Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6262
Peer-review started: June 6, 2023
First decision: July 28, 2023
Revised: July 31, 2023
Accepted: August 15, 2023
Article in press: August 15, 2023
Published online: September 16, 2023
Processing time: 93 Days and 23.7 Hours
Holoprosencephaly (HPE) is a congenital malformation with various degrees of incomplete separation of the cerebral hemispheres due to differentiation disorders of the forebrain. Although HPE with diabetes insipidus due to associated pituitary dysfunction has been reported, HPE with the syndrome of inappropriate antidiuretic hormone secretion (SIADH) is very rare. Tolvaptan, a vasopressin V2 receptor antagonist, is effective in adults with SIADH. However, there is no report of its efficacy in infants with SIADH. The purpose of this report is to demonstrate that tolvaptan is effective for SIADH in infants and that administration of tolvaptan eliminates the need for restriction of water intake and sodium administration.
A 2414-g female infant was born at 38 wk by normal vaginal delivery. Facial anomalies and head magnetic resonance imaging indicated semilobar HPE. After birth, she had hyponatremia due to SIADH and was treated using water and sodium restriction. However, she developed an exaggerated response to the fluid restrictions, resulting in large fluctuations in serum sodium levels. Subsequent administration of tolvaptan improved the fluctuations in serum sodium levels without the need for adjustment of water or sodium administration. Serum sodium was maintained within the normal range after discontinuation of tolvaptan at 80 d of life. There were no side effects, such as hypernatremia or liver dysfunction, during the administration of tolvaptan.
This is the first report on the safety and efficacy of tolvaptan in an infant with SIADH associated with HPE.
Core Tip: Holoprosencephaly with the syndrome of inappropriate antidiuretic hormone secretion (SIADH) is very rare. The main treatments for SIADH are restriction of water intake and sodium administration, which could inhibit the infant’s growth. Tolvaptan, a vasopressin V2 receptor antagonist, is effective in adults with SIADH but has not been reported in infants. In the present neonatal case, the patient developed SIADH, and a restriction of water intake and sodium administration caused the fluctuations in serum sodium levels. Subsequent administration of tolvaptan improved the serum sodium level. Tolvaptan could be a novel standard treatment for infants with SIADH.
- Citation: Mori M, Takeshita S, Nakamura N, Mizuno Y, Tomita A, Aoyama M, Kakita H, Yamada Y. Efficacy of tolvaptan in an infant with syndrome of inappropriate antidiuretic hormone secretion associated with holoprosencephaly: A case report. World J Clin Cases 2023; 11(26): 6262-6267
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6262.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6262
Holoprosencephaly (HPE) is a congenital malformation in which ventralization of the forebrain, which forms the left and right cerebral hemispheres, is incomplete, due to gene mutations involved in ventral induction of the forebrain, such as SHH, SIX3, and ZIC2[1-5]. HPE causes malformations in the cerebral hemispheres, deep brain structures, olfactory bulbs, and visual bulbs, and facial malformations in the form of interocular narrowing, flat nasal bridge, a single nostril, cleft lip and palate, and a single maxillary central incisor[1,6,7]. Furthermore, HPE might be associated with various degrees of hypothalamic-pituitary dysfunction[1,6-10]. In particular, pituitary dysfunction results in partial or global hyposecretion of anterior lobe hormones and hyposecretion of posterior lobe hormones[1,6-10]. Diabetes insipidus (DI) is generally a common complication of HPE[1,6-10], and HPE with the syndrome of inappropriate antidiuretic hormone secretion (SIADH) is very rare.
The main treatments for infants with SIADH are restriction of water intake and sodium administration[11]. However, these treatments might inhibit the infants’ growth, while also increasing morbidity and mortality in neonates[12,13]. In recent years, tolvaptan, a vasopressin V2 receptor antagonist, has been reported to improve hyponatremia by promoting free water excretion via the renal collecting duct in adults with SIADH[14,15]. However, there are no reports of tolvaptan improving SIADH associated with HPE.
We report herein an infant with SIADH associated with HPE in whom tolvaptan improved hyponatremia, with prevention of the fluctuation in serum sodium levels without limitation of water and sodium administration. This is the first case report showing the efficacy and safety of tolvaptan in an infant with SIADH in association with HPE.
A 2414-g female infant was born at 38 wk by normal vaginal delivery. Her Apgar score was 8 and 9 at one and five minutes, respectively. She had no respiratory problems or other symptoms but was admitted to the neonatal intensive care unit promptly after birth because HPE was suspected prenatally.
The patient had been suspected of having HPE on a fetal echo since the prenatal period.
The patient had no significant family history.
The patient had several facial anomalies, including a cleft lip, closely spaced eyes, a depressed nasal bridge, and an absent nasal septum.
At birth, no abnormalities were observed in laboratory examinations.
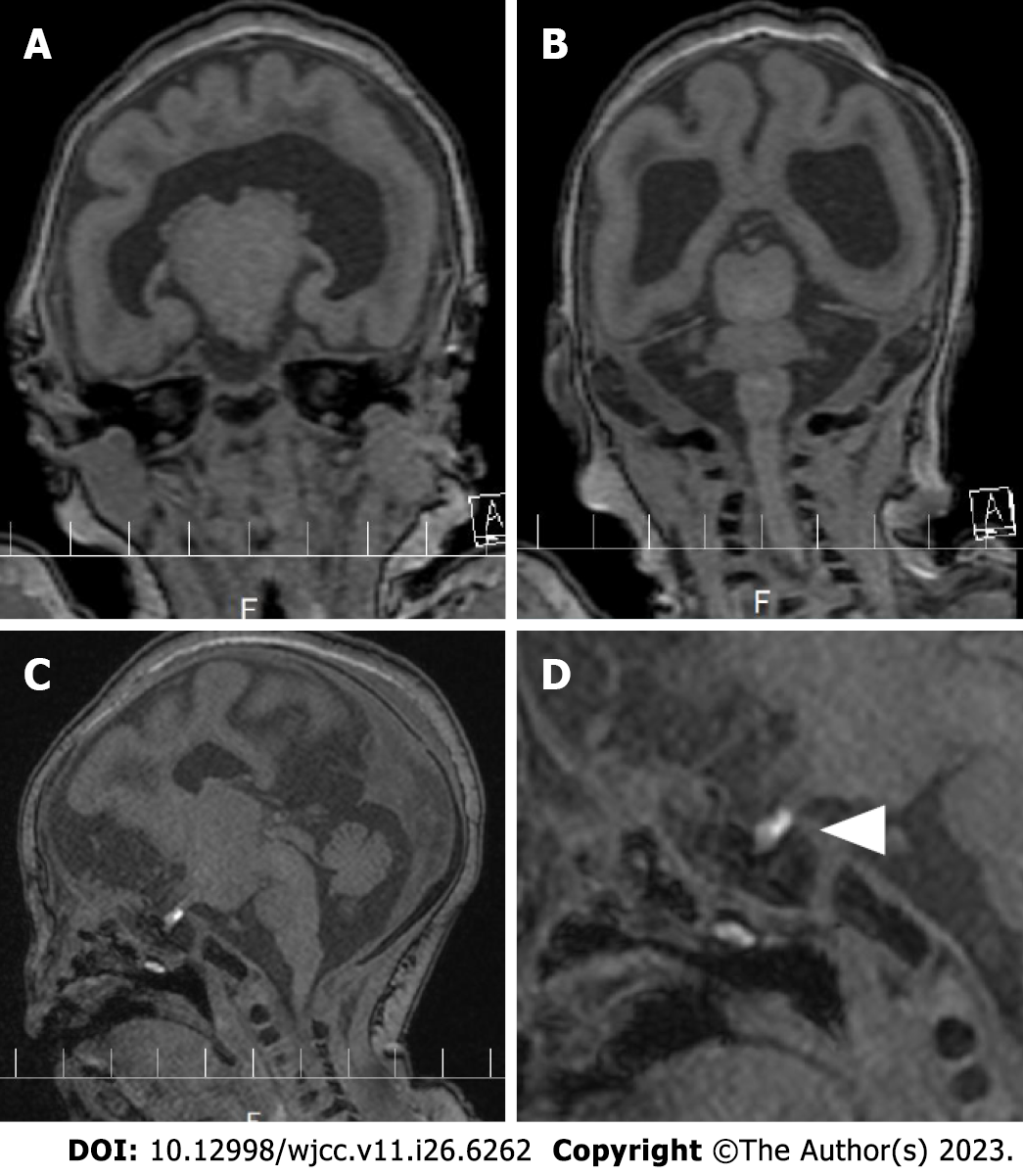
Head magnetic resonance imaging (MRI) was taken at 3.0 Tesla with 0.8 mm slices to evaluate brain morphology on the 3rd d of life, which revealed a fusion of the frontal region, an interhemispheric fissure in the occipital region, and normal pituitary lobes (Figure 1A and B). The posterior pituitary lobe, which is high-signal on T1-weighted images, is small and shifted upward, suggesting an ectopic posterior pituitary stalk (Figure 1C and D).
Based on the MRI results, the patient was diagnosed with semilobar HPE.
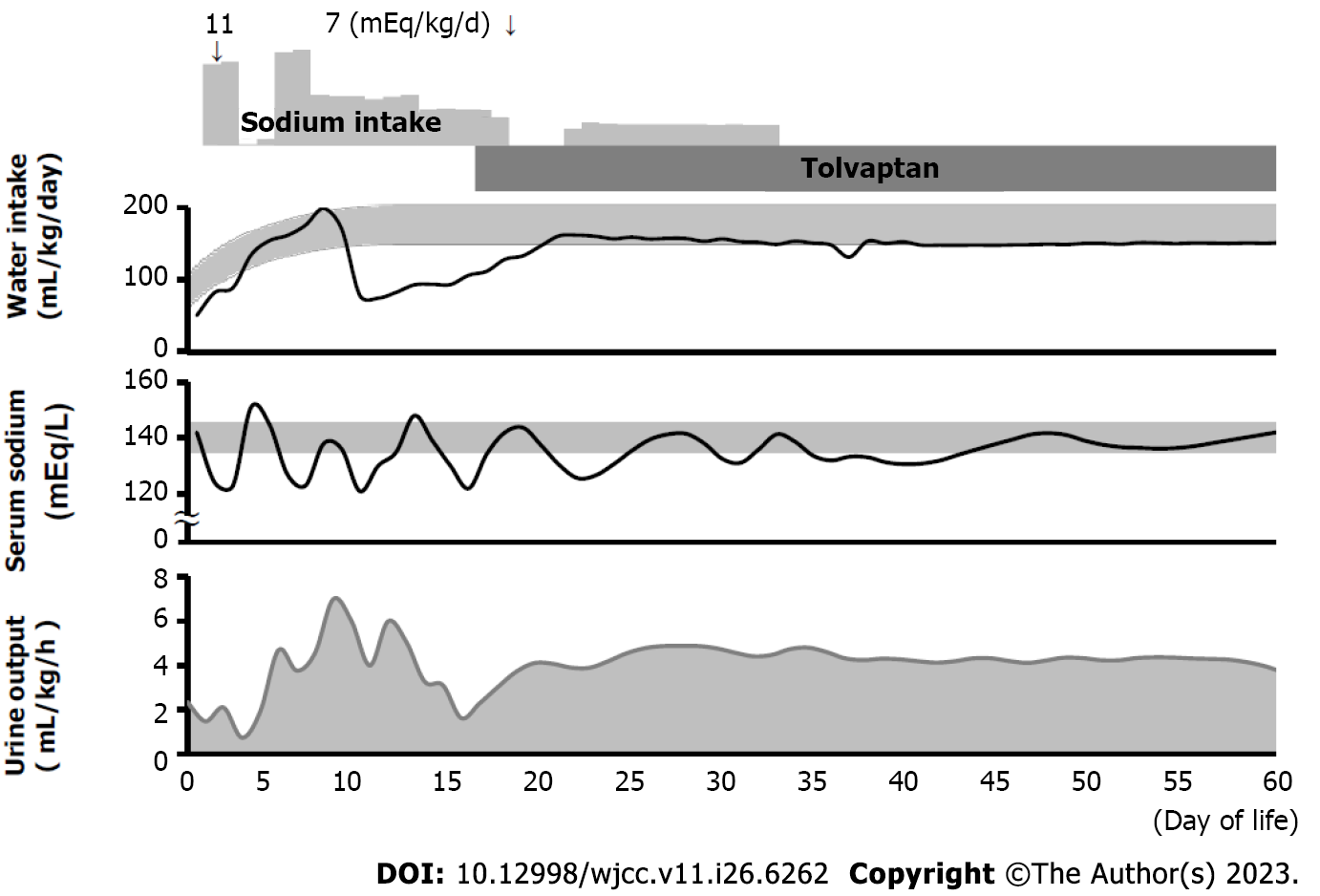
Figure 2 shows the patient’s clinical course of water intake, serum sodium level, and urine output. On the 2nd d of life, her urine output was 2.4 mL/kg/h and her serum sodium level was 123 mEq/L. Urinary sodium concentration at this time was very high, at 150 mEq/L. However, administration of sodium according to the calculated necessary sodium requirement (11 mEq/kg/d) and restriction of water intake (to 88 mL/kg/d) caused an exaggerated reaction, resulting in hypernatremia (serum sodium level 151 mEq/L) and increased urine output (5.1 mL/kg/h) on the next day. Blood hormone evaluation on the 7th d of life showed that serum adrenocorticotropic hormone (ACTH) and cortisol levels were relatively preserved (ACTH: 21.2 pg/mL; cortisol: 7.0 µg/dL), and thyroid function was normal (free T3: 1.94 pg/mL; free T4: 1.37 ng/dL; thyroid-stimulating hormone: 1.032 µIU/mL), suggesting the preservation of anterior pituitary function.
On the 9th d of life, hyponatremia (serum sodium level 119 mEq/L) and decreased urine output (2.8 mL/kg/h) appeared again. Serum osmolality was 246 mOsm/L and urine osmolality was 444 mOsm/L, and serum antidiuretic hormone (ADH) was 1.1 pg/mL. SIADH was diagnosed based on these data[16,17]. Regulation of water intake and sodium administration again caused an exaggerated reaction in terms of serum sodium levels, with no improvement in the fluctuation of serum sodium. Thus, oral tolvaptan was started at a dose of 0.14 mg/kg/d on the 16th d of life. Thereafter, urine output increased and stabilized, and hyponatremia improved without restriction of water or sodium administration.
The patient’s serum sodium levels remained within the normal range even after discontinuation of tolvaptan at 80 d of life. There were no side effects of administration of tolvaptan, such as hypernatremia or liver dysfunction.
Pituitary dysfunction is often present in HPE, and central DI caused by hyposecretion of ADH is one of the most common complications of HPE[1,6-10]. However, this patient had SIADH, and it was difficult to regulate the serum sodium instability with only sodium administration and regulation of water intake. The purpose of this report is to demonstrate that tolvaptan is effective for SIADH in infants and that administration of tolvaptan eliminates the need for restriction of water intake and sodium administration.
Osmoreceptors located in the anteroventral wall of the third ventricle sense changes in plasma osmolality, and transmit signals to ADH neurons in the hypothalamus to regulate ADH secretion[1,6,18]. Four mechanisms of osmotically regulated ADH secretion have been reported in SIADH: Erratic ADH release, reset osmostat, persistent ADH release with low plasma osmolality, and normal osmoregulated ADH secretion[19]. In fact, ADH was secreted in this case despite hyponatremia, in accordance with the diagnostic criteria for SIADH[17,20]. In addition, serum ACTH and cortisol levels were normal, indicating that anterior pituitary function was well preserved. Based on these results, the fluctuations in serum sodium levels might have been due to a disorder in osmoregulation because of posterior pituitary dysfunction.
ADH activates V2 receptors on the basement membrane in renal collecting duct cells, causing aquaporin-2 channels to move to the apical membrane, and allowing the normally impermeable apical membrane to become permeable to water[17,20]. In SIADH, the inappropriately secreted ADH enhances water reabsorption in the renal collecting duct and increases extracellular fluid volume. By promoting free water excretion, tolvaptan reportedly improves hyponatremia in adult SIADH patients[14-16]. In our case, urine output stabilized and serum sodium levels improved after starting tolvaptan administration, without the need for adjustment of water or sodium administration, suggesting its potential efficacy even in infants. Since tolvaptan acts on the renal collecting ducts, it might have potential benefit in stabilizing urine output even in the presence of central nervous system diseases[15,16]. Reportedly, 5.9% of adult SIADH patients treated with tolvaptan experience overly rapid correction of hyponatremia[16]. However, no abnormalities in serum sodium were observed in this patient after the administration of tolvaptan. The previous main treatments for infants with SIADH were restriction of water intake and sodium administration. However, these treatments might inhibit the infants’ growth, while also increasing morbidity and mortality in neonates[13,14]. In our patient, the administration of tolvaptan resulted in adequate control of serum sodium levels without the need for adjustment of water or sodium administration.
There are some limitations to this study. First, because it is a single case report, there is insufficient evidence of tolvaptan’s efficacy. Second, the outcomes without tolvaptan are unknown because of the lack of controlled trials. However, it is anticipated that the accumulation of other cases will provide stronger evidence.
We report an infant with SIADH associated with HPE with an exaggerated response to water restriction and sodium administration. The pathology in this case was presumed to be due to osmoregulatory disorders caused by posterior pituitary dysfunction. Finally, tolvaptan administration enabled stabilization of serum sodium levels. Tolvaptan has the potential to be a novel standard in the treatment of infants with SIADH.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Papadopoulos K, Thailand; Thongon N, Thailand; Zhou Y, United States S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Dubourg C, Bendavid C, Pasquier L, Henry C, Odent S, David V. Holoprosencephaly. Orphanet J Rare Dis. 2007;2:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 260] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Petryk A, Graf D, Marcucio R. Holoprosencephaly: signaling interactions between the brain and the face, the environment and the genes, and the phenotypic variability in animal models and humans. Wiley Interdiscip Rev Dev Biol. 2015;4:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Hong M, Krauss RS. Modeling the complex etiology of holoprosencephaly in mice. Am J Med Genet C Semin Med Genet. 2018;178:140-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Domené S, Roessler E, El-Jaick KB, Snir M, Brown JL, Vélez JI, Bale S, Lacbawan F, Muenke M, Feldman B. Mutations in the human SIX3 gene in holoprosencephaly are loss of function. Hum Mol Genet. 2008;17:3919-3928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Barratt KS, Arkell RM. ZIC2 in Holoprosencephaly. Adv Exp Med Biol. 2018;1046:269-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Raam MS, Solomon BD, Muenke M. Holoprosencephaly: a guide to diagnosis and clinical management. Indian Pediatr. 2011;48:457-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Kauvar EF, Muenke M. Holoprosencephaly: recommendations for diagnosis and management. Curr Opin Pediatr. 2010;22:687-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Tasdemir S, Sahin I, Cayır A, Doneray H, Solomon BD, Muenke M, Yuce I, Tatar A. Holoprosencephaly: ZIC2 mutation in a case with panhypopituitarism. J Pediatr Endocrinol Metab. 2014;27:777-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Levey EB, Stashinko E, Clegg NJ, Delgado MR. Management of children with holoprosencephaly. Am J Med Genet C Semin Med Genet. 2010;154C:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Kourti M, Pavlou E, Rousso I, Economou I, Athanassiadou F. Holoprosencephaly and diabetes insipidus in a 3-month-old infant. J Child Neurol. 2008;23:118-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Grant P, Ayuk J, Bouloux PM, Cohen M, Cranston I, Murray RD, Rees A, Thatcher N, Grossman A. The diagnosis and management of inpatient hyponatraemia and SIADH. Eur J Clin Invest. 2015;45:888-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2014;2014:CD000503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Ottolini KM, Andescavage N, Keller S, Limperopoulos C. Nutrition and the developing brain: the road to optimizing early neurodevelopment: a systematic review. Pediatr Res. 2020;87:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C; SALT Investigators. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 833] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 15. | Jamookeeah C, Robinson P, O’Reilly K, Lundberg J, Gisby M, Ländin M, Skov J, Trueman D. Cost-effectiveness of tolvaptan for the treatment of hyponatraemia secondary to syndrome of inappropriate antidiuretic hormone secretion in Sweden. BMC Endocr Disord. 2016;16:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Ranadive SA, Rosenthal SM. Pediatric disorders of water balance. Endocrinol Metab Clin North Am. 2009;38:663-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med. 2007;120:S1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 335] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 18. | Baylis PH. The syndrome of inappropriate antidiuretic hormone secretion. Int J Biochem Cell Biol. 2003;35:1495-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Verbalis JG, Adler S, Schrier RW, Berl T, Zhao Q, Czerwiec FS; SALT Investigators. Efficacy and safety of oral tolvaptan therapy in patients with the syndrome of inappropriate antidiuretic hormone secretion. Eur J Endocrinol. 2011;164:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Knoers NV. Hyperactive vasopressin receptors and disturbed water homeostasis. N Engl J Med. 2005;352:1847-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |