Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6252
Peer-review started: May 31, 2023
First decision: August 10, 2023
Revised: August 16, 2023
Accepted: August 21, 2023
Article in press: August 21, 2023
Published online: September 16, 2023
Processing time: 99 Days and 14.9 Hours
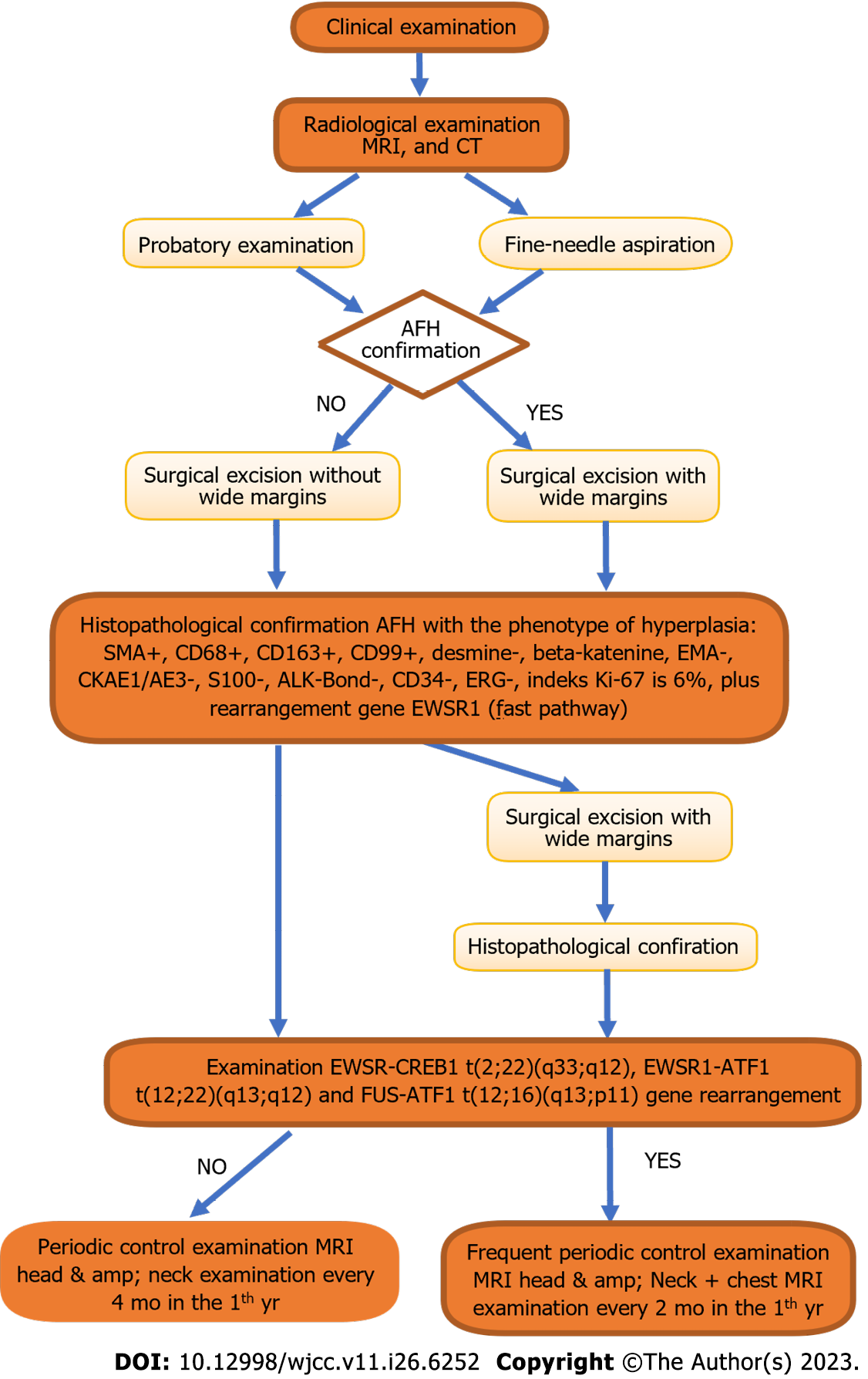
Angiomatoid fibrous histiocytoma (AFH) is a rare, slow-growing soft tissue tumor. It appears mostly on the limbs and trunk in children and young adults. The biology of AFH remains unclear because of the small number of reported cases. Diagnostic testing does not provide definitive results. It has two clinical forms, that differ in terms of gene expression and clinical prognosis. It is important to inform the laboratory which specific gene testing is necessary. Here, we describe a case of rare AFH in the submandibular region using a full genetic panel.
A 13-year-old boy who had been misdiagnosed in the past 6 mo by his dentist visited our clinic because of a lesion in the submandibular area on the right side. The lesion was homogeneous and painless upon palpation. No skin discoloration was observed. Due to the non-specific radiological picture computed tomography (CT), magnetic resonance imaging (MRI), cone-beam CT (CBCT), and ultrasound-guided biopsy were performed. A venous malformation was suspected on the MRI. None of the tests provided a definitive diagnosis. Owing to the non-specific radiological findings, the patient qualified for surgical treatment. The surgical procedure included an excisional biopsy. The diagnostic testing was extended using gene rearrangements. The most distinctive gene translocation in diagnosing AFH is within the EWS RNA-binding protein 1 (EWSR1)-CREB-binding protein. However, in this case, the diagnosis was confirmed by a rearrangement within the EWSR1 gene testing.
AFH in the submandibular location is rare, and surgical treatment with genetic evaluation defines AFH type that affects subsequent procedures.
Core Tip: Angiomatoid fibrous histiocytoma (AFH) is a rare neoplasm of soft tissues with an atypical location and a large morphological spectrum, and is considered a lesion with diagnostic difficulties. Auxiliary diagnostic tests in the field of molecular genetics are also important. The EWS RNA-binding protein 1-CREB-binding protein 1 t(2;22)(q33;q12) fusion assay is an extremely important diagnostic element that should be routinely performed in patients with AFH. In the case of gene translocations, we are obliged to increase the frequency of follow-up examinations. However, owing to the small number of clinical cases, it is difficult to predict the course of the disease and assess patient prognosis.
- Citation: Michcik A, Bień M, Wojciechowska B, Polcyn A, Garbacewicz Ł, Kowalski J, Drogoszewska B. Difficulties in diagnosing angiomatoid fibrous histiocytoma of the head and neck region: A case report. World J Clin Cases 2023; 11(26): 6252-6261
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6252.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6252
Angiomatoid fibrous histiocytoma (AFH) is a rare, slow-growing, soft tissue tumor with a low risk of malignancy[1]. It accounts for 0.3% of all soft tissue sarcomas. It was first described in 1979 as “angiomatoid malignant fibrous histiocytoma” and it was classified by the World Health Organization under the category of “tumors of uncertain differentiation” in 2020. AFH is a lesion that rarely metastasizes[2-4]. It mostly occurs on the superficial extremities of both children and young adults[3,5-7]. It can be misdiagnosed because its clinical, histological, and radiological findings are similar to those of several tumors[5,8].
Here, we present the case of a young male with a lesion in the submandibular area on the right side. It is a rare location. In the available literature, AFH lesions have been described in the upper and lower limbs but less often in the head and neck[9]. Because of the rarity of AFH, we also performed a genetic test using the fluorescent in-situ hybridization (FISH) method, which detected a rearrangement of the EWS RNA binding protein 1 (EWSR1) gene. Diagnostic gene testing was extended to a specific test for the translocation within the EWSR1-CREB-binding protein (EWSR1-CREB). No rearrangement was observed within these genes in the present study (Table 1).
| Time | Name of procedure |
| November 2022 | Laboratory examinations |
| June 2022 | CT Siemens Somatom Definition Flash (Siemens Healthcare, Germany) with standard contrast enhanced protocol |
| July 2022 | First MRI (1.5T MR Magnetom Aera Flash; Siemens Healthcare, Germany) with contrast injection protocol |
| October 2022 | FNA |
| November 2022 | CBCT |
| November 2022 | First surgical procedure |
| January 2023 | Second MRI (1.5T MR Magnetom Aera Flash; Siemens Healthcare, Germany) with contrast injection protocol |
| January 2023 | Second surgical procedure |
A 13-year-old boy was admitted to the hospital because of a lesion in the right submandibular area that had persisted for six months.
The patient was referred from a dentist, and was generally healthy and without allergies.
There was no obvious history of illness related to the disease.
There was no significant personal or family history related to the disease.
Clinically, the lesion was firm, homogeneous, compact, painless to palpation, and 2.5 cm × 2.0 cm in size. No skin discoloration was observed. The boy did not complain of any sensory disorders and denied any growth changes.
Routine blood test (November 2022) results were as follows: Neutrophil cell count, 3.88 × 109/L (reference value: 2-7 × 109/L); neutrophil percentage: 58.6% (reference value: 40%-74%).
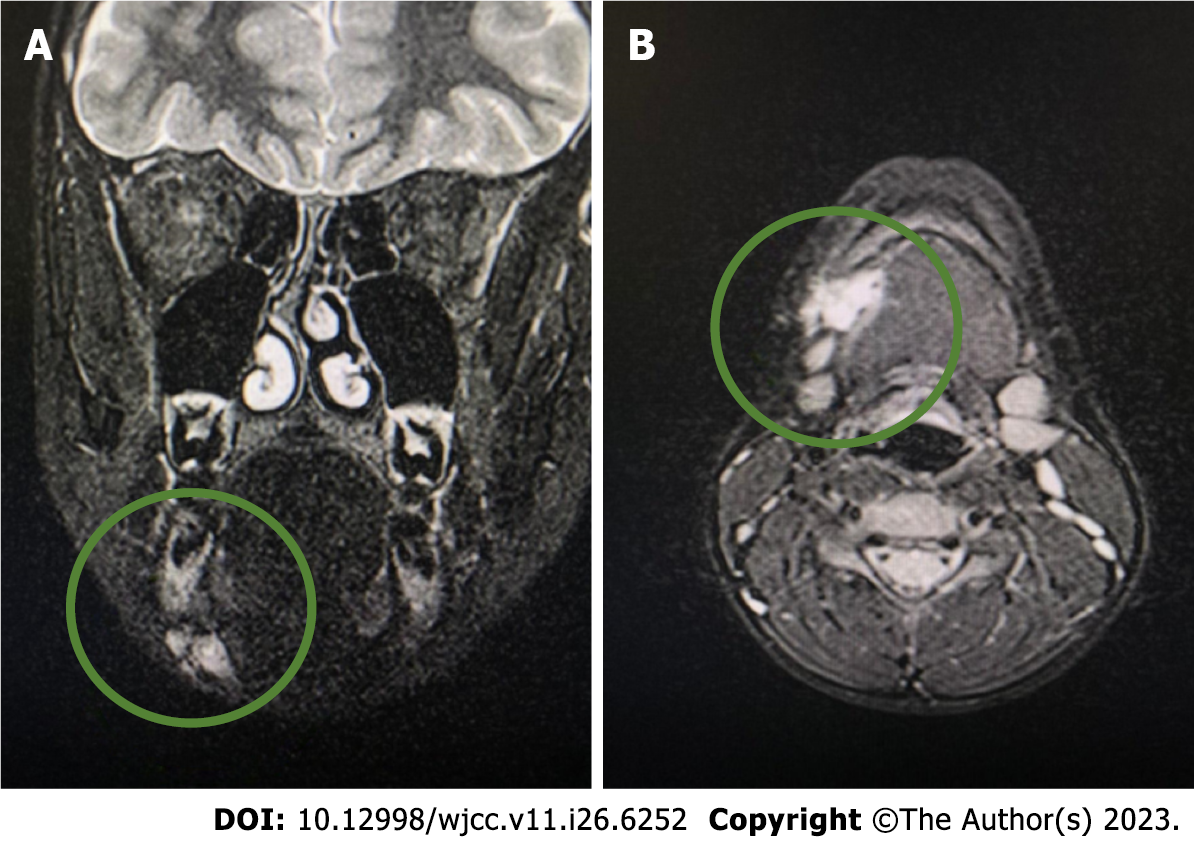
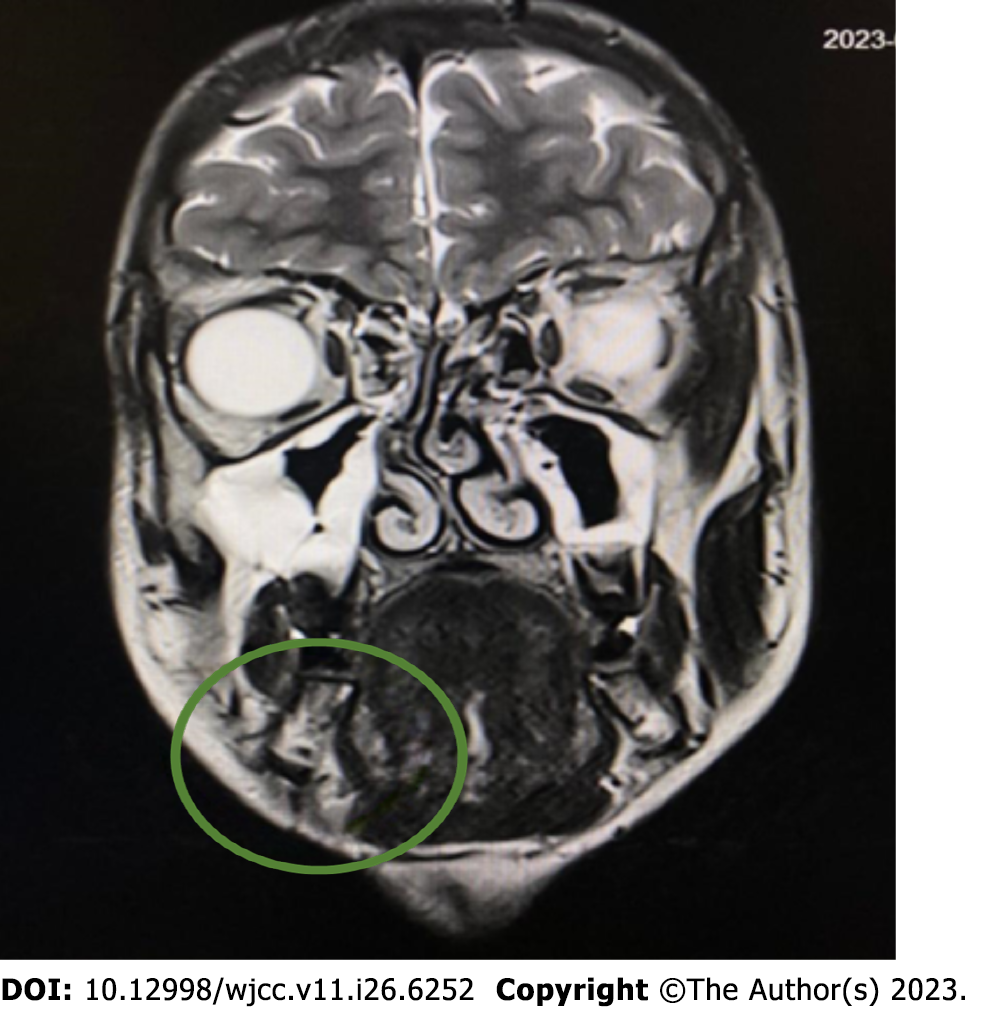
The first computed tomography (CT) (Siemens Somatom Definition Flash; Siemens Healthcare, Germany) was performed on June 23, 2022. It showed a lesion under the mandible on the right side with smooth edges. It was not connected to the sublingual or submandibular glands. Owing to the nonspecific CT image, magnetic resonance imaging (MRI) (1.5T MR Magnetom Aera Flash; Siemens Healthcare, Germany) with contrast (Gadovist, 5 mL) was performed on July 25, 2022. In the submandibular space on the right side, heterogeneous high signal intensity was observed on T2-weighted images, with a hemorrhagic component measuring 18 mm × 25 mm × 13 mm. The lesion adhered to the digastric muscle and the body of the mandible and involved the platysma (Figure 1).
Conclusion: A venous malformation was suspected. The radiologist suggested extending the diagnosis using ultrasonography to plan the treatment. The large interval between diagnostic imaging and testing is due to the patient’s lack of cooperation and not reporting for follow-up to the Department of Maxillofacial Surgery.
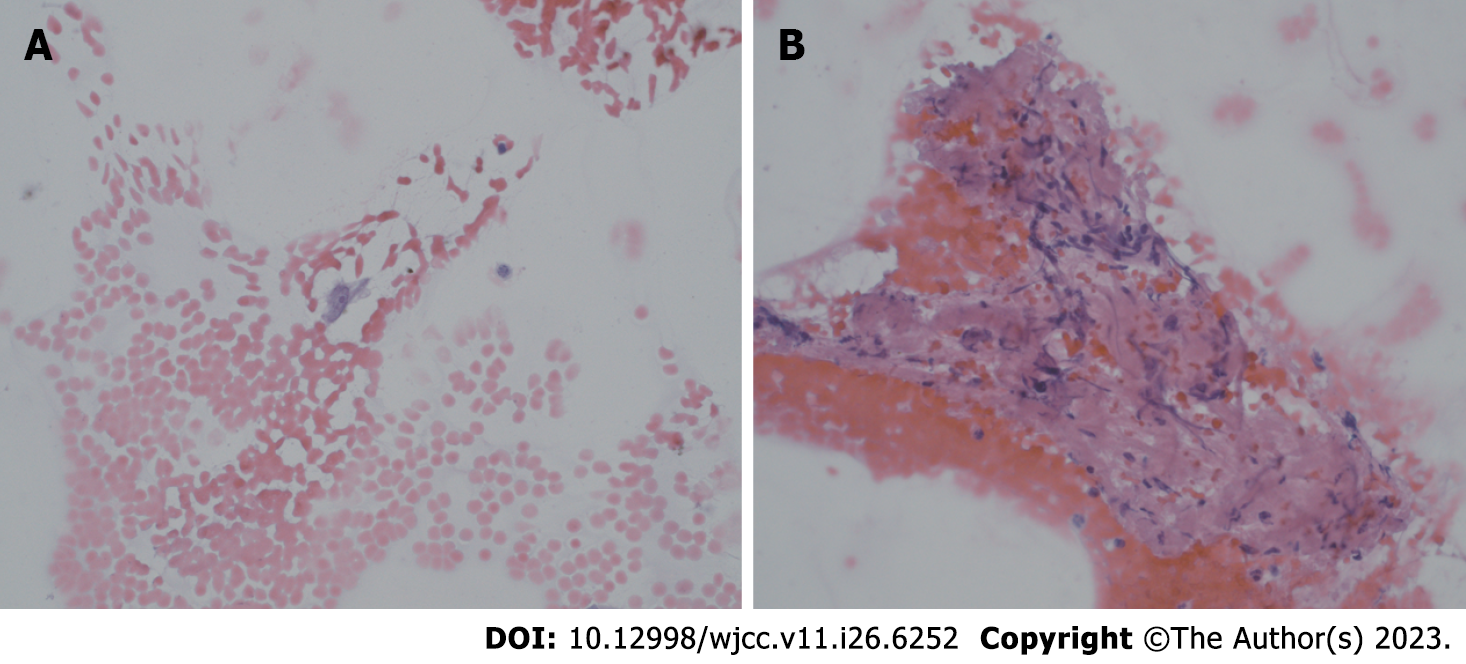
Fine-needle aspiration (FNA) was performed on October 27, 2022. In the submandibular space on the right side, visible polycyclic, solid, isoechogenic, and hypoechogenic lesions up to 23 mm × 24 mm × 14 mm were observed. The mass was hard with congenital vascularity. It was not equivalent to lymphatic or lymphatic-venous malformations.
Microscopic results of FNA: The cervical smear included sparse, incorrupt extravasation of blood and several crushed parts of tissues. Furthermore, a few cells were similar to each other, such as cells with an oval nucleus and no mitosis. FNA does not reveal the characteristics of the lesion. Surgical excision of the entire lesion was necessary (Figure 2).
Single bland cell with oval nucleus, smooth nuclear membranes, and conspicuous nucleolus.
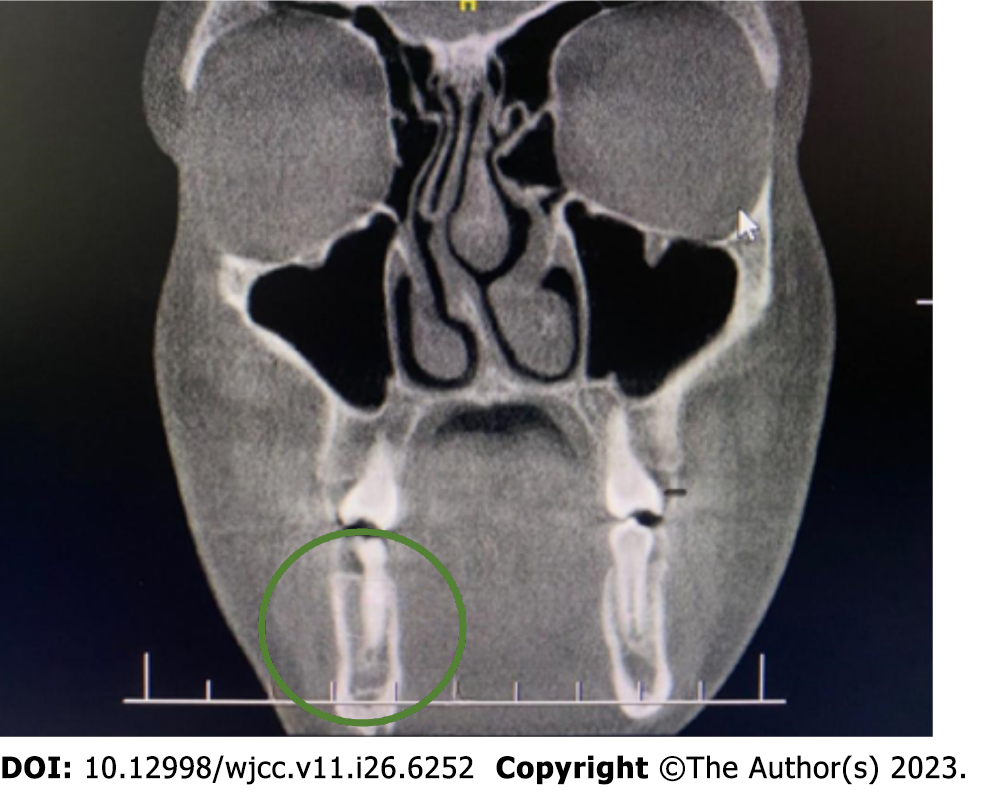
None of these tests provided a diagnosis, and the patient was eligible for surgery under general anesthesia. The surgical procedure included an excisional biopsy. Before that, we extended the diagnostics with cone beam CT (CBCT) on November 10, 2022, which revealed a 3 mm lesion in the cortical layer of the mandible on the right side (Figure 3).
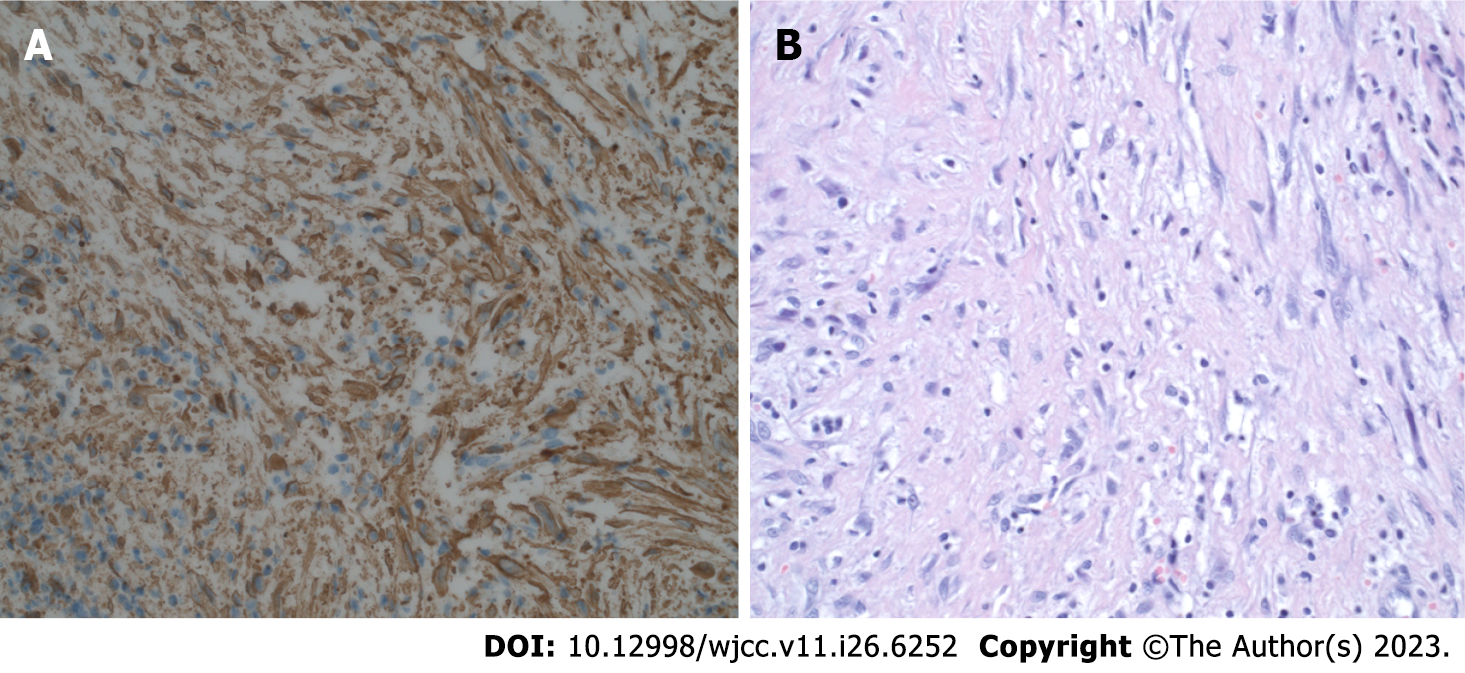
Histopathological diagnosis: Irregular fibrohistiocytic hyperplasia with a variety of cells was observed between fibrous tissue, fatty tissue, and fibrous skeletal muscles. They were surrounded by multiple blood vessels. In addition, minor lymphocytes are rare in this circuit. Hyperplasia is an invasive and expandable condition. The phenotype of hyperplasia is as follows: SMA+, CD68+, CD163+, CD99+, desmine-, beta-catenin-, EMA-, CKAE1/AE3-, S100-, ALK-Bond-, CD34-, ERG-, and indeks Ki-67 IS 6% (Figure 4).
The cumulative findings suggested fibrohistiocytic hyperplasia with an intermediate degree of malignancy, AFH.
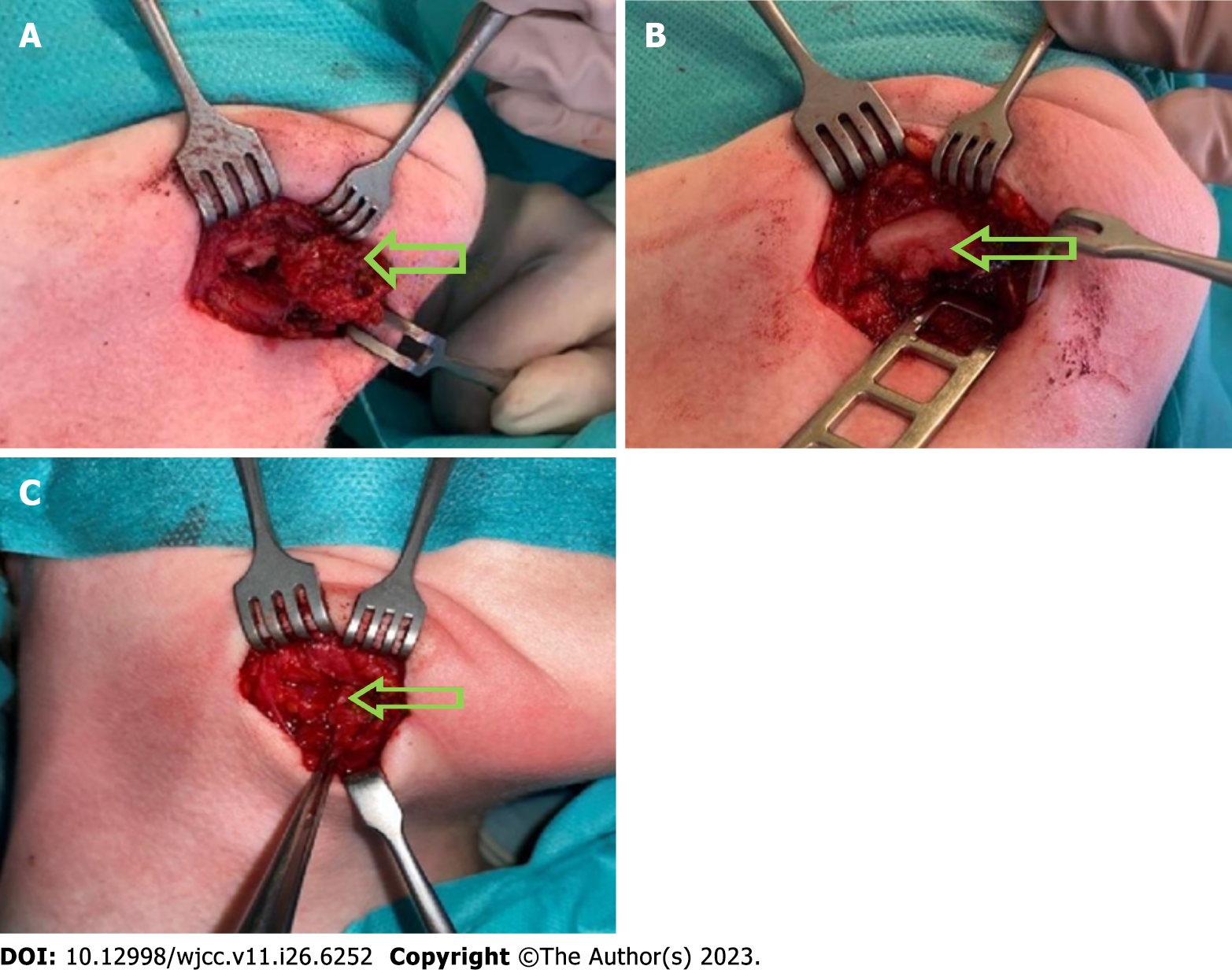
The surgery was performed on November 29, 2022, under general anesthesia with oral intubation. A skin incision was made on the neck and the mass alone was removed, avoiding macroscopically unchanged tissues, which was considered the best solution. The affected external layer of the bone was exposed during the procedure. Along with the tumor, a bone sample was sent for histopathological examination. No complications occurred (Figure 5A and B).
Because of the rarity of AFH, we also performed a genetic test using the FISH method to check for the presence of the genetic translocation, that is, rearrangement gene EWSR1. Rearrangement gene EWSR1 was confirmed. The genetic study was conducted at the Laboratory of the Genetics Clinic of UCK in Gdansk. Pathomorphological and genetic studies validated the diagnosis of AFH.
As we know from previous research, the most aggressive form of AFH is associated with the involvement of both EWSR1 and CREB1 gene rearrangements. Tumors harboring these two genes exhibit recurrence and metastasis. Additional testing was performed to rule out the possibility that the tumor was aggressive.
EDTA- and formalin-fixed, paraffin-embedded samples were submitted for testing. A total of 750 genes were tested, including CREB1, methylthioadenosine phosphorylase gene, tumor protein p53, and MRE11 gene (MRE 11).
The novel allele frequency, which is the frequency at which a mutated allele occurs in the sequencing data, was 100%. The observed frequencies are influenced by the tumor content and copy number alterations and do not directly correlate with the frequency of the variant in the tumor. Somatic alterations were classified according to their functional effect on protein levels into the following categories: inactivating/activating/function altered likely inactivating/activating/function altered unknown and benign.
The detected germline variant c.1867+5G > T; p,,? in gene MRE11 is predicted to cause aberrant splicing and might therefore disrupt protein function. Based on the ACMG criteria, the variant was ranked as a variant of uncertain significance in the germline (Tables 2 and 3).
| Item | Description |
| Gene | MRE11 (germline) |
| Functional category | Splice_region |
| Variant | c.1867+5G > T;P.? |
| NAF in germline | 0.52 |
| Effect on protein function | Probably inactivating |
| Therapeutic option for discussion in the MTB | PARP inhibitor |
| Approved by EMA/FDA | EMA & FDA |
| Approved for current entity | Po |
| Drug name | Tumor entity | Approval | Approval limited to biomarkers/others | Approval in combination with other drugs |
| Niraparib, PARP inhibitor | Prostate cancer | EMA | BRCA1, BRCA2 | Abiraterone |
| Olaparib, PARP inhibitor | Fallopian tube carcinoma | EMA | HRD | Bevacizumab |
| Olaparib, PARP inhibitor | Fallopian tube carcinoma | FDA | HRD | Bevacizumab |
| Olaparib, PARP inhibitor | Ovarian cancer | EMA | Cancer associated with HRD | Bevacizumab |
| Olaparib, PARP inhibitor | Ovarian cancer | FDA | HRD | Bevacizumab |
| Olaparib, PARP inhibitor | Primary peritoneal carcinoma | EMA | Cancer associated with HRD | Bevacizumab |
| Olaparib, PARP inhibitor | Primary peritoneal carcinoma | FDA | HRD | Bevacizumab |
| Olaparib, PARP inhibitor | Prostate cancer | EMA | Adult patients, metastatic castraction- resitant cancer, chemotherapy not indicated | Abiraterone, prednisone |
| Olaparib, PARP inhibitor | Prostate cancer | FDA | BRCA1, BRCA2 | Abiraterone, prednisone |
The variant 28/*28 detected in UDP-glucuronosyltransferase family 1 member A1 is a homozygous germline variant. For this genotype [also known as (TA)7/(TA)7rs8175347 or rs3064744], there is a possible increase in toxicity upon treatment with irinotecan-based chemotherapeutics (Table 4).
| Item | Description |
| Gene | UGT1A1 |
| Functional category | Upstream_gene |
| Variant | c.-41_-40dupAT;p.?(*28/*28) |
| Transcript-ID | NM_000463.3 |
| Zygosity in germline | Homozygous |
| Effect on protein function | Inactivating |
| Therapeutic option | Topoisomerase inhibitor |
| Phenotype | Poor metabolizer |
No other genes or translocations, such as EWSR1-CREB, were found.
We also followed-up with a new MRI with contrast (Gadovist, 5.5 mL) on January 3, 2023 (Figure 6).
Based on histopathological examination results, a second surgery was conducted to completely remove the lesions with wide margins.
The second surgery was performed on January 24, 2023, under general anesthesia with oral intubation. A skin incision was made within the scar from the initial surgery, and part of the platysma was removed, as well as part of the increased cohesion tissue located under the body of the mandible and surrounding lymph nodes. The specimen was sent for histopathological examination. Intraoperative course was without complications. Intravenous pharmacotherapy administered after the first surgery was repeated (Figure 5C).
Based on the results of the histopathological examination extended by genetic examination, the presence of an extremely rare soft-tissue sarcoma, AFH, was confirmed. To the best of our knowledge, this is the first reported case involving this location. Since gene translocation has not been demonstrated, particularly in EWSR1-CREB, surgery can be considered the main and final treatment (Figure 7). The patient is currently reporting for routine check-ups at the Oral and Maxillofacial Surgery Clinic. Locally, there were no signs of recurrence.
AFH, first discovered in 1979, is a very rare soft tissue tumor with a relatively good prognosis and medium malignancy potential[10,11]. It accounts for 0.3% of all soft tissue sarcomas. It is considered an intermediate tumor. In the past, it was classified as a malignant variant, but owing to its course and treatment, it was found to have a favorable prognosis[12]. It occurs most often in the deep dermis and subcutaneous tissues of the upper and lower limbs[6]. It mainly affects children and young adults, and there have been a few reported cases involving older adults[12]. The tumor growth is slow and painless. Patients are often unaware of this disease[2]. This may be accompanied by fever and general weakness. However, this criterion does not define a unit[11].
Based on the case presented, diagnosis is difficult and yields ambiguous results. From the clinical and radiological perspectives, lymphatic malformations, lymphovenous malformations, bone aneurysmal cysts, hematomas, heman
Surgical treatment alone enabled us to obtain additional information. Based on the available literature, surgery involving the removal of a lesion with a wide margin of macroscopically unchanged tissue is the best solution. In the head and neck area, performing a wide excision is often clinically impossible and is associated with disease recurrence in the future. However, local recurrence of the lesion depends on the size and depth of tissue invasion[11] and occurs in 15%[13] or, according to another source, in 2%-12% of cases[5].
Based on immunohistochemistry results, CD68, CD99, EMA, and wimentyne (EP21) were positive for epithelial membrane antigens, whereas cytokeratin and S100 protein were negative[5,11,12]. However, desmine was positive and negative in two different assays[2,5,12].
Because the diagnosis of AFH can be difficult owing to variable histological features and the lack of highly sensitive immunohistochemical markers, the immunohistochemical markers TLE1 and BCOR have been tested and proven useful. The sensitivity and specificity of TLE1 marker for detecting AFH were 100.0% and 94.4%, respectively. Therefore, TLE1 can be considered as a highly sensitive immunohistochemical marker[14].
In addition, FISH tests can detect gene rearrangements. The three most characteristic AFH translocations are: EWSR-CREB1 t(2;22)(q33;q12), EWSR1-ATF1 t(12;22)(q13;q12), and FUS-ATF1 t(12;16)(q13;p11)[5,15,11,16-18]. The most common translocation is EWSR-CREB1 (90%)[19,20]. This is associated with the risk of distant metastases. Therefore, we decided to keep the patient under observation and scheduled follow-up visits. Distant metastases occur in 5% of cases and are mainly located in the regional lymph nodes or lungs[13]. The EWSR1-CREB fusion is also found in gastrointestinal clear cell sarcoma and primary myxoid lung sarcoma (PPMS)[19]. CCSGT is a rare cancer that occurs in the walls of the small intestine, mainly in young adults and is similar to AFH. In contrast, PPMS is a rare lung cancer that arises intrabronchial[19].
The histology of AFH consists of a multinodular growth of spindle-shaped cells. These cells have a characteristic syncytial appearance. The pseudovascular spaces are filled with cancer cells and blood. Histologically, a thick, fibrous pseudocapsule with deposited hemosiderin together with lymphoplasmacytic cuffs can also be distinguished[21]. Regarding the morphology, AFH showed a wide spectrum. Multinodular cell growth was consistent. A specific feature of the morphology is the presence of small, clear cells with scanty cytoplasm. This resembles Ewing’s Sarcoma[21].
Ultrasonography is the least invasive radiological examination. The image shows a heterogeneous hypoechoic structure that may contain cystic spaces or fluid levels[6,19]. On CT, AFH is described as a heterogeneous soft tissue lesion with hyperdense areas suggestive of hemorrhage and hypotensive areas that could indicate necrosis or a cystic/myxoid lesion[6]. MRI may show hypointense pseudocapsules (double-rim sign), cystic changes, peritumoral edema, and fluid-fluid levels. On T1-weighted images, the lesion is most often isotensive to the muscles, whereas on T2-weighted images, it is heterogeneously hyperintense[6,19,21,22].
Favorable prognosis has been established for patients with AFH after treatment. As mentioned above, local recurrence occurs in up to 15% of cases, whereas distant metastases occur in < 5% of cases. The presence of gene translocations, especially EWSR1-CREB, is also important because of the increased risk of distant metastases. Patients with distant metastases have been treated with systemic chemotherapy; however, no benefits have been reported. AFH has an unspecified course that is rarely predictable[21].
AFH is a rare neoplasm of soft tissues with an atypical location and a large morphological spectrum, and is considered a lesion with diagnostic difficulties. In the case of AFH, diagnostics are crucial because they allow us to plan further diagnostics and extend the treatment to distant tumors, in addition to local treatment (in the case of metastases). Auxiliary diagnostic tests in the field of molecular genetics (reverse transcription polymerase chain reaction or FISH) are also important. The EWSR1-CREB1 t(2;22)(q33;q12) fusion assay is an extremely important diagnostic element that should be routinely performed in patients with AFH. In the case of gene translocation, we are obliged to increase the frequency of follow-up examinations, including chest CT imaging. However, owing to the small number of clinical cases, it is difficult to clearly predict the course of the disease and assess the prognosis of patients. Publishing the treatment results for patients with AFH will deepen our knowledge about this tumor.
Words of thanks to Bień M and Wojciechowska B who have made genuine contributions to the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salvi M, Italy S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Corley EA, Pace E, Barnacle AM, Patel PA, Thway K, Chisholm JC. Evidence of Chemoresponsiveness in Unresectable Metastatic Angiomatoid Fibrous Histiocytoma. J Pediatr Hematol Oncol. 2023;45:e279-e284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 2. | Maqbool H, Bashir S, Hassan U, Hussain M, Mushtaq S, Ishtiaq S. Angiomatoid Fibrous Histiocytoma: A Tumor With Uncertain Behavior and Various Clinicopathological Presentations. Cureus. 2022;14:e28985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 3. | Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives. Pathologica. 2021;113:70-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 555] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 4. | Cazzato G, Lupo C, Casatta N, Riefoli F, Marzullo A, Colagrande A, Cascardi E, Trabucco SMR, Ingravallo G, Moretti B, Maiorano E, Pesce V, Resta L. Angiomatoid Fibrous Histiocytoma (AFH) of the Right Arm: An Exceptional Case with Pulmonary Metastasis and Confirmatory EWSR1::CREB1 Translocation. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Alzahim MA, Abed AH, Mashrah HT, Almahdaly AM, Shaheen M. Angiomatoid Fibrous Histiocytoma: A Series of Three Cases. Cureus. 2021;13:e16465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Bauer A, Jackson B, Marner E, Gilbertson-Dahdal D. Angiomatoid fibrous histiocytoma: a case report and review of the literature. J Radiol Case Rep. 2012;6:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Chen G, Folpe AL, Colby TV, Sittampalam K, Patey M, Chen MG, Chan JK. Angiomatoid fibrous histiocytoma: unusual sites and unusual morphology. Mod Pathol. 2011;24:1560-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Pasricha S, Durga G, Sharma A, Pruthi M, Kamboj M, Gupta G, Jajodia A, Mahawar V, Babu Koyyala VP, Mehta A. Angiomatoid fibrous histiocytoma: Report of two cases, initially construed as sarcoma with unusual clinico-pathological features. Indian J Pathol Microbiol. 2022;65:921-924. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Lemnos L, Salle H, Caire F, Duchesne M. Angiomatoid fibrous histiocytoma: an atypical brain location newly described as intracranial mesenchymal tumor FET-CREB fusion-positive. Acta Neurol Belg. 2023;123:691-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Kim NR, Kim SI, Park JW, Park CK, Chung CK, Choi SH, Yun H, Park SH. Brain parenchymal angiomatoid fibrous histiocytoma and spinal myxoid mesenchymal tumor with FET: CREB fusion, a spectrum of the same tumor type. Neuropathology. 2022;42:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Tabibkhooei A, Asgarzadeh L, Sadeghipour A, Vafaee Shahi M. Intracranial angiomatoid fibrous histiocytoma: report of a rare case. Iran J Child Neurol. 2022;16:205-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Deshmukh AV, Shivkumar VB, Atram M, Bhoyar MP, Gangane NM. Angiomatoid Fibrous Histiocytoma in an Elderly Male: An Unusual Presentation of a Rare Case. Oman Med J. 2023;38:e468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Ngo C, Grinda T, Boilève A, Levy A, Le Pechoux C, Haddag L, Valent A, Lazure T, Briand S, Honoré C, Faron M, Mir O, Bahleda R, Verret B, Le Cesne A. Durable response to crizotinib in metastatic angiomatoid fibrous histiocytoma with EWSR1-CREB1 fusion and ALK overexpression. Ann Oncol. 2022;33:848-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Pan H, Byers J, Yin H, Rytting H, Logan S, He M, Yu Z, Wang D, Mangray S, Zhou S. The utility of TLE1 and BCOR as immunohistochemical markers for angiomatoid fibrous histiocytoma. Int J Clin Exp Pathol. 2023;16:32-39. [PubMed] |
| 15. | Thway K, Fisher C. Angiomatoid fibrous histiocytoma: the current status of pathology and genetics. Arch Pathol Lab Med. 2015;139:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Sloan EA, Chiang J, Villanueva-Meyer JE, Alexandrescu S, Eschbacher JM, Wang W, Mafra M, Ud Din N, Carr-Boyd E, Watson M, Punsoni M, Oviedo A, Gilani A, Kleinschmidt-DeMasters BK, Coss DJ, Lopes MB, Raffel C, Berger MS, Chang SM, Reddy A, Ramani B, Ferris SP, Lee JC, Hofmann JW, Cho SJ, Horvai AE, Pekmezci M, Tihan T, Bollen AW, Rodriguez FJ, Ellison DW, Perry A, Solomon DA. Intracranial mesenchymal tumor with FET-CREB fusion-A unifying diagnosis for the spectrum of intracranial myxoid mesenchymal tumors and angiomatoid fibrous histiocytoma-like neoplasms. Brain Pathol. 2021;31:e12918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 17. | Dermawan JK, Vanoli F, Herviou L, Sung YS, Zhang L, Singer S, Tap WD, Benayed R, Bale TA, Benhamida JK, Dickson BC, Antonescu CR. Comprehensive genomic profiling of EWSR1/FUS::CREB translocation-associated tumors uncovers prognostically significant recurrent genetic alterations and methylation-transcriptional correlates. Mod Pathol. 2022;35:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Trecourt A, Macagno N, Ngo C, Philip CA, Lopez J, Ferreira J, Alves-Vale C, Ray-Coquard I, Genestie C, Agaimy A, Devouassoux-Shisheboran M. CREB fusion-associated epithelioid mesenchymal neoplasms of the female adnexa: three cases documenting a novel location of an emerging entity and further highlighting an ambiguous misleading immunophenotype. Virchows Arch. 2023;482:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 19. | Colangeli M, Rimondi E, Spinnato P, Donati DM, Manfrini M. Difficult diagnosis of Angiomatoid Fibrous Histiocytoma of the leg mimicking a benign condition. J Radiol Case Rep. 2019;13:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Kleinschmidt-DeMasters BK, Gilani A. Some CNS sarcomas seen: A 22-year series. Clin Neuropathol. 2023;42:54-65. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Saito K, Kobayashi E, Yoshida A, Araki Y, Kubota D, Tanzawa Y, Kawai A, Yanagawa T, Takagishi K, Chuman H. Angiomatoid fibrous histiocytoma: a series of seven cases including genetically confirmed aggressive cases and a literature review. BMC Musculoskelet Disord. 2017;18:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Martinez SJ, Moreno CC, Vinson EN, Dodd LG, Brigman BE. Angiomatoid fibrous histiocytoma: novel MR imaging findings. Skeletal Radiol. 2016;45:661-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |