Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6280
Peer-review started: June 7, 2023
First decision: August 4, 2023
Revised: August 8, 2023
Accepted: August 17, 2023
Article in press: August 17, 2023
Published online: September 16, 2023
Processing time: 92 Days and 20.8 Hours
Coronavirus disease 2019 (COVID-19)-associated invasive pulmonary aspergillosis presents a diagnostic challenge due to its non-specific clinical/ imaging features, as well as the fact that the proposed clinically diagnostic algorithms do not necessarily apply to COVID-19 patients. In addition, Fusarium spp. is a rare cause of opportunistic life-threatening fungal infections. Disseminated Fusarium infection in an immunocompromised host is intractable, with a high likelihood of resulting mortality. To our knowledge, this is the first case of secondary pulmonary infection by Fusarium solani (F. solani) and Aspergillus niger (A. niger) during systemic steroid treatment for COVID-19.
A 62-year-old male was transported to our hospital by ambulance with a complaint of fever and dyspnea. We established a diagnosis of pneumococcal pneumonia, complicated with COVID-19 and septic shock, together with acute renal failure. He was admitted to the intensive care unit, to be treated with piperacillin/tazobactam, vancomycin, and 6.6 mg per day of dexamethasone sodium phosphate, along with noradrenaline as a vasopressor, ventilator management, and continuous hemodiafiltration. His condition improved, and we finished the vasopressor on the fifth hospital day. We administered dexamethasone for ten days, and finished the course of treatment. On the eleventh day, patient respiratory deterioration was observed, and a computed tomography scan showed an exacerbation of bilateral ground-glass-opacity-like consolidation, together with newly appeared cavitary lesions in the lung. we changed antibiotics to meropenem plus vancomycin. In addition, a fungal infection was considered as a possibility based on microscopic findings of sputum, and we began coadministration of voriconazole. However, the pneumonia worsened, and the patient died on the seventeenth day of illness. Later, F. solani and A. niger were identified from sputum collected on the twelfth day. It was believed that he developed a cell-mediated immune deficiency during COVID-19 treatment, which led to the complication of pneumonia caused by the above-mentioned fungi, contributing to his death.
Because early initiation of intense antifungal therapy offers the best chance for survival in pulmonary fusariosis, computed tomography scans and appropriate microbiologic investigations should be obtained for severely immunocompromised patients.
Core Tip: Coronavirus disease 2019 (COVID-19)-associated invasive pulmonary aspergillosis presents a diagnostic challenge due to its non-specific clinical/imaging features, as well as the fact that the proposed clinically diagnostic algorithms do not necessarily apply to COVID-19 patients. In addition, disseminated Fusarium infection in an immunocompromised host is intractable, with a high likelihood of resulting mortality. To our knowledge, we experienced the first case of secondary pulmonary infection by Fusarium solani and Aspergillus niger during systemic steroid treatment for COVID-19. A cell-mediated immunodeficiency during COVID-19 treatment with a systemic steroid led to this opportunistic infection.
- Citation: Usuda D, Kato M, Sugawara Y, Shimizu R, Inami T, Tsuge S, Sakurai R, Kawai K, Matsubara S, Tanaka R, Suzuki M, Shimozawa S, Hotchi Y, Osugi I, Katou R, Ito S, Mishima K, Kondo A, Mizuno K, Takami H, Komatsu T, Oba J, Nomura T, Sugita M. Secondary pulmonary infection by Fusarium solani and Aspergillus niger during systemic steroid treatment for COVID-19: A case report. World J Clin Cases 2023; 11(26): 6280-6288
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6280.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6280
Invasive fungal infections occurring in coronavirus disease 2019 (COVID-19) patients have drawn significant attention of late[1]. Patients suffering from severe forms of COVID-19, including patients treated in the intensive care unit (ICU) with prolonged use of steroids, represent a population especially vulnerable to secondary bacterial and fungal infections[1]. Particularly in immunocompromised patients, disseminated systemic mycosis is a life-threatening condition[1]. Invasive fungal infections (IFIs) can cause complications during the clinical course of COVID-19, and are, particularly in critically ill ICU patients, associated with significantly increased mortality[2]. Often, COVID-19 patients are characterized by the presence of known candidemia risk factors, including broad-spectrum antibiotics, in-dwelling vascular catheters, and mechanical ventilation[2]. Secondary IFIs, most frequently diagnosed in patients who are severely immunocompromised, indicate that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is able to stun the host’s immune system; these secondary IFIs include Pneumocystis jirovecii (P. jirovecii) pneumonia, cryptococcosis, histoplasmosis, coccidioides infections, pulmonary infections due to Fusarium spp., and pulmonary infections due to Scedosporium[2].
Opportunistic fungal infections occur primarily in patients who have chemotherapy-induced neutropenia, acquired immunodeficiency syndrome, or immunosuppression after transplantation of a solid organ or bone marrow[3]. In this past decade, these infections have been found to be significant causes of both morbidity and mortality in immunocompromised patients[3,4]. The most frequent IFI presentation in patients with hematologic malignancies, and in hematopoietic stem cell transplant recipients, is fungal pneumonia[5]. The most common causes include Aspergillus, Candida, Fusarium, and Mucor species[5]. Of these, non-Aspergillus molds (including Mucorales, Fusarium, Scedosporium,
A 62-year-old Japanese male was transported to our hospital by ambulance with a complaint of fever and dyspnea.
One day prior, he had developed fever and dyspnea, and symptoms persisted.
His medical history included untreated hypertension.
He smoked 80 cigarettes per day, with a 20-year history of smoking, and drank 1750 mL of beer per day, with a 40-year history of alcohol consumption. He did not undergo regular medical check-ups. He was engaged in transport work, and had no known allergies. He was single and lived alone. He did not require any assistance for everyday life activities. Additionally, the patient had no family history of hereditary diseases or malignant diseases.
The patient was 175 cm tall and weighed 70 kg (body mass index: 22.9). At the emergency department (ED), his vital signs were abnormal: His blood pressure was 190/90 mmHg, his heart rate was 150 regular beats/min, his body temperature was 39.5°C, his oxygen saturation was 77% under 10 L/min oxygen on a reservoir face mask, his respiratory rate was 40/min, and his Glasgow Coma Scale score was 15 points (E4V5M6). Upon physical examination, fine crackling was heard bilaterally throughout his chest. Nothing else abnormal was detected upon physical examination, including skin and neurological findings.
A routine laboratory examination, taken upon arrival at the ED, revealed increased values for aspartate aminotransferase, lactate dehydrogenase, creatine kinase, blood urea nitrogen, creatinine, uric acid, C-reactive protein, procalcitonin, prothrombin time-international normalized ratio, activated partial thromboplastin time, fibrin/fibrinogen degradation products, and d-dimer, and deceased values for white blood cells, platelets, albumin, sodium, chloride, and blood sugar. On the other hand, other values, including complete blood count and biochemistry, were normal (Table 1). A urine qualitative test revealed positive results for occult blood and protein. A rapid urinary antigen detection kit, BinaxNOW™ Streptococcus pneumoniae (S. pneumoniae) (Abbott, United States), tested positive. A blood gas analysis taken from an artery, under 10 L/min oxygen on a reservoir face mask, revealed increased values for anion gap and lactate, and decreased values for potential of hydrogen, partial pressure of arterial oxygen and carbon dioxide, bicarbonate ions, base excess, and arterial oxygen saturation (Table 2). An ID NOW™ COVID-19 assay (Abbott, United States), an isothermal nucleic acid amplification near-patient test that is marketed as providing a qualitative result (positive, negative, or invalid) in 15 min, tested positive.
| Parameter (unit) | Measured value | Normal value |
| White blood cells (103/µL) | 1.5 | 3.9–9.7 |
| Neu (%) | 56 | 37–72 |
| Lym (%) | 32 | 25–48 |
| Mon (%) | 12 | 2–12 |
| Eos (%) | 0 | 1–9 |
| Bas (%) | 0 | 0–2 |
| Hemoglobin (g/dL) | 15.4 | 13.4-17.1 |
| Platelets (103/µL) | 103 | 153–346 |
| Aspartate transaminase (IU/L) | 209 | 5–37 |
| Alanine aminotransferase (IU/L) | 21 | 6–43 |
| Lactic acid dehydrogenase (U/L) | 614 | 124–222 |
| Alkaline phosphatase (U/L) | 69 | 38–113 |
| Gamma-glutamyl transpeptidase (IU/L) | 54 | 0–75 |
| Total bilirubin (mg/dL) | 0.5 | 0.4–1.2 |
| Total protein (g/dL) | 6.5 | 6.5–8.5 |
| Albumin (g/dL) | 3.3 | 3.8–5.2 |
| Creatine kinase (U/L) | 2906 | 57–240 |
| Blood urea nitrogen (mg/dL) | 63 | 9–21 |
| Creatinine (mg/dL) | 4.2 | 0.6–1 |
| Amylase (IU/L) | 198 | 43–124 |
| Uric acid (mg/dL) | 7.7 | 3.5-6.9 |
| Sodium (mEq/L) | 130 | 135–145 |
| Potassium (mEq/L) | 5 | 3.5–5 |
| Chloride (mEq/L) | 89 | 96–107 |
| C-reactive protein (mg/dL) | 39.3 | 0–0.29 |
| Procalcitonin (ng/mL) | 160.4 | 0-0.5 |
| Plasma glucose (mg/dL) | 48 | 65–109 |
| Glycated hemoglobin (NGSP) (%) | 5.1 | 4.6–6.2 |
| Activated partial thromboplastin time (seconds) | 42.6 | 23–36 |
| Prothrombin time (International normalized ratio) | 1.12 | 0.85–1.15 |
| Fibrinogen and fibrin degradation products (μg/mL) | 14.7 | 0–10 |
| D-dimer (μg/mL) | 3.3 | 0–1 |
| Parameter (unit) | Measured value |
| pH | 7.216 |
| Partial pressure of arterial carbon dioxide (mmHg) | 31.3 |
| partial pressure of arterial oxygen (mmHg) | 51.7 |
| Bicarbonate ion (mmol/L) | 12.7 |
| Base excess (mmol/L) | -13.9 |
| Arterial oxygen saturation (%) | 76.7 |
| Anion gap (mmol/L) | 20 |
| Lactate (mmol/L) | 8.2 |
| Fraction of inspiratory oxygen (%) | > 90 |
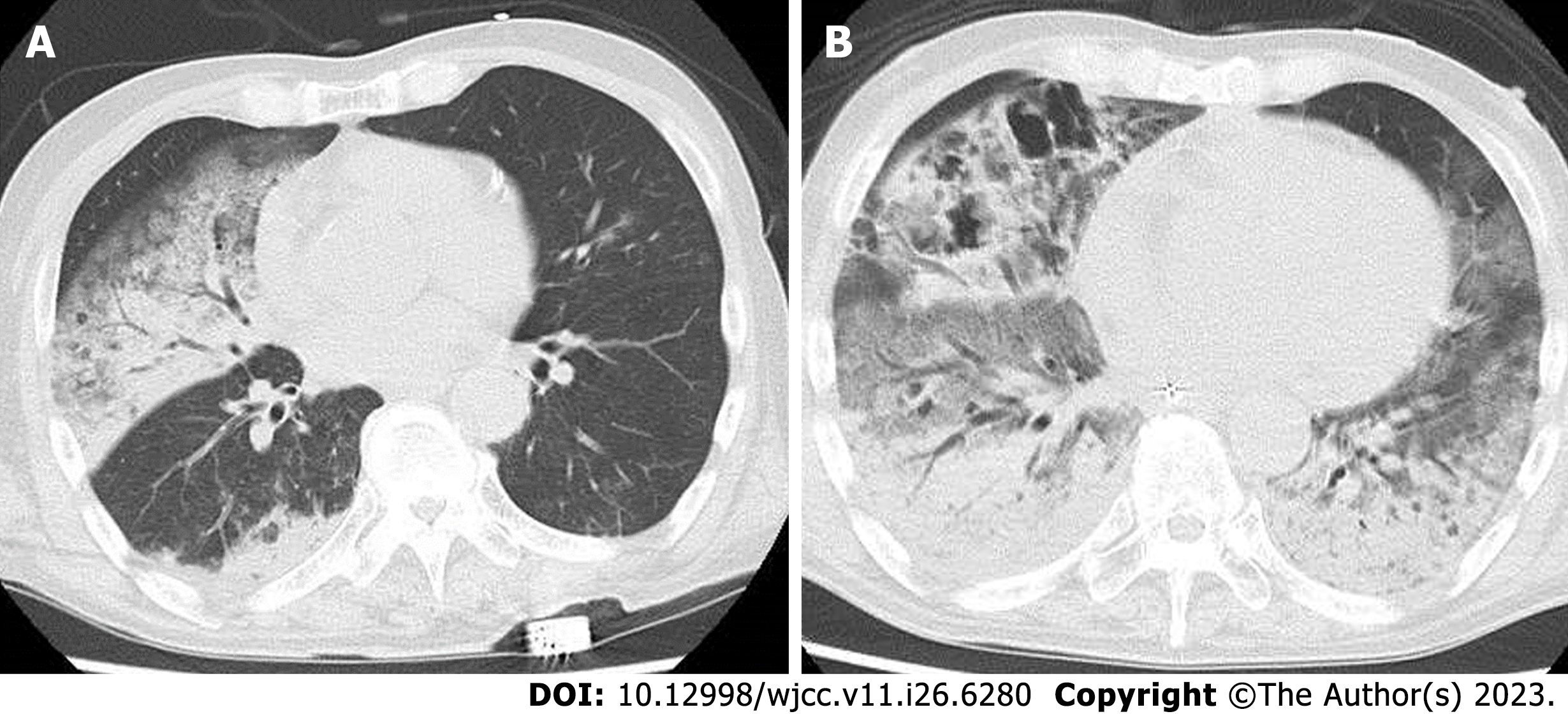
An electrocardiogram revealed sinus tachycardia. A computed tomography (CT) scan revealed bilateral ground grass opacity in the right middle and lower lung field, and the left lower lung field (Figure 1A).
At this point, we established a diagnosis of pneumococcal pneumonia, complicated with COVID-19 and septic shock, together with acute renal failure and rhabdomyolysis.
The patient was suspected to be in septic shock. He was admitted to the ICU, to be treated with piperacillin/tazobactam, vancomycin, and 6.6 mg per day of dexamethasone sodium phosphate, along with noradrenaline as a vasopressor, ventilator management, and continuous hemodiafiltration.
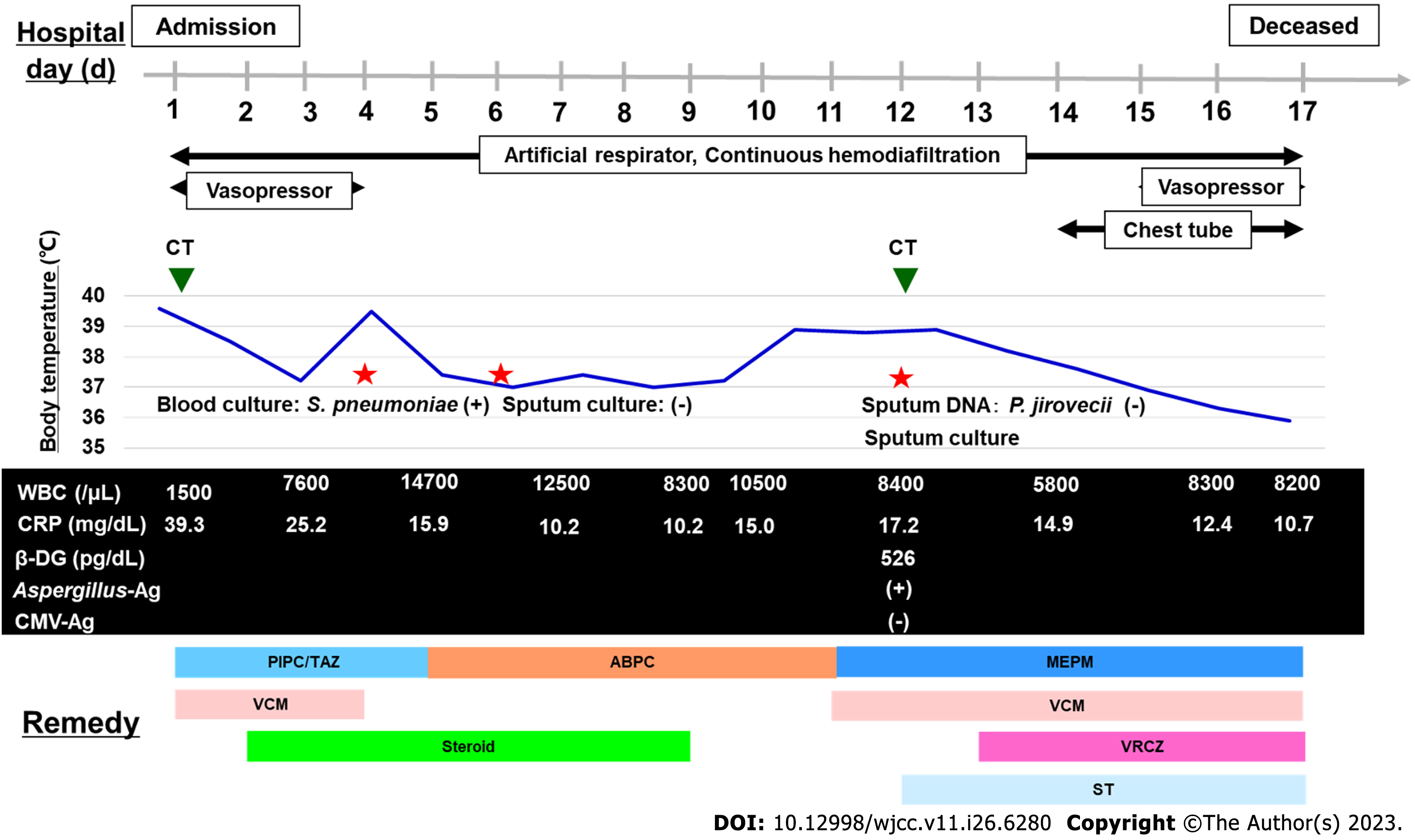
On the fourth day, blood cultures taken on admission revealed penicillin-susceptible S. pneumoniae, and a diagnosis was made of invasive pneumococcal disease (IPD). His condition improved, and we finished the vasopressor and changed the antibiotics to ampicillin on the fifth hospital day. We administered dexamethasone sodium phosphate for ten days, and finished the course of treatment. However, on the eleventh day, patient respiratory deterioration was observed, and a CT scan taken on the twelfth day showed an exacerbation of bilateral ground-glass-opacity-like consolidation, together with newly appeared cavitary lesions in the right lung (Figure 1B). A sputum culture was collected on the twelfth day, and we changed antibiotics to meropenem plus vancomycin. In the sputum culture, black and white colonies were observed on blood agar/chocolate agar (Figure 2A). In addition, microscopic findings with lactophenol cotton blue stain (200 × magnification) revealed growth of the following: multicellular, banana-like macroconidium, and clavate monocellular or bicellular small-sized conidiospores (Figure 2B), and smooth colored conidiophores and conidia, among which the conidiophores were protrusions from a septate and hyphae, with conidial heads appearing radially and splitting into columns (biseriate) (Figure 2C). Based on these findings, a fungal infection was considered as a possibility, and we began coadministration of voriconazole.
However, the pneumonia worsened on both sides, and the patient died on the seventeenth day of illness. Later, F. solani and A. niger were identified from sputum collected on the twelfth day. On the other hand, a blood culture and sputum deoxyribonucleic acid of P. jirovecii on the twelfth day were negative. Blood examination on the same day revealed that cytomegalovirus antigenemia test was negative and Aspergillus galactomannan enzyme-linked immunosorbent assay was positive. It was believed that he developed a cell-mediated immune deficiency during COVID-19 treatment, which led to the complication of pneumonia caused by the above-mentioned fungi, contributing to his death. The clinical course of the patient is shown in Figure 3.
We present the first case of secondary pulmonary infection by F. solani and A. niger during systemic steroid treatment for COVID-19. Consequently, there is value in reporting this event.
SARS-CoV-2 infections affect different people differently[7]. Although the majority of individuals will recover after a mild to moderate disease, some go on to develop severe COVID-19, which can lead to serious, life-threatening complications such as acute respiratory failure that can necessitate hospitalization and intensive critical care[7,8]. The SARS-CoV-2 virus can cause COVID-19, a mild to moderate disease in 80% of laboratory-confirmed cases, which may be community-managed[9]. In at-risk populations such as the elderly, there is a considerable age-dependent mortality, but among young, healthy individuals, mortality remains less than 0.5%[9]. The most common etiological agents of secondary infections in COVID-19 patients are Gram-positive and Gram-negative bacteria; however, a number of molds and yeasts have been described as being deadly secondary pathogens, as well[2]. Due to the serious damage to the lung parenchyma, there is reason to fear opportunistic infections[8]. Corticosteroid treatment brings both advantages and disadvantages, and methodological limitations in the available evidence mean that corticosteroids remain a controversial treatment[10]. We acknowledge the potential risks of treating COVID-19 pneumonia using high doses of corticosteroids, which include secondary infections, prolonged virus shedding, and even long-term complications[10]. As the medical evidence, COVID-19 patients have inadequate immunity and there is a possibility of reduced virus clearance after treating dexamethasone, the patient can be more immune-compromised if dexamethasone is used[11]. Consequently, expert consensus is to eschew liberal use of corticosteroids, with only prudent use of low to moderate doses of corticosteroids for short courses recommended for critically ill COVID-19 pneumonia patients[10].
Despite the prevalence of COVID-19-associated pulmonary mold infections (CAPMIs), the lack of any appropriate antifungal treatment highlights the fact that this complication affecting mechanically ventilated COVID-19 patients admitted to ICUs remains neglected[12]. COVID-19-associated invasive pulmonary aspergillosis (CAPA) presents a diagnostic challenge due not only to its non-specific clinical imaging features, but also to the fact that the clinical diagnostic algorithms that have been proposed do not necessarily apply to COVID-19 patients[2]. In addition, Fusarium spp. is known to be a rare cause of life-threatening opportunistic fungal infections[13].
To date, there has been only one report of secondary pulmonary infection by Fusarium spp. during systemic steroid treatment for COVID-19, making this case exceedingly rare. We will discuss Fusarium spp. later, together with a review of the literature[14]. It should also be noted that we were unable to find any articles mentioning S. pneumoniae infections including IPD and Fusarium spp. or Aspergillus spp. It is therefore reasonable to consider that IPD would not lead to secondary pulmonary infection by F. solani and A. niger.
Fusarium is a mold (specifically a hyaline septate filamentous fungus) widely distributed in soil and water; it is the cause of various diseases in plants, as well as, occasionally, in animals[4,15]. Fusarium is a common genus found in the human mycobiota[16]. Fusarium species are frequent onychomycosis and fungal keratitis agents, and occasionally invasive disease agents[17]. They are the causes of a broad spectrum of infections in humans, the severity of which is generally dependent upon the host’s immune status, with the most severe and invasive infections occurring in immunocompromised individuals[15]. Furthermore, all pulmonary fusariosis patients have had underlying hematologic malignancies[18]. The most prevalent manifestations of fusarium infections in humans include skin infections, sinusitis, and pneumonia[15]. Severely immunodeficient patients run the risk of the disease becoming invasive and disseminated, leading to fungemia[15]. The clinical spectrum of pulmonary fusariosis includes allergic disease (allergic bronchopulmonary fusariosis), hypersensitivity pneumonitis, preexisting cavity colonization, and pneumonia[17]. Fusarial pneumonia has been known to occur almost without exception in patients who are severely immunocompromised, particularly acute leukemia patients and allogeneic cell transplant recipients[17]. In these cases, invasive fusariosis is generally disseminated, with pneumonia occurring in almost half of these patients[17]. In this case, fusarial pneumonia occurred during systemic steroid treatment, and the patient’s status was suspected to be immunocompromised. Therefore, the etiology of fusarial pneumonia in this case is believed to be compatible with previous reports.
Opportunistic fungal pneumonia’s radiologic features can vary significantly, and are frequently nonspecific[3]. In addition, pulmonary fusariosis has previously been described as being indistinguishable in imaging from other invasive mold diseases, such as aspergillosis, alveolar infiltrates, nodules with or without halo sign, ground-glass infiltrates, and pleural effusions[17,19]. Aspects that differ from aspergillosis include positive blood cultures and the frequent occurrence of disseminated nodular and papular skin lesions[17]. Radiological findings remain unable to discriminate between different types of mold infections; however, if a chest CT scan shows pulmonary infiltrates with the hypodense sign, but without the occluded-vessel or halo signs, then it is reasonable to suspect invasive fusariosis[19]. On the other hand, some articles have mentioned that the radiographic manifestations of pulmonary fusariosis resemble those of an angioinvasive mold[18]. The following characteristics have also been reported regarding pulmonary fusariosis imaging: Masses and/or nodules are the most common findings on CT scans, seen in 82% of patients (compared to only 45% for chest radiography); no halo sign is seen; chest radiographs show nonspecific findings for 30% of patients, and findings were normal at time of presentation in 25% of patients[18].
Culture samples collected from bronchoalveolar lavage (BAL) and serum revealed that Aspergillus (76%) and Fusarium (21%) collectively constituted 97% of the molds isolated from COVID-19 patients[12]. Regarding mycological findings in CAPMI patients, 38% met our definition of probable CAPMI[12]. None had proven CAPMI[7]. 28% had a positive BAL culture[12]. CAPA was found in 76% of patients, and among these, Aspergillus flavus (64%) was the most commonly seen agent, followed by Aspergillus fumigatus and Aspergillus japonicus (14% each), then A. niger (9%)[12]. In patients with COVID-19-associated pulmonary fusariosis, Fusarium incarnatum (50%) was the most commonly seen, followed by Fusarium fujikuroi (17%) and Fusarium equiseti and F. solani (17% each)[12]. From a microbiology perspective, the two fungi we identified, F. solani and A. niger, were not common ones.
Diagnosis requires knowledge of these infections’ various modes of presentation, radiologic manifestations, and epidemiology[3]. Sputum cultures are generally considered unreliable as diagnostics, as many of these organisms are able to colonize the upper airway[3]. Rather, definitive diagnoses require culturing of fungus taken from infected tissue, or demonstrating the organism upon microscopic examination[3]. In this case, it is believed that the patient developed a cell-mediated immune deficiency during COVID-19 treatment, which led to the complication of pneumonia caused by the above-mentioned fungi, contributing to his death. On the other hand, other appropriate treatments are available for COVID-19 or IPD. In addition, no other microorganisms were identified in the sputum or blood culture. Therefore, we failed to consider that these could have contributed to the patient’s worsening respiratory status on the eleventh hospital day.
Regarding treatment, hyaline septated filamentous fungi, such as Fusarium species, have increasingly been reported as being the cause of invasive mycoses refractory to amphotericin B therapy[20]. Fusarium spp. has been found to have an extremely high resistance profile, with very few effective antifungal agents: The drug of choice for invasive fusariosis treatment is either voriconazole or liposomal amphotericin B[4,13,17,20,21]. Due to species-dependent variability in in vitro susceptibility profiles of Fusarium spp., as well as the immunocompromised hosts’ poor fusariosis prognoses, particularly in hosts with disseminated diseases, some recommend combination antifungal regimens initially in such settings, with the goal of ensuring the activity of at least one agent[22,23]. A handful of in vitro studies have suggested that certain combinations of antifungal agents (e.g. voriconazole plus terbinafine, or amphotericin B plus voriconazole) could provide synergistic effects, though possible antagonism remains a potential concern, especially for the latter combination[24,25]. Compared to immunocompetent patients, immunocompromised patients may run a greater risk of in-hospital complications and mortality in the event of COVID-19 hospitalization[26,27]. The adjusted odds ratios (95% confidence interval) for death among patients receiving systemic steroids, compared to immunocompetent patients, were 2.16 (1.80–2.61)[27]. Fusariosis outcomes are generally poor, and ordinarily depend on the host’s immune status recovery, particularly for neutropenia[17]. In immunocompromised hosts, disseminated Fusarium infections are intractable, and result in high mortality[28]. In one study of pulmonary fusariosis, 65% of patients died within one month of the diagnosis[18]. Early initiation of intense antifungal therapy offers the greatest chance for patient survival of pulmonary fusariosis, so for severely immunocompromised patients, early CT scans and the appropriate microbiologic investigations should be obtained[18]. From the perspective of treatment and prognosis, we should have initiated antifungal combination therapy sooner, based on the smear findings of the sputum culture; had we done so, we could have saved this patient.
This case study has a limitation: It reviews only a single case report and case series of secondary pulmonary infection by F. solani and A. niger during systemic steroid treatment for COVID-19. Therefore, the actual situation and nature of the disease may differ from the results of the literature review, as a result of reporting bias. Additional studies are needed to further evaluate the impact of clinical presentation, laboratory, microbiology, imaging examinations, and treatment patterns, and the outcomes of these fungal infections. In addition, microbiological examinations do not have 100% sensitivity nor specificity, meaning that we cannot fully rule out the possibility of involvement of other organisms not identified through culturing, which may have caused the patient’s deteriorating condition on the eleventh hospital day.
In conclusion, this is, to our knowledge, the first case of secondary pulmonary infection by F. solani and A. niger during systemic steroid treatment for COVID-19. A cell-mediated immunodeficiency during COVID-19 treatment with systemic steroids can lead to opportunistic infections caused by various pathogens, including fungi, viruses, and acid-fast bacteria, which can become lethal in some cases. Therefore, clinicians should suspect pathogens based on microbiological, serological, and radiological examinations, and start appropriate treatment without delay, especially for opportunistic infections.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li JC, China; Naseef PP, India S-Editor: Lin C L-Editor: A P-Editor: Cai YX
| 1. | Manda D, Sen I, Thakral P, Das SS, Cb V, Malik D. Invasive Fungal Infection in COVID-19-Recovered Patient Detected on 18F-FDG-Labeled Leukocytes PET/CT Scan. Clin Nucl Med. 2022;47:e177-e179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Casalini G, Giacomelli A, Ridolfo A, Gervasoni C, Antinori S. Invasive Fungal Infections Complicating COVID-19: A Narrative Review. J Fungi (Basel). 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Connolly JE Jr, McAdams HP, Erasmus JJ, Rosado-de-Christenson ML. Opportunistic fungal pneumonia. J Thorac Imaging. 1999;14:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Walsh TJ, Groll AH. Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transpl Infect Dis. 1999;1:247-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 190] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Godoy MCB, Ferreira Dalla Pria HR, Truong MT, Shroff GS, Marom EM. Invasive Fungal Pneumonia in Immunocompromised Patients. Radiol Clin North Am. 2022;60:497-506. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Li Y, Wang H, Hou X, Huang JJ, Wang PC, Xu YC. Identification by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and Antifungal Susceptibility Testing of Non-Aspergillus Molds. Front Microbiol. 2020;11:922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Jain M, Brar AS, Rath S, Kelgaokar A, Behera HS. Fulminant fungal endogenous endophthalmitis following SARS-CoV-2 infection: A case report. Indian J Ophthalmol. 2022;70:1819-1821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Poignon C, Blaize M, Vezinet C, Lampros A, Monsel A, Fekkar A. Invasive pulmonary fusariosis in an immunocompetent critically ill patient with severe COVID-19. Clin Microbiol Infect. 2020;26:1582-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Mølhave M, Agergaard J, Wejse C. Clinical Management of COVID-19 Patients - An Update. Semin Nucl Med. 2022;52:4-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 424] [Cited by in RCA: 448] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 11. | Ghazanfari M, Arastehfar A, Davoodi L, Yazdani Charati J, Moazeni M, Abastabar M, Haghani I, Mirzakhani R, Mayahi S, Fang W, Liao W, Nguyen MH, Perlin DS, Hoenigl M, Pan W, Hedayati MT. Pervasive but Neglected: A Perspective on COVID-19-Associated Pulmonary Mold Infections Among Mechanically Ventilated COVID-19 Patients. Front Med (Lausanne). 2021;8:649675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Alshaya OA, Saleh RA, Alshehri SD. Voriconazole-Induced Hepatotoxicity Resolved after Switching to Amphotericin B in Fusarium dimerum Central Line-Associated Bloodstream Infection. Am J Case Rep. 2021;22:e932544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Fekkar A, Lampros A, Mayaux J, Poignon C, Demeret S, Constantin JM, Marcelin AG, Monsel A, Luyt CE, Blaize M. Occurrence of Invasive Pulmonary Fungal Infections in Patients with Severe COVID-19 Admitted to the ICU. Am J Respir Crit Care Med. 2021;203:307-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 14. | Calcaterra D, Karam K, Suzuki Y. Computed tomography findings in a patient with fungal aortitis: acute aortic syndrome secondary to fusariosis. Interact Cardiovasc Thorac Surg. 2013;17:171-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Shankar J. Food Habit Associated Mycobiota Composition and Their Impact on Human Health. Front Nutr. 2021;8:773577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Nucci F, Nouér SA, Capone D, Anaissie E, Nucci M. Fusariosis. Semin Respir Crit Care Med. 2015;36:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Marom EM, Holmes AM, Bruzzi JF, Truong MT, O'Sullivan PJ, Kontoyiannis DP. Imaging of pulmonary fusariosis in patients with hematologic malignancies. AJR Am J Roentgenol. 2008;190:1605-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Sassi C, Stanzani M, Lewis RE, Vianelli N, Tarsi A, Poerio A, Cavo M, Battista G. Radiologic findings of Fusarium pneumonia in neutropenic patients. Mycoses. 2017;60:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Fleming RV, Walsh TJ, Anaissie EJ. Emerging and less common fungal pathogens. Infect Dis Clin North Am. 2002;16:915-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Kleinschmidt-Demasters BK. Disseminated Fusarium infection with brain abscesses in a lung transplant recipient. Clin Neuropathol. 2009;28:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Ho DY, Lee JD, Rosso F, Montoya JG. Treating disseminated fusariosis: amphotericin B, voriconazole or both? Mycoses. 2007;50:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Vazquez JA. Clinical practice: combination antifungal therapy for mold infections: much ado about nothing? Clin Infect Dis. 2008;46:1889-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Córdoba S, Rodero L, Vivot W, Abrantes R, Davel G, Vitale RG. In vitro interactions of antifungal agents against clinical isolates of Fusarium spp. Int J Antimicrob Agents. 2008;31:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Ortoneda M, Capilla J, Javier Pastor F, Pujol I, Guarro J. In vitro interactions of licensed and novel antifungal drugs against Fusarium spp. Diagn Microbiol Infect Dis. 2004;48:69-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Turtle L, Thorpe M, Drake TM, Swets M, Palmieri C, Russell CD, Ho A, Aston S, Wootton DG, Richter A, de Silva TI, Hardwick HE, Leeming G, Law A, Openshaw PJM, Harrison EM; ISARIC4C investigators, Baillie JK, Semple MG, Docherty AB. Outcome of COVID-19 in hospitalised immunocompromised patients: An analysis of the WHO ISARIC CCP-UK prospective cohort study. PLoS Med. 2023;20:e1004086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 56] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 26. | Suárez-García I, Perales-Fraile I, González-García A, Muñoz-Blanco A, Manzano L, Fabregate M, Díez-Manglano J, Aizpuru EF, Fernández FA, García AG, Gómez-Huelgas R, Ramos-Rincón JM; SEMI-COVID-19 Network. In-hospital mortality among immunosuppressed patients with COVID-19: Analysis from a national cohort in Spain. PLoS One. 2021;16:e0255524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 27. | Okada H, Hamatani S, Kondo M, Imai T, Itoh S, Isobe K, Onishi S. Successful treatment of disseminated Fusarium infection in an infant with leukemia. Int J Hematol. 2000;72:494-498. [PubMed] |
| 28. | Akter F, Araf Y, Hosen MJ. Corticosteroids for COVID-19: worth it or not? Mol Biol Rep. 2022;49:567-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |