Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6268
Peer-review started: June 9, 2023
First decision: July 28, 2023
Revised: August 9, 2023
Accepted: August 23, 2023
Article in press: August 23, 2023
Published online: September 16, 2023
Processing time: 91 Days and 2.6 Hours
Bronchial Dieulafoy’s disease (BDD) is characterized by the erosion of an anomalous artery in the submucosa of the bronchus. The etiology of pediatric BDD is mainly congenital dysplasia of bronchus and pulmonary arteries, which is different from chronic inflammatory injury of the airway in adult patients. The internal thoracic artery, subclavian artery, and intercostal artery are known to be involved in the blood supply to the BDD lesion in children.
We report a case of BDD in a 4-year-old boy with recurrent hemoptysis for one year. Selective angiography showed a dilated right bronchial artery, and anastomosis of its branches with the right lower pulmonary vascular network. Bronchoscopy showed nodular protrusion of the bronchial mucosa with a local scar. Selective embolization of the bronchial artery was performed to stop bleeding. One month after the first intervention, the symptoms of hemoptysis recurred. A computed tomography angiogram (CTA) showed another tortuous and dilated feeding artery in the right lower lung, which was an abnormal ascending branch of the inferior phrenic artery (IPA). The results of angiography were consistent with the CTA findings. The IPA was found to be another main supplying artery, which was not considered during the first intervention. Finally, the IPA was also treated by microsphere embolization combined with coil interventional closure. During the one-year follow-up, the patient never experienced hemoptysis.
The supplying arteries of the bleeding lesion in children with BDD may originate from multiple different aortopulmonary collateral arteries, and the IPA should be considered to reduce missed diagnosis. CTA is a noninvasive radiological examination for the screening of suspected vessels, which shows a high coincidence with angiography, and can serve as the first choice for the diagnosis of BDD.
Core Tip: Bronchial Dieulafoy’s disease (BDD) in children is mostly caused by congenital dysplasia of bronchus and/or pulmonary arteries, which is different from chronic inflammatory injury of the airway in adult patients. We report a 4-year-old male BDD patient with repeated hemoptysis. Selective embolization of the responsible bronchial artery was performed to stop bleeding in the first time. One month after the interventional operation, however, the patient repeated the symptoms of hemoptysis. And the inferior phrenic artery (IPA) was found to be another supplying artery that caused hemoptysis in addition to the bronchial arteries. Finally, the artery was also treated by microsphere embolization with coil interventional closure. The responsible arteries of the bleeding lesion in children with BDD may originate from multiple different aortopulmonary collateral arteries, and the IPA should be considered to reduce missed diagnosis. Computed tomography angiogram is a noninvasive radiological examination for the screening of the responsible vessels, which shows a high coincidence with angiography, and can serve as the first choice for the diagnosis of BDD in suspected patients.
- Citation: Wang F, Tang J, Peng M, Huang PJ, Zhao LJ, Zhang YY, Wang T. Recurrent hemoptysis in pediatric bronchial Dieulafoy’s disease with inferior phrenic artery supply: A case report. World J Clin Cases 2023; 11(26): 6268-6273
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6268.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6268
Bronchial Dieulafoy’s disease (BDD) is a type of tortuous malformation of a submucosal artery of the bronchial wall, which may spontaneously rupture under the action of external factors, resulting in acute lumen hemorrhage with massive hemoptysis. To the best of our knowledge, there are more than 70 cases of BDD reported to date in the literature, of which only two have been reported in children (8 mo and 13 years old, respectively)[2]. The etiology and pathogenesis of BDD remain unclear, but may be related to congenital vascular dysplasia or chronic inflammatory injury of pulmonary vessels[1]. BDD is an important cause of pulmonary hemorrhage with massive hemoptysis. Most of the auxiliary examinations performed show normal results, and chest imaging does not show any specific findings, which may lead to misdiagnosis of cases. There is no consensus on the diagnosis and treatment of BDD. Selective embolization of the bronchial artery is considered to be an effective method to stop bleeding. However, 52.6% of patients with bronchial artery embolization required surgical intervention because of embolization failure or recurrent bleeding[3]. Collectively, all these reports indicate the need to optimize the diagnosis and treatment of BDD through further exploration.
A 4-year-old boy was admitted to the hospital with a history of “recurrent hemoptysis for one year and massive hemoptysis once”.
One year ago, the child suffered from recurrent hemoptysis symptoms, and was treated with anti-hemostasis and anti-infection therapies, but the condition continued to deteriorate. Massive hemoptysis occurred one hour before admission, with about 100 mL of blood loss.
The child suffered from recurrent coughing after birth, and was hospitalized for pneumonia with bronchiectasis 3-5 times every year. After hospitalization, the bronchoscopy performed during initial episodes of bronchiectasis showed chronic endobronchial inflammation.
No associated inherited metabolic disorders or familial clustering disease were identified. And there was no history of tuberculosis (TB) or any contact with TB patients.
The patient had shortness of breath, with positive three concave sign of nails and mild clubbing of the toes. The respiratory sounds of the right lower lung were weak, and no dry and wet rales were found.
Routine blood tests, biochemical function, electrolyte levels, and bleeding and coagulation related indicators were not abnormal.
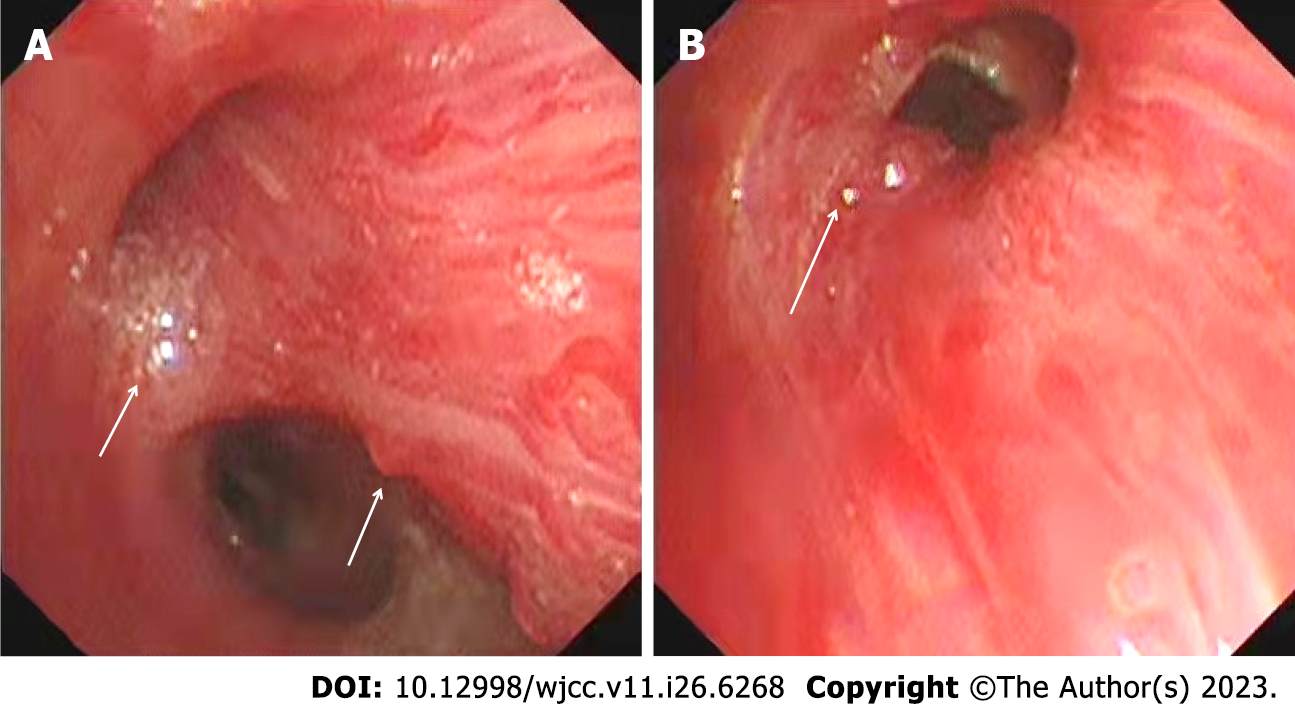
Fiberoptic bronchoscopy showed multiple nodular protrusions in the right middle and lower lobar bronchial mucosa, local scar formation, rough and pale surface, and easy bleeding of the mucosa (Figure 1A). Partial bronchial branch openings were deformed, with sputum bolt and old bleeding (Figure 1B). Computed tomography (CT) showed uneven thickening of the bronchial wall of the basal segment of the right lower lobe, which was partially protruding into the lumen. The right bronchial artery was tortuous and thickened, and branched into a descending vessel, which was obviously dilated at the distal end and anastomosed with the vascular network of the right lower pulmonary artery (Figure 2A). Selective angiography showed a dilated right bronchial artery, and anastomosis of its branches with the right lower pulmonary vascular network. The diameter at the beginning was 2.7 mm, and 2.6 mm at the entrance to the lung (Figure 2B).
The patient was clinically diagnosed as having BDD.
First, 500 μm polyvinyl alcohol (PVA) particles were injected through a 2.7F microcatheter, and then 3-3 coils were inserted through a 4F cobra catheter to embolize the vessels of each branch (Figure 2C). Selective embolization of the bronchial artery was performed to stop bleeding. However, one month after the first intervention, the symptoms of hemoptysis recurred.
A CT angiogram (CTA) showed another tortuous and dilated feeding artery in the right lower lung, which was an abnormal ascending branch of the inferior phrenic artery (IPA). The IPA originated from the abdominal aorta celiac trunk, which moved along the right upper diaphragm and anastomosed with the vascular network of right lower pulmonary artery (Figure 2D). The blood vessels responsible for the hemoptysis were suspected to be the branches of the right bronchial artery and IPA.
The abnormal branch of the IPA moved along the right upper diaphragm (Figure 2E). A 2.7F microcatheter was injected with 500 μm PVA particles, and a 3-3 coil was inserted through a 4F cobra catheter to embolize the abnormal branch vessels (Figure 2F). No residual shunt was detected after intervention.
After one-year follow-up, the growth and development of the patient were normal, and he had no episodes of recurrent hemoptysis (Figure 3).
BDD is very rare among children, and the youngest patient reported with BDD is eight months old. Normally, the disease is considered in heavy smokers when recurrent massive hemoptysis is present without any other abnormal findings, or in patients with chronic respiratory diseases. Congenital vascular dysplasia may be considered in pediatric patients with such findings. Smith et al[4] speculated that the disease was caused by the failure of the submucosal artery with a constant diameter to branch into capillaries. Stoopen et al[5] suggested that the variations in the right bronchial artery during embryonic development might cause an increased risk of abnormal right vessels.
Most of the abnormalities in arteries reported in BDD cases originated from branches of the right bronchial artery, which accounted for about two-third of all cases. Relative to adults, BDD in children is rare, which is mainly considered to be associated with congenital vascular malformations. In the present case, the pulmonary hemorrhagic lesion had two supplying arteries, the abnormal right bronchial artery branch and a branch from the IPA, which was involved in pulmonary hemorrhage. If the IPA is ignored, it may lead to treatment failure in patients with BDD. Importantly, for BDD related to congenital angiogenesis, it is necessary to consider the ectopic origin of the supplying vessels such as the IPA, which has not yet been reported.
CTA and angiography can clearly show the tortuous and dilated bronchial artery in BDD patients[6]. The blood vessels of BDD lesions traverse through the bronchial mucosa and could pass through the bronchial cartilage ring. The diameter of the diseased artery is significantly increased and remains constant, which is called “constant diameter artery”. The bronchial artery suddenly disappears at the point of entering the pulmonary segment, showing a “truncated” change. Part of the tortuous and dilated artery extends into the bronchial lumen, forming local protuberant lesions or small nodules covered with normal mucosa, and these signs could be easily detected by CTA and bronchoscopy. Bronchoscopy is an important method to detect BDD, where the typical microscopic lesions are manifested as nodular lesions protruding into the lumen. The surface mucosa of the lesions is normal and complete, which is usually misdiagnosed as a tumor or TB. If biopsy is performed for confirmation due to misdiagnosis as a tumor, it may result in massive hemorrhage with profuse and fatal hemoptysis. A retrospective study analyzed 73 BDD patients reported worldwide from 1995 to 2019. Among them, 19 underwent bronchoscopy, 17 suffered from bleeding, and six died[2]. Hence, CTA is recommended as a low-risk noninvasive method, which can be used as the preferred diagnostic method for suspected cases of BDD.
At present, there is no consensus or guidelines for the accurate diagnosis and treatment of BDD. The two reported pediatric BDD cases were characterized by recurrent hemoptysis. Among them, one female developed pulmonary hemorrhage at the age of eight months, and was diagnosed as having BDD at the age of five years. The lesion was located in the upper right bronchus. The pulmonary hemorrhage did not improve after two bronchial artery embolizations, and the right lung upper lobotomy was subsequently performed[7]. The other case was a 13-year-old boy with recurrent hemoptysis three months after bronchial artery embolization, who subsequently underwent lobotomy. Both patients had no symptoms of hemoptysis after surgery[8]. Selective bronchial artery embolization is considered to be an effective way to prevent bleeding and reduce the mortality rate. However, studies have found that 52.6% of patients with bronchial artery embolization require surgical intervention because of embolization failure or recurrent bleeding, which may be due to local tissue revascularization and neovascularization, as well as the proliferation and expansion of adjacent vascular system[3]. We believe that the vascular network is very rich in BDD, with many collateral branches. Microsphere embolization is recommended as the first choice for the surgical intervention of BDD. Consequently, selective occlusion of the main feeding arteries with coils can significantly reduce the risk of local rebleeding. A few patients with uncontrollable bleeding or failed arterial embolization may require further surgical treatment. In our case, the two supplying arteries responsible for hemoptysis were treated by microsphere embolization combined with coil interventional closure. After one-year follow-up, the patient had no recurrent hemoptysis, indicating that the combined intervention was effective.
The supplying arteries of the bleeding lesion in children with BDD may originate from multiple different aortopulmonary collateral arteries, and the IPA should be considered to reduce missed diagnosis. CTA is a noninvasive radiological examination for the screening of the suspected vessels, which shows a high coincidence with angiography, and can serve as the first choice for the diagnosis of BDD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Cardiovasology Group of the Chinese Medical Association, No. 8050570; Children Blood Pressure and Health Group of China Rehabilitation Medicine, No. 7970560; Hospital Building and Equipment Branch Committee (Women and Children) of China, No. 2022698.
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pavlovic M, Serbia; Pop TL, Romania S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Qian X, Du Q, Wei N, Wang M, Wang H, Tang Y. Bronchial Dieulafoy’s disease: a retrospective analysis of 73 cases. BMC Pulm Med. 2019;19:104. [PubMed] [DOI] [Full Text] |
| 2. | Viola P, Amat Villegas I, Dusmet M, Nicholson AG, Montero Fernandez MA. Dieulafoy’s disease of the airways: a comprehensive review of a rare entity. Histopathology. 2016;69:886-890. [PubMed] [DOI] [Full Text] |
| 3. | Sheth HS, Maldonado F, Lentz RJ. Two cases of Dieulafoy lesions of the bronchus with novel comorbid associations and endobronchial ablative management. Medicine (Baltimore). 2018;97:e9754. [PubMed] [DOI] [Full Text] |
| 4. | Smith B, Hart D, Alam N. Dieulafoy’s disease of the bronchus: a rare cause of massive hemoptysis. Respirol Case Rep. 2014;2:55-56. [PubMed] [DOI] [Full Text] |
| 5. | Stoopen E, Baquera-Heredia J, Cortes D, Green L. Dieulafoy’s disease of the bronchus in association with a paravertebral neurilemoma. Chest. 2001;119:292-294. [PubMed] [DOI] [Full Text] |
| 6. | Walker CM, Rosado-de-Christenson ML, Martínez-Jiménez S, Kunin JR, Wible BC. Bronchial arteries: anatomy, function, hypertrophy, and anomalies. Radiographics. 2015;35:32-49. [PubMed] [DOI] [Full Text] |
| 7. | Eyssartier E, Ang P, Bonnemaison E, Gibertini I, Diot P, Carpentier E, Chantepie A, Leclair MD, Brouard J, Boutard P, Deneuville E, Marie-Cardine A, Lardy H. Characteristics of endobronchial primitive tumors in children. Pediatr Pulmonol. 2014;49:E121-E125. [PubMed] [DOI] [Full Text] |
| 8. | Ganganah O, Guo S, Chiniah M, Sah SK, Wu J. Endobronchial ultrasound and bronchial artery embolization for Dieulafoy’s disease of the bronchus in a teenager: A case report. Respir Med Case Rep. 2015;16:20-23. [PubMed] [DOI] [Full Text] |