Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6231
Peer-review started: May 30, 2023
First decision: July 17, 2023
Revised: July 30, 2023
Accepted: August 18, 2023
Article in press: August 18, 2023
Published online: September 16, 2023
Processing time: 100 Days and 20.3 Hours
Hepatocellular carcinoma (HCC) is one of the most frequent cancers and the main cause of cancer-related death worldwide. Ectopic HCC, an extremely rare type of HCC, exhibits a wide range of clinical signs and radiographic features, making preoperative identification challenging.
A 47-year-old man underwent routine abdominal color ultrasonography, which identified an asymptomatic tumor in the left upper abdomen. The patient had no history of hepatitis, did not drink alcohol, and had no family history of cancer. Abdominal contrast-enhanced computed tomography (CT) revealed a heterogeneously enhanced lesion between the spleen and stomach that had invaded the diaphragm, with blood supplied by the left inferior phrenic artery. The patient underwent laparoscopic surgery, and HCC was identified by postoperative pathology. Additionally, specific immunohistochemical staining was performed to assess the molecular biological characteristics of the HCC. The patient underwent two rounds of hepatic arterial interventional chemotherapy after surgery. Abdominal plain and enhanced magnetic resonance imaging and lung CT 3 mo postoperatively revealed no signs of local recurrence or distant metastasis.
This asymptomatic ectopic HCC case described achieved an excellent result due to early detection, radical resection, and systematic surveillance.
Core Tip: Ectopic hepatocellular carcinoma (HCC) is relatively rare, and there is no acknowledged diagnostic or treatment standard for this type of tumor. Here, we describe a 47-year-old man with diaphragmatic invasion caused by a heterogeneously enhanced lesion between his spleen and stomach that was eventually diagnosed as HCC. We thoroughly describe the patient’s information, course of treatment, and excellent curative result.
- Citation: Liu HB, Zhao LH, Zhang YJ, Li ZF, Li L, Huang QP. Left epigastric isolated tumor fed by the inferior phrenic artery diagnosed as ectopic hepatocellular carcinoma: A case report. World J Clin Cases 2023; 11(26): 6231-6239
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6231.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6231
Hepatocellular carcinoma (HCC) is one of the most prevalent cancers and a major contributor to the global mortality rate due to cancer[1]. HCC has a dismal prognosis, with a relative 5-year survival rate of only approximately 18%[2]. Ectopic HCC is an entity of HCC that has no connection to the mother liver and is an extremely rare condition and poses a significant diagnostic challenge before surgery. Ectopic liver tissue, which has an incidence of 0.23% to 0.7%, is hypothesized to be the main source of ectopic HCC[3]. We describe a man with an isolated mass in his left upper abdomen that ultimately proved to be HCC.
A 47-year-old male patient was referred to our hospital due for a routine medical examination more than 1 mo ago, which revealed an asymptomatic tumor in the left upper abdomen.
The asymptomatic tumor was suspected to be a gastrointestinal stromal tumor during routine abdominal color ultrasonography.
The patient had no medical history, no history of viral hepatitis, and no history of smoking or consuming alcohol.
There was no history of malignancy in the family.
Physical examination revealed no obvious mass or tenderness. Bowel sounds were within an acceptable range, while rectal palpation and ascites examinations were negative.
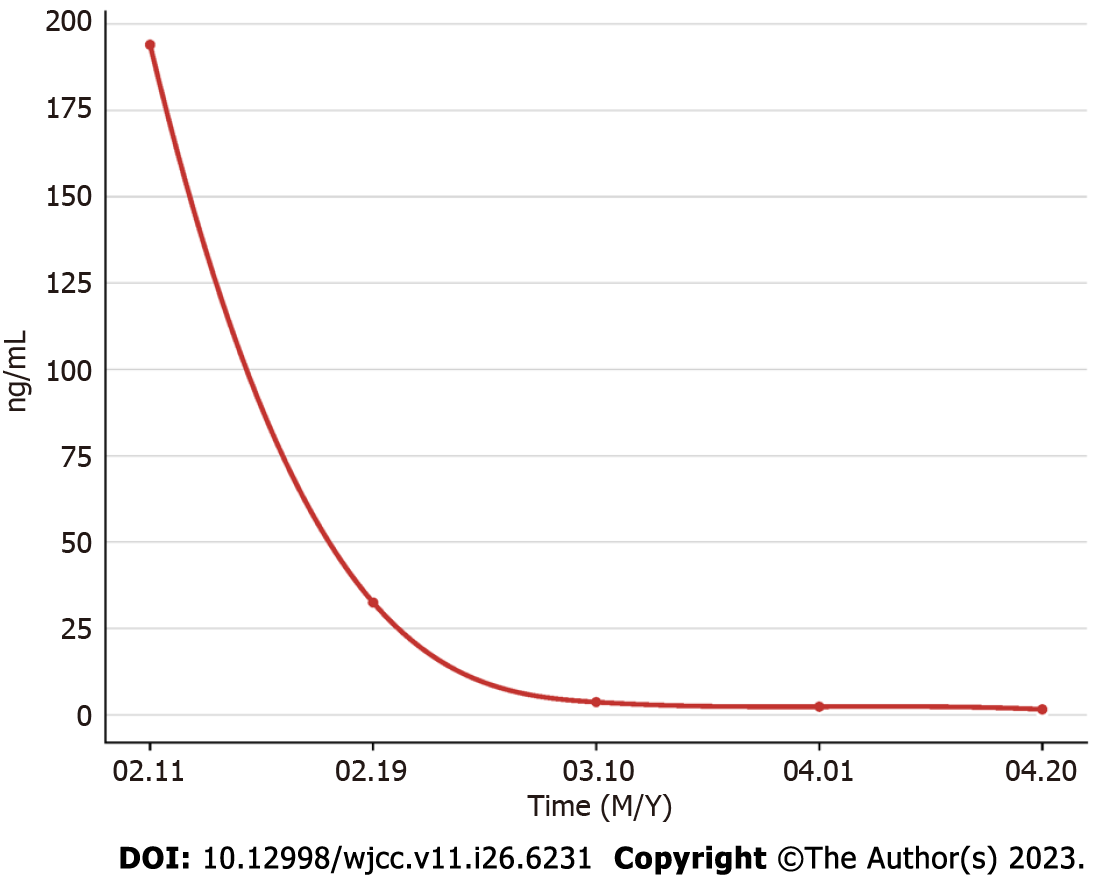
On laboratory examination, the only specific abnormality was an elevated α-fetoprotein (AFP) concentration (194.38 ng/mL). The results of other laboratory tests, namely hemoglobin, gamma glutamyl transferase, alkaline phosphatase, anti-hepatitis C virus, hepatitis B virus (HBV) surface antigen, carcinoembryonic antigen, and cancer antigen 19–9, were within normal limits.
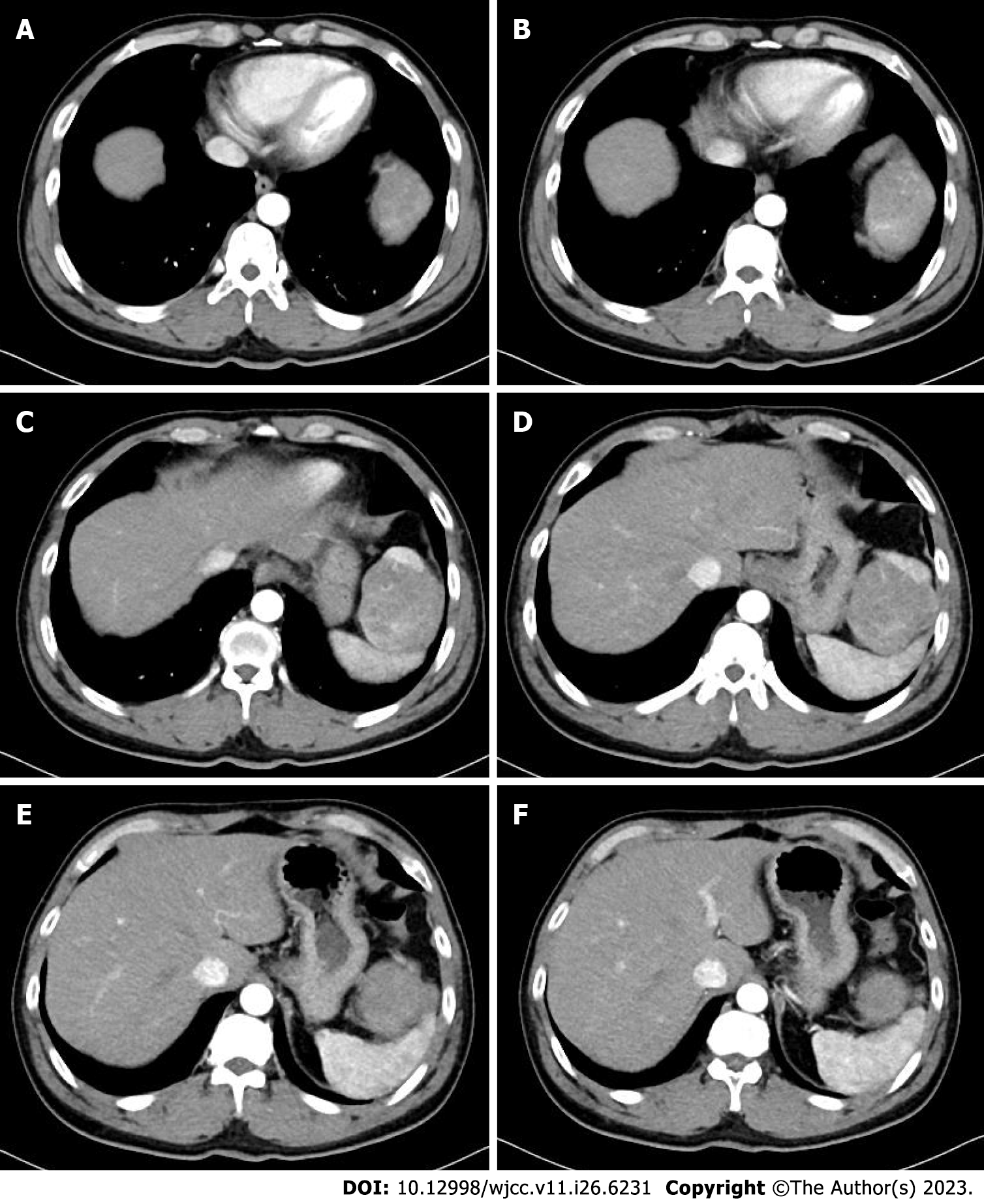
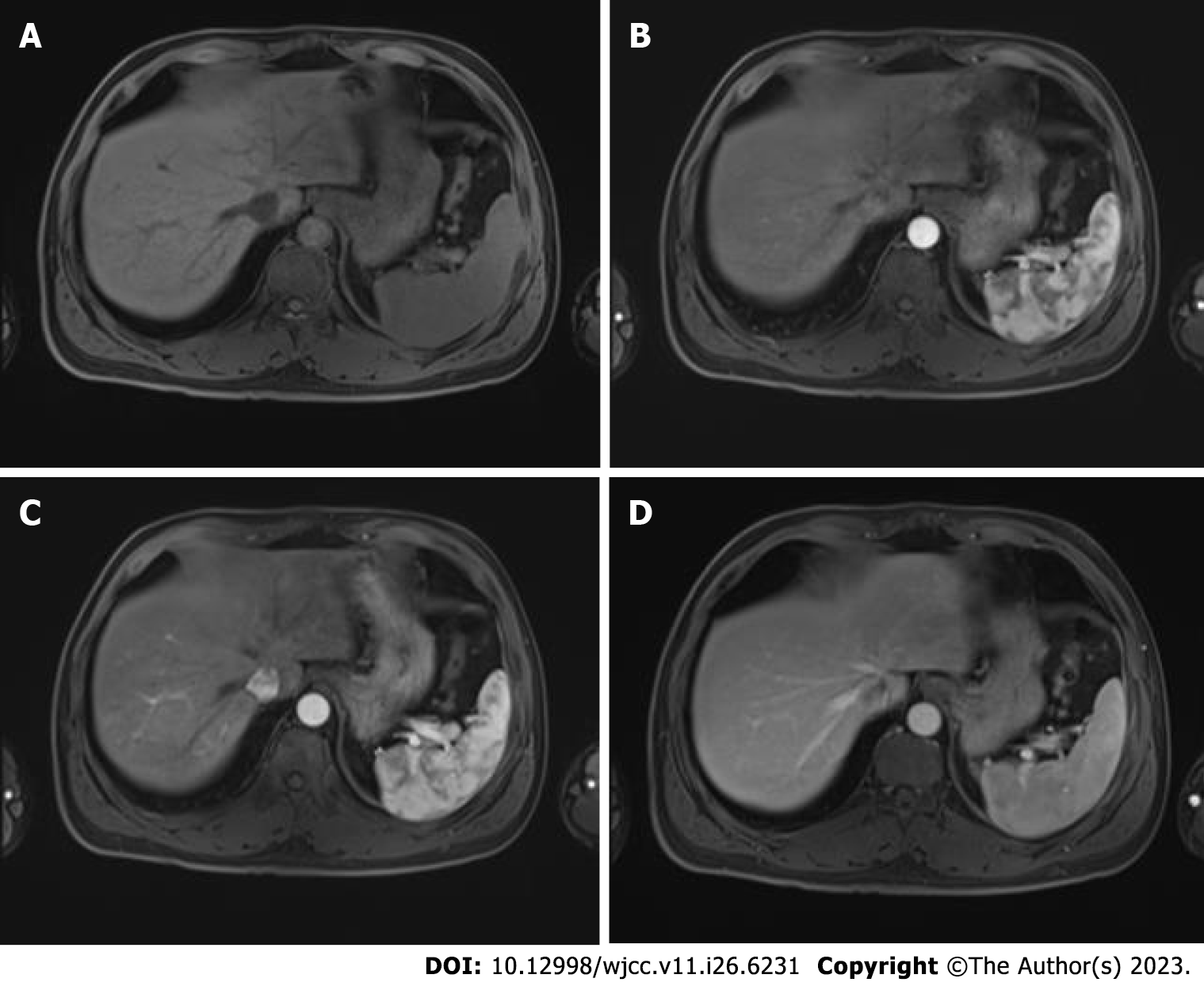
Abdominal ultrasonography revealed a heterogeneous mass measuring approximately 6 cm × 9 cm between the spleen and stomach. Contrast-enhanced computed tomography (CT) showed that the shape and size of the liver were normal, the edges were smooth, the proportion of each lobe was balanced, and no obvious abnormal density shadows were identifiable in the liver parenchyma. A soft tissue mass was observed in the splenic and gastric spaces, with a CT value of 48 HU and size of 6.3 cm × 9.3 cm. Enhanced CT showed uneven enhancement (Figure 1). CT values during the arterial, portal, and extended stages were 71-125 HU, 82-112 HU, and 72-85 HU, respectively (Supplementary Figure 1). The tumor feeding artery originated from the left inferior phrenic artery.
An ectopic HCC with diaphragmatic involvement was eventually diagnosed.
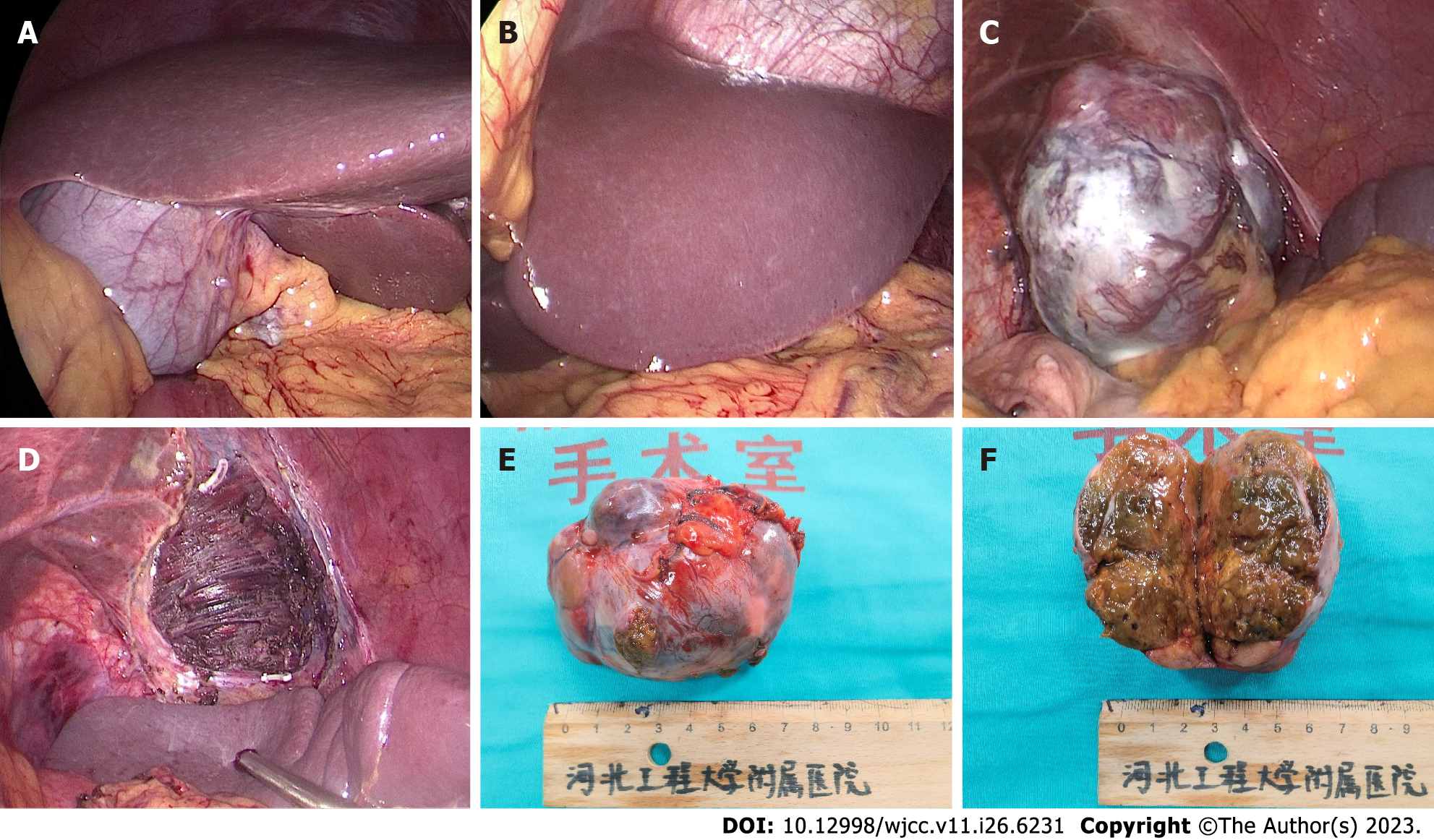
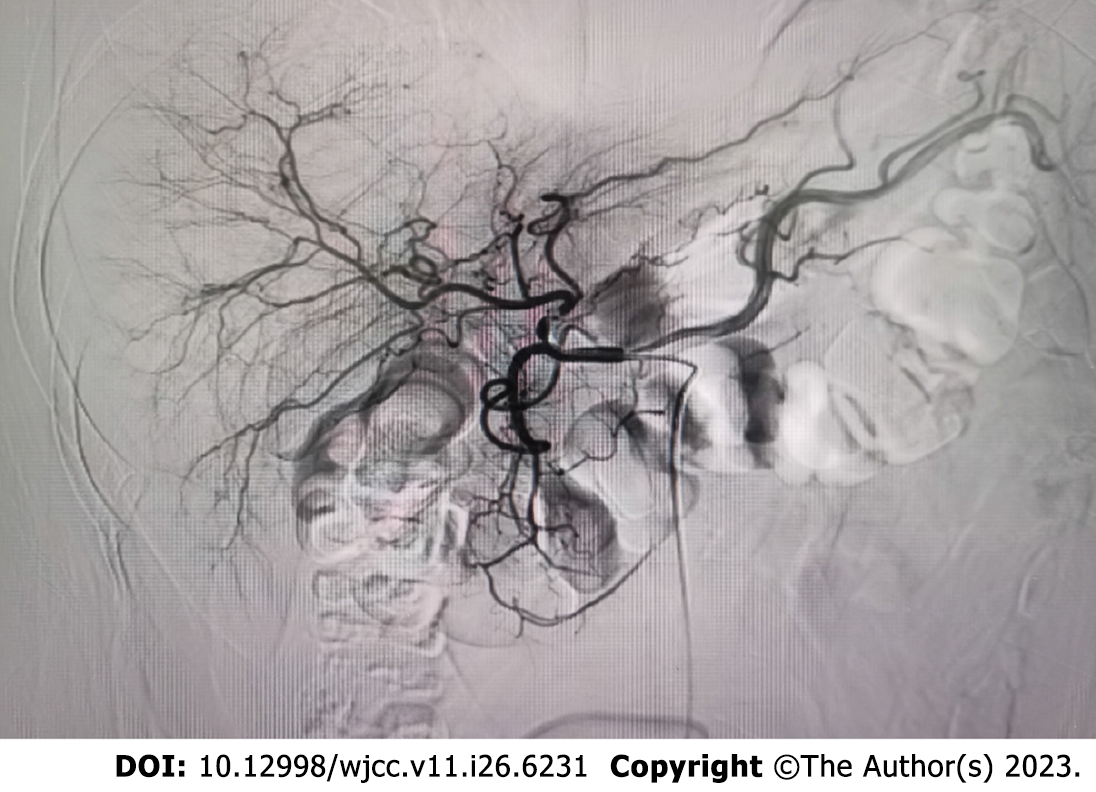
The tumor was located in the left subdiaphragm, which was a challenge to access with open surgical techniques. Therefore, we performed laparoscopic surgery. During the operation, no ascites was found, and the liver was normal in size, color, and consistency. A tumor with an intact envelope was found between the greater curvature of the stomach and the spleen. It was not related to the hepatic parenchyma and had invaded the diaphragm (Figure 2). We removed part of the diaphragm to ensure that the tumor was entirely resected as the tumor had direct diaphragmatic involvement. Preventive hepatic arterial infusion chemotherapy (HAIC) was administered to the patient twice, 1 mo apart. The potential for intrahepatic microneoplasms, such as micrometastases or a microprimary tumor with metastasis to the left upper abdomen, was ruled out by hepatic arteriography (Figure 3).
Regular monitoring and follow-up were performed after surgery as advised during a multidisciplinary team meeting that examined AFP (Figure 4), lung and skull CT scan, and liver magnetic resonance imaging (Figure 5).
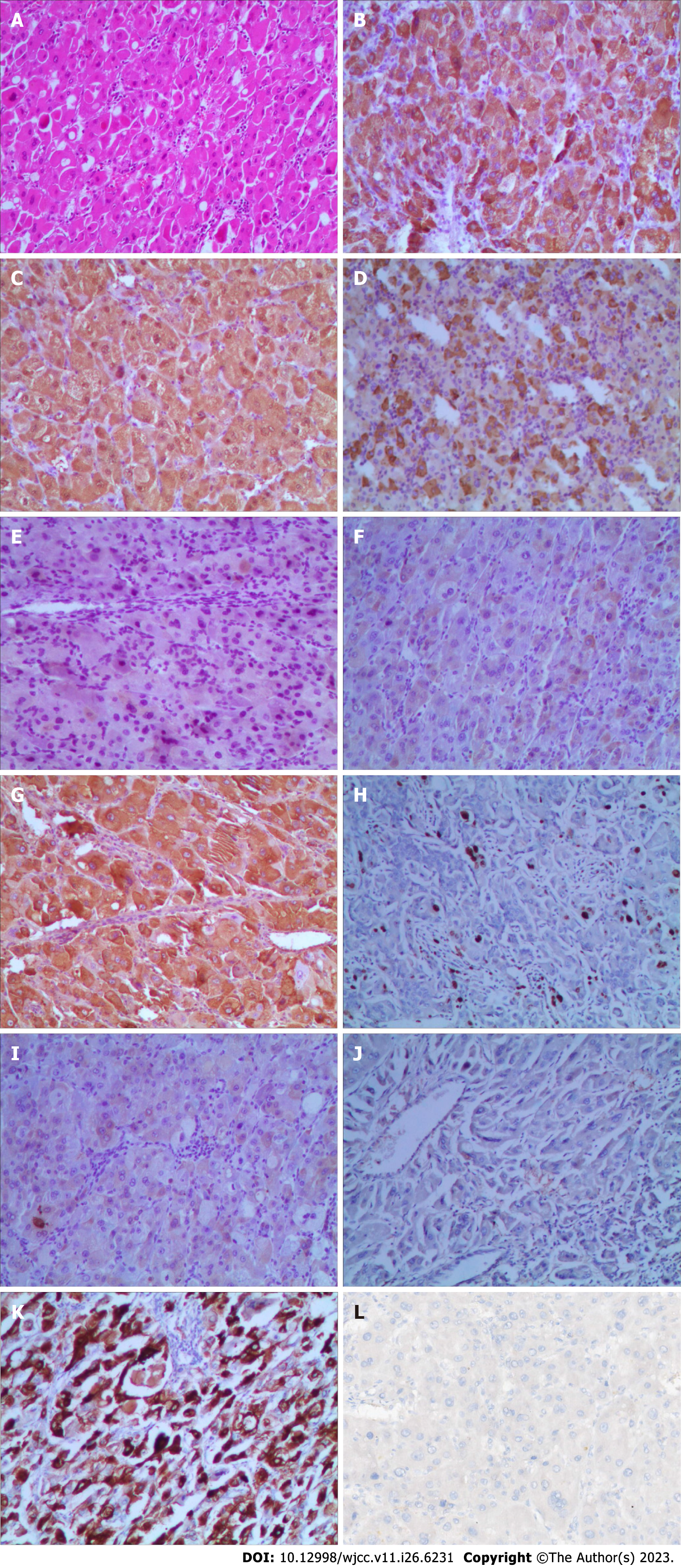
Hematoxylin and eosin staining, and immunohistochemistry were used to thoroughly assess the tumor type and its immunological markers (Figure 6). Capsule formation is related to better tumor cell differentiation and tumor biological characteristics compared with no capsule formation, and the pathology results of this patient suggested a well-to-moderately differentiated adenocarcinoma. Immunohistochemical study revealed the following: Hepatocyte antigen (+), arginase-1 (+), AFP (+), inhibin-α (−), cytokeratin (CK) (+/−), GPC-3 (+), Ki-67 (hot regions: 5%), CK19 (−), CK8/18 (−), and hepatocyte paraffin-1 (+). To determine whether the patient could benefit from immunotherapy, we also performed programmed death-ligand 1 testing. However, the results were negative. Consequently, intensive postoperative immunotherapy was not administered.
HCC is a common malignancy of the digestive system with high morbidity and mortality worldwide. However, only 27 cases of ectopic HCC have been reported as of 2022[4]. The risk factors for ectopic HCC are unrelated to those for typical HCC (such as chronic viral hepatitis B and C; alcohol abuse or alcoholic steatohepatitis; and nonalcoholic fatty liver disease or nonalcoholic steatohepatitis). Ectopic HCC may be caused by problems with biliary drainage, insufficient arterial blood flow, and insufficient venous drainage[3,5]. Prolonged exposure to these carcinogenic factors promotes carcinogenesis. In the present case, the patient had no substantial coexisting conditions or HCC risk factors, and the mother liver was not cirrhotic. The most common extra-hepatic feeding artery of HCC was found to be the inferior phrenic artery (IPA)[6]. Our patient presented with an isolated mass in the left upper abdomen, invaded the diaphragm and fed by the left IPA.
Owing to the distinct features of ectopic HCC, patients present with a wide spectrum of clinical signs and radiological presentations, which makes it challenging to obtain a preoperative diagnosis[7]. In the case described herein, the isolated tumor with no connection to the liver and supplied by diaphragmatic arteries, made preoperative diagnosis challenging. Anatomically, part of ectopic HCC shows a significantly better overall survival than that for HCC in the orthotopic liver due to encapsulation and the possibility of wide resection margins with less vascular invasion[8]. Our patient had well-to-moderately differentiated histological characteristics, with a low Ki-67 labeling index, which may indicate a highly favorable overall survival compared with other histological subtypes.
Some HCCs can attach to and potentially infiltrate nearby tissues, such as the diaphragm, which presents therapeutic difficulty. During the preoperative examination, whether the diaphragm is actually invaded by HCC is difficult to identify; therefore, a postoperative pathological diagnosis or intraoperative frozen pathology is necessary to evaluate whether the diaphragm has been invaded or is just showing diaphragmatic fibrous adhesion[9]. As there is a risk of tumor rupture, tumor spread, and bleeding when the diaphragm is separated from a tumor that has gross diaphragmatic involvement, the grossly involved section of the diaphragm must be excised to ensure radical cure[10]. However, whether diaphragmatic excision is necessary and beneficial for individuals with subclinical adhesion and a smooth, intact tumor capsule is debatable. In the present case, part of the diaphragm was resected to ensure radical resection without postoperative complications. However, extrapolating general implications from a single instance might be challenging.
Laparoscopic techniques are widely used and well recognized by the majority of liver surgeons owing to reduced patient stress and rapid postoperative recovery[11]. However, the use of these techniques in HCC with diaphragmatic involvement has not been widely reported[12]. We have found that this approach is reliable and efficient, helps reduce surgical complexity and operation time, and enhances surgical safety; however, high-quality clinical trials are required.
Surgery is the first option for ectopic HCC patients who can be cured. However, some ectopic HCC patients may experience a recurrence following surgery. Liu et al[4] reported that four of the six ectopic HCC recurrence cases were in the mother's liver. It is advised that HAIC was a potential option for preventing early recurrence even though no lesion was seen in the mother liver during the initial therapy[4,13]. Despite the lack of a sufficient and significant amount of high-quality research assistance, we conducted prophylactic HAIC twice with the full knowledge and strong request of the patient and his family due to the high expectations of the patient.
With advanced-stage HCC, comprehensive postoperative care is required, including preventive transcatheter arterial chemoembolization, anti-HBV therapy, immunotherapy, and targeted therapy. Close, frequent monitoring and follow-up are also necessary following surgery, as with resection or radiofrequency ablation of recurring foci[5]. In the present case, we performed two sessions of prophylactic HAIC. To date, there has been no evidence of recurrence.
We report a patient with an isolated tumor between the spleen and the stomach that was diagnosed as ectopic HCC. Excellent curative results were obtained following treatment.
We appreciate the patient’s informed consent so that some of the photographs might be used in the publication of this report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Boteon YL, Brazil; Sudhamshu K, Nepal; Wani I, India S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11436] [Article Influence: 3812.0] [Reference Citation Analysis (4)] |
| 3. | Martinez CA, de Resende HC Jr, Rodrigues MR, Sato DT, Brunialti CV, Palma RT. Gallbladder-associated ectopic liver: A rare finding during a laparoscopic cholecystectomy. Int J Surg Case Rep. 2013;4:312-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Liu Q, Li J, Pan Y, Zheng X, Gao B. Challenge in Diagnosis and Treatment of Ectopic Hepatocellular Carcinoma: A Case Report and Literature Review. Front Surg. 2022;9:827006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1233] [Article Influence: 411.0] [Reference Citation Analysis (41)] |
| 6. | Kim HC, Miyayama S, Choi JW, Kim GM, Chung JW. Hepatocellular Carcinoma Supplied by the Inferior Phrenic Artery or Cystic Artery: Anatomic and Technical Considerations. Radiographics. 2023;43:e220076. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Adachi Y, Hayashi H, Yusa T, Takematsu T, Matsumura K, Higashi T, Yamamura K, Yamao T, Imai K, Yamashita YI, Baba H. Ectopic hepatocellular carcinoma mimicking a retroperitoneal tumor: A case report. World J Gastroenterol. 2020;26:2268-2275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Yang Y, Lu Q, Li Z, Wang C, Li Y. A large ectopic hepatocellular carcinoma with adrenal infiltration: a rare case report. Front Oncol. 2023;13:1116684. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Wu Q, Zhang Z, Dong H, Mei B. Combined resection for hepatocellular carcinoma with diaphragmatic invasion: a systematic review and meta-analysis. Minerva Med. 2020;111:354-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Liu YC, Mao YZ, Wang JC, Wang J, Lao XM, Chen MS, Li SP. Hepatocellular carcinoma with en bloc diaphragmatic resection: A single-center experience over 14 years. Int J Surg. 2018;53:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Mohammed MM, Basset HSA, Elsayed MAA, Hegazi AAB. Assessment of Safety and Applicability of Laparoscopic vs Open Non-Anatomical Resection of Hepatocellular Carcinoma in Cirrhotic Patients. QJM-INT J MED. 2021;114 Supple1. [DOI] [Full Text] |
| 12. | You N, Wang Z, Wu K, Wang L, Li J, Zheng L. Laparoscopic surgical management of hepatocellular carcinoma patients with diaphragmatic involvement. Surg Endosc. 2023;37:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Singh V, Sinha RJ, Sankhwar SN, Kumar S, Mehrotra B, Puri M, Sengottayan VK. Primary hepatocellular carcinoma in ectopic liver masquerading as left adrenal carcinoma: a rare occurrence. Rare Tumors. 2010;2:e35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |