72-4 61.5 U/mL; CEA 5.48 ng/mL; and hemoglobin 80 g/L. Normal ranges as for Case 1.
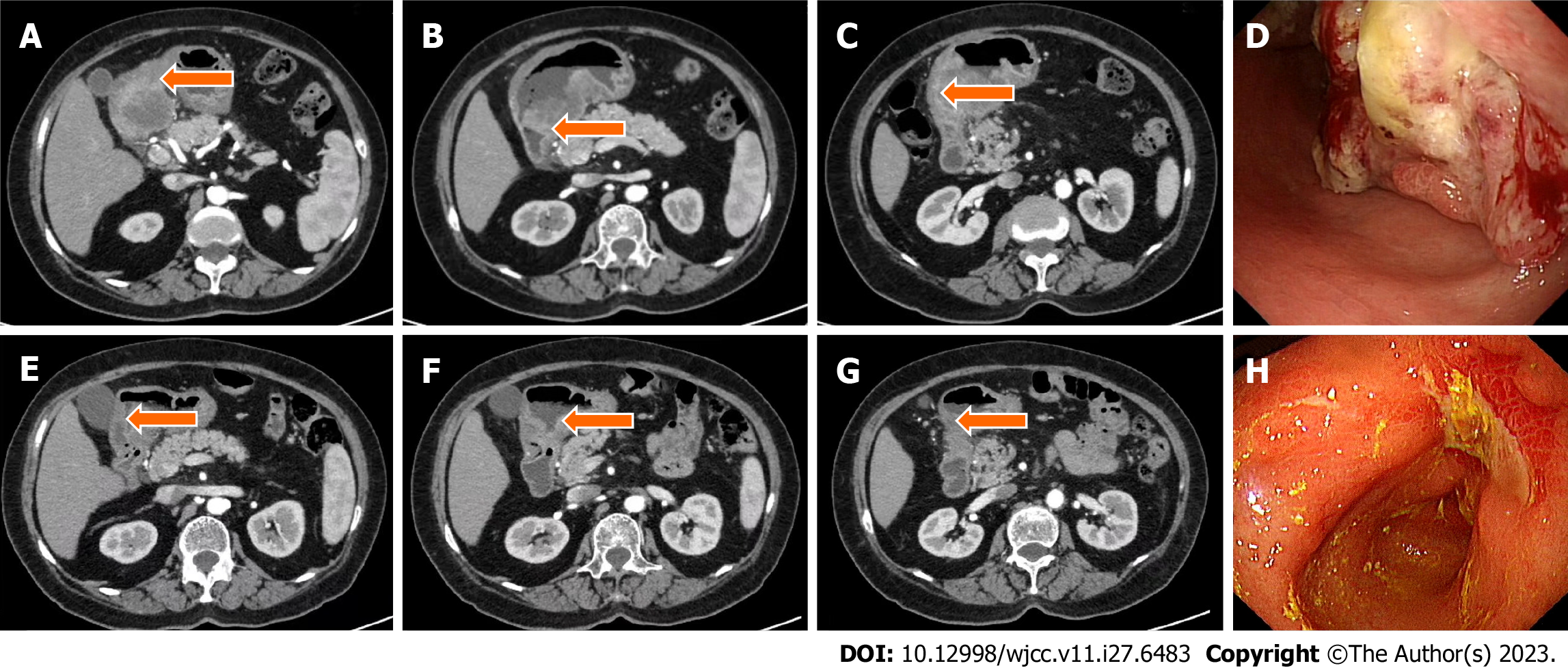
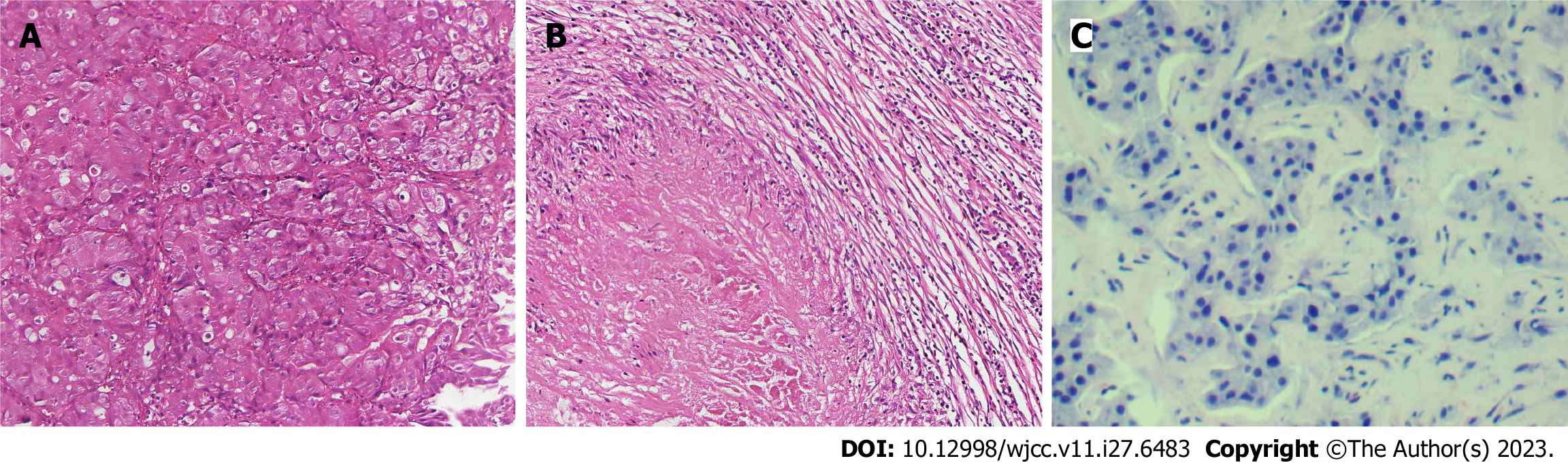
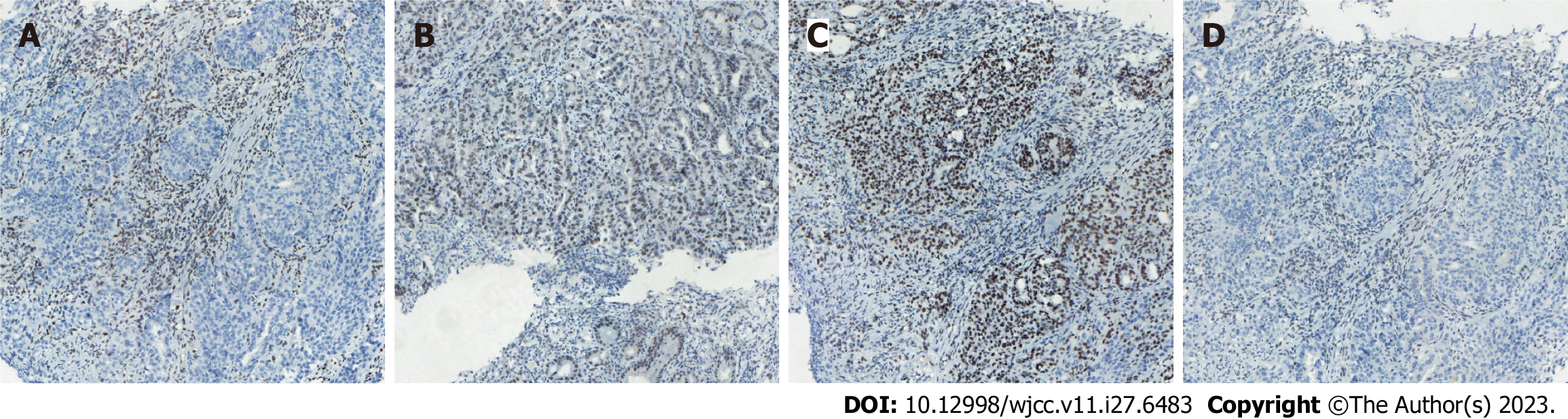
Case 1: Computed tomography (CT) was performed on the abdomen, showing gastric wall thickening in the lesser curvature of the antrum, gastric cavity stenosis with increased density of perigastric fat space, and multiple enlarged lymph nodes (Figure 1A-C). Gastroscopy showed an ulcerative mass in the antrum, and pathology showed adenocarcinoma (Figures 1D and 2A). Detection of MMR protein showed dMMR (Figure 3).
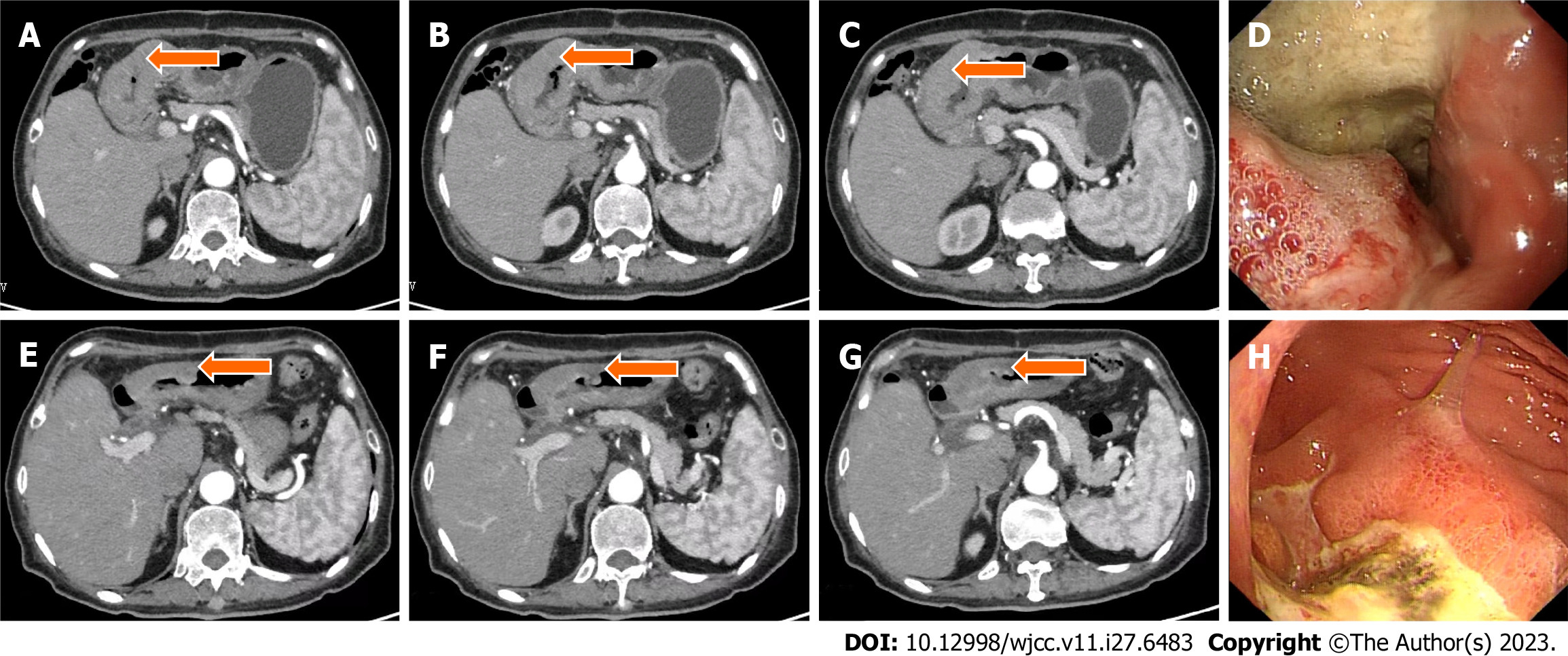
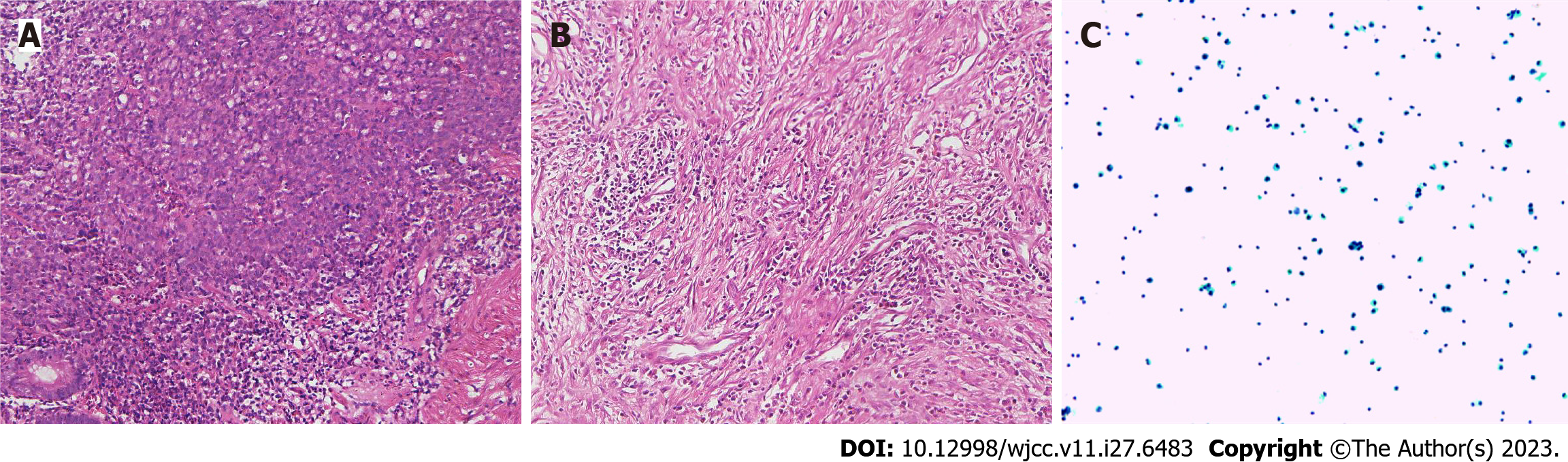
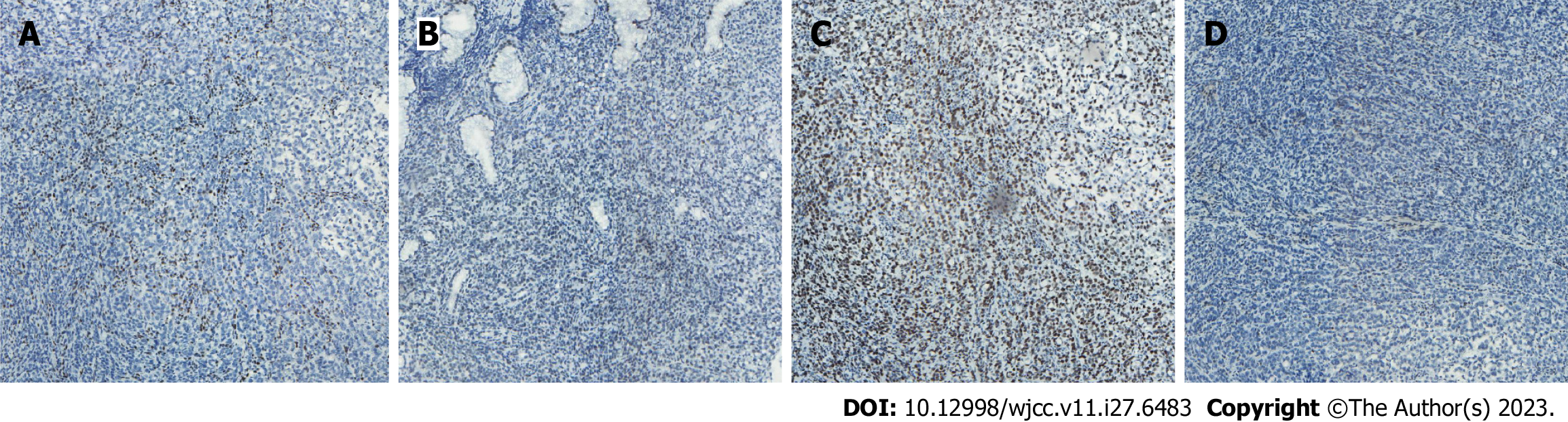
Case 2: Gastroscopy showed masses in the antrum and gastric horn, and pathology showed heterospecific cells and signet-ring cells. CT of the abdomen showed local irregular thickening of the gastric wall in the antrum and gastric horn with clumped soft tissue shadows and enlargement of multiple lymph nodes around the stomach (Figures 4A-D and 5A). MMR protein detection showed dMMR (Figure 6).
Case 1: According to the Eighth American Joint Committee on Cancer/Union for International Cancer Control TNM Classification of Malignant Tumors, clinical stage cT4N2M0 gastric adenocarcinoma.
Case 2: Clinical stage cT4N1M0 gastric adenocarcinoma, according to the above classification.
This patient benefited from immunosuppressive therapy. From March to May 2021, the patient, without chemotherapy contraindication, received three cycles of SOX chemotherapy (intravenous oxaliplatin 130 mg/m2 on day 1, S-1 80 mg/m2 after breakfast and dinner twice daily on days 1–14), plus the PD-1 inhibitor (camrelizumab) 200 mg intravenous glucose tolerance test (IVGTT) on day 1, every 3 wk. The treatment was well tolerated, and no immune-related adverse effects were observed based on tests for hematotoxicity and multiorgan function. After three cycles of treatment, abdominal CT scan on June 2, 2021 showed a significant reduction in the antral mass and perigastric lymph nodes. The efficacy of therapy was defined as partial response (PR) (Figure 1E–H). Laparoscopic comprehensive exploration and peritoneal lavage showed no metastasis and negative tumor cytology. Finally, gastric cancer D2 surgery was performed (June 2021), and the patient recovered and was discharged smoothly.
This patient benefited from immunosuppressive therapy. From March to April 2021, she received two cycles of SOX chemotherapy in addition to PD-1 inhibitor (camrelizumab) (200 mg IVGTT on day 1, every 3 wk). The treatment was well tolerated, and no immune-related adverse effects were observed based on tests for hematotoxicity and multiorgan function. After two cycles of treatment, the patient underwent abdominal CT on April 14, 2021, which showed a decrease in the antral mass and lymph nodes around the stomach. The efficacy of treatment was defined as PR (Figure 4E–H). Laparoscopic comprehensive exploration and peritoneal lavage showed no metastasis and negative tumor cytology. Finally, gastric cancer D2 surgery was performed (May 2021), and the patient recovered and was discharged smoothly.
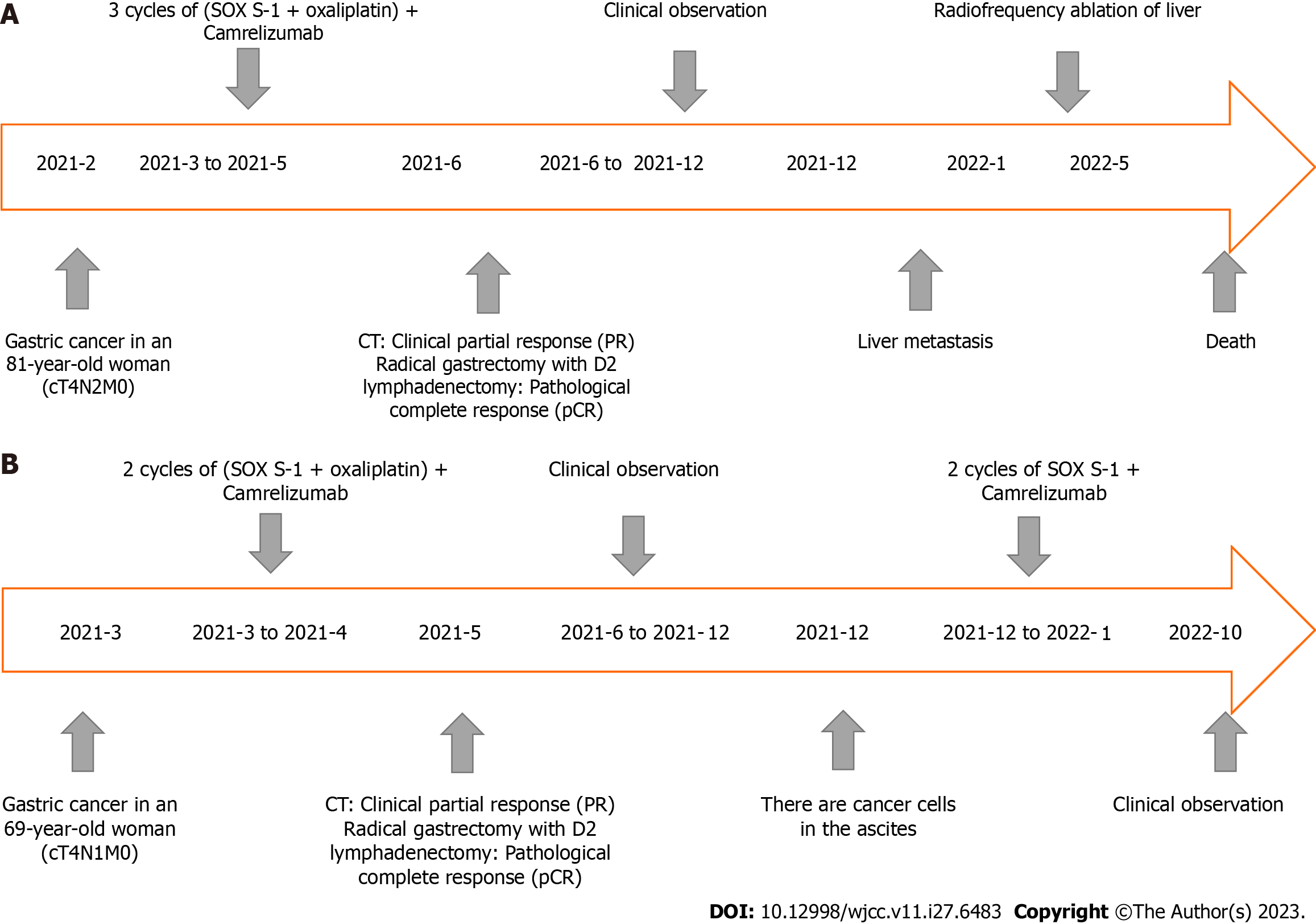
After the D2 operation for gastric cancer, postoperative pathological examination showed the pathological stage and ypCR of ypT0N0M0 (Figure 2B). Regular follow-up was required after postoperative communication with the patient. In December 2021, liver ultrasound examination showed that two lesions in the right anterior lobe of the liver were considered metastatic, with liver biopsy indicating adenocarcinoma (Figure 2C). Radiofrequency therapy for liver tumors was performed on January 5, 2022. Because the patient refused chemotherapy after the operation, she was followed up regularly and died on May 2, 2022. The entire diagnosis and treatment schedule of the patient is shown in Figure 7A.
After the D2 operation for gastric cancer, postoperative pathological examination showed the pathological stage and ypCR of ypT0N0M0 (Figure 5B). Because the patient insisted on continuing postoperative treatment and could not tolerate therapy after one cycle with camrelizumab 200 mg IVGTT, she requested regular follow-up. In December 2021, the patient had apparent abdominal distension caused by ascities. Tumor cell examination showed dispersed nuclear heterogeneous cells in the ascites (Figure 5C). The patient was treated with a combination of PD-1 inhibitor and S-1 for two cycles because of intraperitoneal metastasis. Because a gastrointestinal reaction occurred again, the patient did not tolerate the treatment and was given regular follow-up observations as well as nutritional support. The last follow-up time was October 2022. The entire diagnosis and treatment schedule of the patient is shown in Figure 7B.
Gastric cancer constitutes a significant global health challenge, representing a leading cause of morbidity and mortality in the realm of gastrointestinal malignancies. As such, the standard treatment strategy for patients with a clinical assessment of T ≥3, or advanced gastric cancer accompanied by lymph node metastasis, entails neoadjuvant therapy, surgery, and postoperative adjuvant therapy. However, the ypCR rate following neoadjuvant chemotherapy for advanced gastric cancer remains low and lacks a universally accepted treatment protocol[1]. Increasingly, therapeutic combinations involving checkpoint inhibitors and other antitumor agents are being proposed for various types of cancers[2-4]. Of note, recent research within the field of gastric cancer has demonstrated a growing focus on immunotherapy, with several studies underscoring the efficacy of immune checkpoint inhibitors targeting PD-1/PD-ligand 1 in patients with advanced gastric cancer[5-8]. Consequently, combining a PD-1 inhibitor with chemotherapy has demonstrated considerable benefits in terms of overall survival, progression-free survival, and tolerable safety profiles in comparison to chemotherapy alone[9]. Microsatellite instability high (MSI-H)/dMMR is a recognized marker for forecasting the effectiveness of immunotherapy in gastric cancer[10]. Additionally, the National Comprehensive Cancer Network clinical practice guidelines for gastric cancer advocate the use of PD-1 inhibitors for MSI-H/dMMR gastric cancer, which demonstrates progression post-chemotherapy[11]. Literature reveals a ypCR rate of 6% to 20% for neoadjuvant chemotherapy[12-14]. However, studies focusing on the ypCR status after neoadjuvant immunotherapy in gastric cancer are sparse. The ongoing GERCOR NEONIPIGA II study is a promising development in this area and its forthcoming results are eagerly awaited[15]. Although there is little evidence for adjuvant chemotherapy in patients with ypCR after neoadjuvant therapy[16], a retrospective analysis involving 2676 patients with advanced gastric or gastroesophageal junction adenocarcinoma suggests that ypCR does not necessarily guarantee a cure. Surprisingly, ypCR patients had a high recurrence rate of 23%, including 36% developing brain metastases, compared to non-ypCR patients with a 4% incidence of brain metastases[17]. This raises questions about the effectiveness of adjuvant chemotherapy and whether there is undiscovered biological information behind ypCR. Therefore, more studies are expected to compare the choices of treatment mode after ypCR.
There is a growing body of evidence suggesting that the augmented antitumor effect resulting from the synergistic action of chemotherapy and immunotherapy leads to a commendable remission rate and survival benefit. Nevertheless, the rational and efficient amalgamation of chemotherapy and immunotherapy still presents a myriad of challenges, such as identifying the patient cohort that would optimally benefit from immunotherapy. Achieving ypCR is an excellent short-term treatment response, but it does not necessarily predict long-term survival. The potential for a less favorable prognosis underpins the necessity for rigorous postoperative surveillance and a collaborative effort across multiple disciplines, including medicine, surgery, radiotherapy, pathology, and translational medicine. It is anticipated that advancements in translational medicine can yield a more profound understanding of molecular biological information, as well as provide a more nuanced interpretation of tumor behavior. This, in turn, could pave the way for tailored treatment strategies for such patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Rotondo JC, Italy; Shah OJ, India; Taheri S, Iran S-Editor: Liu JH L-Editor: Kerr C P-Editor: Zhao S
| 1. | Kaltenmeier C, Althans A, Mascara M, Nassour I, Khan S, Hoehn R, Zureikat A, Tohme S. Pathologic Complete Response Following Neoadjuvant Therapy for Gastric Adenocarcinoma: A National Cancer Database Analysis on Incidence, Predictors, and Outcomes. Am Surg. 2021;87:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Li X, Su X, Liu R, Pan Y, Fang J, Cao L, Feng C, Shang Q, Chen Y, Shao C, Shi Y. HDAC inhibition potentiates anti-tumor activity of macrophages and enhances anti-PD-L1-mediated tumor suppression. Oncogene. 2021;40:1836-1850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 3. | Mazziotta C, Lanzillotti C, Gafà R, Touzé A, Durand MA, Martini F, Rotondo JC. The Role of Histone Post-Translational Modifications in Merkel Cell Carcinoma. Front Oncol. 2022;12:832047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Borcoman E, Kamal M, Marret G, Dupain C, Castel-Ajgal Z, Le Tourneau C. HDAC Inhibition to Prime Immune Checkpoint Inhibitors. Cancers (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic C, Chung HC, Muro K, Goekkurt E, Mansoor W, McDermott RS, Shacham-Shmueli E, Chen X, Mayo C, Kang SP, Ohtsu A, Fuchs CS; KEYNOTE-061 investigators. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 991] [Article Influence: 141.6] [Reference Citation Analysis (0)] |
| 6. | Huo G, Liu W, Chen P. Efficacy of PD-1/PD-L1 inhibitors in gastric or gastro-oesophageal junction cancer based on clinical characteristics: a meta-analysis. BMC Cancer. 2023;23:143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Brar G, Shah MA. The role of pembrolizumab in the treatment of PD-L1 expressing gastric and gastroesophageal junction adenocarcinoma. Therap Adv Gastroenterol. 2019;12:1756284819869767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Voutsadakis IA. A Systematic Review and Meta-analysis of PD-1 and PD-L1 Inhibitors Monotherapy in Metastatic Gastric and Gastroesophageal Junction Adenocarcinoma. Euroasian J Hepatogastroenterol. 2020;10:56-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1836] [Article Influence: 459.0] [Reference Citation Analysis (1)] |
| 10. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 4913] [Article Influence: 614.1] [Reference Citation Analysis (0)] |
| 11. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 929] [Article Influence: 309.7] [Reference Citation Analysis (0)] |
| 12. | Thuss-Patience PC, Hofheinz RD, Arnold D, Florschütz A, Daum S, Kretzschmar A, Mantovani-Löffler L, Bichev D, Breithaupt K, Kneba M, Schumacher G, Glanemann M, Schlattmann P, Reichardt P, Gahn B. Perioperative chemotherapy with docetaxel, cisplatin and capecitabine (DCX) in gastro-oesophageal adenocarcinoma: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO){dagger}. Ann Oncol. 2012;23:2827-2834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Schulz C, Kullmann F, Kunzmann V, Fuchs M, Geissler M, Vehling-Kaiser U, Stauder H, Wein A, Al-Batran SE, Kubin T, Schäfer C, Stintzing S, Giessen C, Modest DP, Ridwelski K, Heinemann V. NeoFLOT: Multicenter phase II study of perioperative chemotherapy in resectable adenocarcinoma of the gastroesophageal junction or gastric adenocarcinoma-Very good response predominantly in patients with intestinal type tumors. Int J Cancer. 2015;137:678-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg H, Probst S, Koenigsmann M, Egger M, Prasnikar N, Caca K, Trojan J, Martens UM, Block A, Fischbach W, Mahlberg R, Clemens M, Illerhaus G, Zirlik K, Behringer DM, Schmiegel W, Pohl M, Heike M, Ronellenfitsch U, Schuler M, Bechstein WO, Königsrainer A, Gaiser T, Schirmacher P, Hozaeel W, Reichart A, Goetze TO, Sievert M, Jäger E, Mönig S, Tannapfel A. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 506] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 15. | Cohen R, Pudlarz T, Garcia-Larnicol ML, Vernerey D, Dray X, Clavel L, Jary M, Piessen G, Zaanan A, Aparicio T, Louvet C, Tournigand C, Chibaudel B, Tougeron D, Guimbaud R, Benouna J, Adenis A, Sokol H, Borg C, Duval A, Svrcek M, André T. [Localized MSI/dMMR gastric cancer patients, perioperative immunotherapy instead of chemotherapy: The GERCOR NEONIPIGA phase II study is opened to recruitment]. Bull Cancer. 2020;107:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Wang T, Wang N, Zhou H, Zhou A, Jin J, Chen Y, Zhao D. Long-term survival results of patients with locally advanced gastric cancer and pathological complete response after neoadjuvant chemotherapy and resection. Transl Cancer Res. 2020;9:529-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Fields RC, Strong VE, Gönen M, Goodman KA, Rizk NP, Kelsen DP, Ilson DH, Tang LH, Brennan MF, Coit DG, Shah MA. Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastrooesophageal adenocarcinoma. Br J Cancer. 2011;104:1840-1847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |