Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6200
Peer-review started: May 25, 2023
First decision: June 15, 2023
Revised: July 24, 2023
Accepted: August 18, 2023
Article in press: August 18, 2023
Published online: September 16, 2023
Processing time: 106 Days and 5 Hours
Mixed-phenotype acute leukemia (MPAL) is characterized by acute undifferentiated leukemia with blasts co-expressing myeloid and lymphoid antigens. However, consensus regarding the ideal management strategy for MPAL is yet to be established, owing to its rarity.
A 55-year-old male was diagnosed with T/myeloid MPAL. Vincristine, pred
CR was maintained in a patient with MPAL who underwent haploidentical peripheral blood stem-cell transplantation after additional venetoclax/decitabine cycles.
Core Tip: We report a 55-year-old male diagnosed with T/myeloid mixed-phenotype acute leukemia (MPAL) who received induction chemotherapy with vincristine, prednisolone, daunorubicin, and L-asparaginase. The patient experienced septic shock 10 days post-induction therapy and received antibiotic therapy to treat extended-spectrum beta-lactamase-positive bacteremia. Bone marrow examination post-sepsis recovery revealed refractory disease. To reduce the infection risk, venetoclax and decitabine were administered as chemotherapy-free induction therapy. After two additional venetoclax/decitabine cycles, the patient underwent haploidentical peripheral blood stem-cell transplantation, subsequently achieving complete response. This is the third report documenting the successful treatment of refractory T/myeloid MPAL with venetoclax and a hypomethylating agent.
- Citation: Park S, Jeong EJ, Kang JH, Lee GW, Go SI, Lee DH, Koh EH. T/myeloid mixed-phenotype acute leukemia treated with venetoclax and decitabine: A case report. World J Clin Cases 2023; 11(26): 6200-6205
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6200.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6200
The term "mixed-phenotype acute leukemia" (MPAL) refers to a heterogeneous group of uncommon acute leukemias accounting for ≤ 5% of all acute leukemias, characterized by co-expression of myeloid and lymphoid antigens on the same blasts or by the presence of two separate cell populations expressing various lineage traits[1]. In 2022, the World Health Organization (WHO) recognized T/myeloid MPAL as one of five MPAL subtypes of acute leukemia of uncertain lineage[2]. The prognosis and consequences of MPAL are worse than those of standard-risk acute myeloid leukemia (AML) or acute lymphocytic leukemia (ALL)[1]. The prognosis is exceedingly dismal for patients with relapsed/refractory (R/R) AML who are unsuitable candidates for intensive chemotherapy regimens or allogeneic hematopoietic stem-cell transplantation (allo-HSCT).
Given the rarity of MPAL, an ideal strategy for disease management is yet to be established. AML- and ALL-based regimens should be prospectively validated, given the inevitable bias inherent in retrospective studies and small case series. Currently, an ALL-like induction protocol is recommended, then allogeneic stem cell transplantation (allo-SCT) is performed after the initial complete remission. Moreover, the treatment strategy has not been well established since introducing the concept of MPAL. Salvage treatment for relapsed/refractory (R/R) acute leukemias of ambiguous lineage, not otherwise specified (ALAL-NOS), remains a major concern. Currently, allo-HSCT is the only treatment strategy for ALAL-NOS; however, the prognosis remains poor if complete response (CR) is not achieved before allo-HSCT[3]. Chemotherapy is ineffective for ALAL-NOS. Therefore, salvage treatments are required to enhance the CR before allo-HSCT.
To the best of our knowledge, the current report is the third instance of successful treatment with venetoclax and a hypomethylating agent (HMA) in a patient with refractory T/myeloid MPAL. Future studies should examine the effectiveness of this approach.
A 55-year-old male patient with dizziness was admitted to the hospital.
Beginning one week prior, the patient developed dizziness and difficulty breathing when climbing stairs.
He had a history of hypertension and epilepsy and was taking levetiracetam.
No specific findings were recorded.
There were no specific findings on palpation of the spleen, the face appeared markedly pale, and there were no bruises on the skin.
Neutropenia (1.12 × 103/mm3), anemia (4.0 g/dL), and thrombocytopenia (12 × 103/mm3) were detected in peripheral blood. Figure 1 describes the findings of the bone marrow examination. Flow cytometry showed the expression of cMPO+, CD 13+, CD33+, CD64+, CD117+, CD34+, and high cytoplasmic CD3. Chromosomal karyotyping revealed a 48, XY, add(11)(q23), add(12)(p13).-20,-22,+4mar karyotype. Fluorescence in situ hybridization revealed KMT2A positivity. Next-generation sequencing results were negative for the BCR-ABL gene or other mutations.
No specific findings were observed on the chest X-ray.
A diagnosis of T/myeloid MPAL was confirmed by bone marrow aspiration, which was suggestive of acute leukemia.
Vincristine, prednisolone, daunorubicin, and L-asparaginase (VPDL) were administered as induction therapy. Ten days after initiating induction chemotherapy, the patient developed septic shock with hypotension (blood pressure 80/40 mmHg) and high fever (39.2°C). Effective antibiotics (colistin, meropenem, and ciprofloxacin) were initiated after a blood culture-confirmed bacteremia caused by extended-spectrum beta-lactamase-positive Klebsiella pneumoniae. Bone marrow examination after recovery from sepsis revealed refractory disease, and flow cytometry for minimal residual disease detected the presence of blasts (expressing cCD3+, CD33+, and CD34+). To reduce the risk of infection, venetoclax (Day 1: 10 mg; Day 2: 20 mg; Day 3: 30 mg; Day 4-28: 100 mg), posaconazole, and decitabine (20 mg/m2, mixed with normal saline, 150 mL for 5 d) were administered as chemotherapy-free induction therapy. Thereafter, no serious infection, such as febrile neutropenia, occurred. The patient underwent haploidentical peripheral blood cell transplantation after two additional cycles of venetoclax/decitabine.
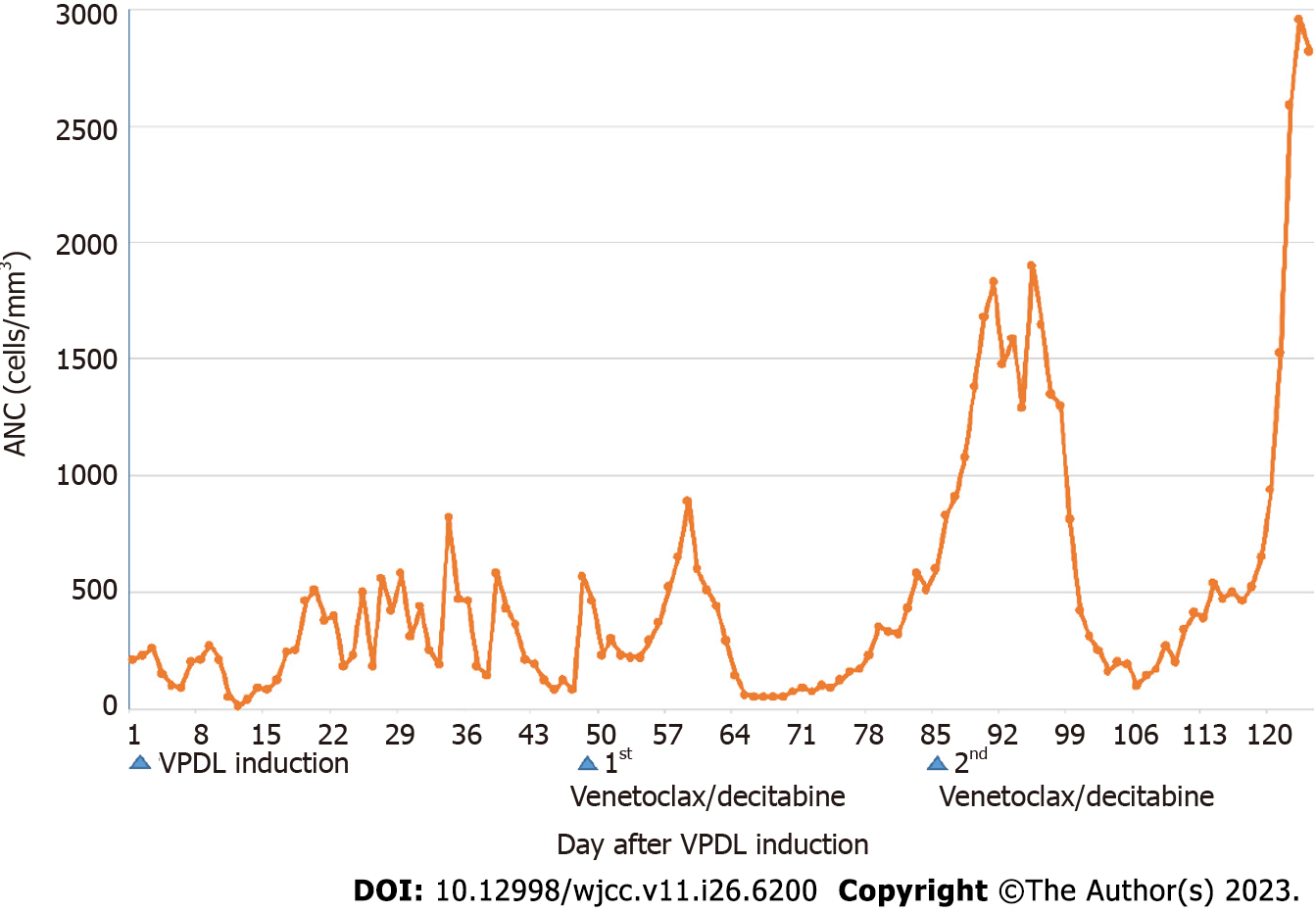
The patient achieved CR one year post-HSCT. Figure 2 summarizes the absolute neutrophil counts from initiating the VPDL regimen to the end of the second venetoclax + decitabine cycle. Neutropenia resolved early during the second cycle. The ANC was less than 500 for two weeks and did not decline to less than 100.
Considering patients with MPAL, the three common treatment modalities include ALL-directed, AML-directed, and hybrid strategies (ALL plus AML). Based on retrospective data, experts favor ALL-based chemotherapy over AML-based chemotherapy owing to its superior remission rates[4,5]. However, accumulated data are retrospective, and validation in multicenter, prospective clinical trials is pending. Additionally, adverse effects associated with conventional treatment modalities, such as deadly infections and bleeding, present a persistent challenge to the survival of patients with MPAL[6]. A recent meta-analysis of 1300 patients found that patients who received an induction regimen with ALL-directed therapy were significantly more likely to achieve CR and were twice as likely to survive than patients who received an AML-directed regimen. The study included case reports of patients with MPAL (WHO) or biphenotypic leukemia (European Group for the Immunological Classification of Leukemias) as well as small international series and case reports. A multivariate examination of comprehensive clinical data, including the type of therapy, MPAL subgroup, and patient age, revealed that the overall survival (OS) rates between ALL and AML induction regimens did not differ (3-year OS, 48, 6.9% vs 47, 5.0%, respectively).
Hematopoietic stem cell transplantation has been shown to substantially increase the 5-year survival rate in patients with MPAL[7], and bridging therapy should ideally be associated with less toxicity and complications. Patients' survival was demonstrated to be considerably decreased by a combination regimen (a mix of ALL and AML therapy) (3-year OS, 23% 8.6%, P = 0.001), probably because of higher toxicity[4].
In November 2018, the Food and Drug Administration approved the administration of venetoclax along with an HMA in older or more vulnerable patients with recently diagnosed AML[8]. Venetoclax, a powerful oral BCL-2 inhibitor, has shown clinical success in treating various hematologic cancers[9]. In hematologic malignancies, HMAs act by inhibiting deoxyribonucleic acid (DNA) methyltransferase-1, which results in DNA demethylation, cell differentiation, or apoptosis[10]. The pathogenesis of myeloid and lymphoid malignancies is facilitated by aberrant DNA methylation, which has been linked to the possibility that hematopoietic stem cells in MPAL undergo aberrant transformation[11]. According to recent real-world evidence, decitabine with venetoclax can yield superior outcomes to decitabine monotherapy in patients with AML[12].
Moreover, several clinical studies have indicated the potential feasibility of decitabine in B-cell ALL and T-cell ALL, especially R/R ALL, although data in this field remain limited[13-17]. Some case reports have indicated the efficacy of venetoclax in patients with MPAL[18]. The characteristics of T/myeloid MPAL are distinct from those of B/myeloid MPAL, another type of MPAL. A case of T/myeloid MPAL relapse following effective treatment of allo-HSCT with venetoclax + decitabine[19]. Given the challenges in conducting clinical trials targeting only the T/myeloid MPAL patient population, we present the third case report of a patient with T/myeloid MPAL successfully treated with venetoclax and an HMA.
Herein, we present a patient with MPAL who experienced serious complications with standard chemotherapy. Subsequently, the patient received combination therapy with venetoclax and HMA and underwent hematopoietic stem cell transplantation. Therefore, it is necessary to establish whether combination therapy with venetoclax and HMA could be beneficial as bridging therapy pre-transplantation. Accordingly, future investigations should compare the potential of venetoclax and HMA with traditional chemotherapy in patients who can eventually undergo transplantation.
The authors gratefully acknowledge the patient and his family for their cooperation and kind help.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elpek GO, Turkey; Gica N, Romania S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Shi R, Munker R. Survival of patients with mixed phenotype acute leukemias: A large population-based study. Leuk Res. 2015;39:606-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, Chen W, Chen X, Chng WJ, Choi JK, Colmenero I, Coupland SE, Cross NCP, De Jong D, Elghetany MT, Takahashi E, Emile JF, Ferry J, Fogelstrand L, Fontenay M, Germing U, Gujral S, Haferlach T, Harrison C, Hodge JC, Hu S, Jansen JH, Kanagal-Shamanna R, Kantarjian HM, Kratz CP, Li XQ, Lim MS, Loeb K, Loghavi S, Marcogliese A, Meshinchi S, Michaels P, Naresh KN, Natkunam Y, Nejati R, Ott G, Padron E, Patel KP, Patkar N, Picarsic J, Platzbecker U, Roberts I, Schuh A, Sewell W, Siebert R, Tembhare P, Tyner J, Verstovsek S, Wang W, Wood B, Xiao W, Yeung C, Hochhaus A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36:1703-1719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 2155] [Article Influence: 718.3] [Reference Citation Analysis (0)] |
| 3. | Shimizu H, Saitoh T, Machida S, Kako S, Doki N, Mori T, Sakura T, Kanda Y, Kanamori H, Miyawaki S, Okamoto S; Kanto Study Group for Cell Therapy (KSGCT). Allogeneic hematopoietic stem cell transplantation for adult patients with mixed phenotype acute leukemia: results of a matched-pair analysis. Eur J Haematol. 2015;95:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Maruffi M, Sposto R, Oberley MJ, Kysh L, Orgel E. Therapy for children and adults with mixed phenotype acute leukemia: a systematic review and meta-analysis. Leukemia. 2018;32:1515-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Wolach O, Stone RM. Optimal therapeutic strategies for mixed phenotype acute leukemia. Curr Opin Hematol. 2020;27:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Xu XQ, Wang JM, Lü SQ, Chen L, Yang JM, Zhang WP, Song XM, Hou J, Ni X, Qiu HY. Clinical and biological characteristics of adult biphenotypic acute leukemia in comparison with that of acute myeloid leukemia and acute lymphoblastic leukemia: a case series of a Chinese population. Haematologica. 2009;94:919-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Munker R, Brazauskas R, Wang HL, de Lima M, Khoury HJ, Gale RP, Maziarz RT, Sandmaier BM, Weisdorf D, Saber W; Center for International Blood and Marrow Transplant Research. Allogeneic Hematopoietic Cell Transplantation for Patients with Mixed Phenotype Acute Leukemia. Biol Blood Marrow Transplant. 2016;22:1024-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, Arellano M, Frattini MG, Kantarjian H, Popovic R, Chyla B, Xu T, Dunbar M, Agarwal SK, Humerickhouse R, Mabry M, Potluri J, Konopleva M, Pollyea DA. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 560] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 9. | Eyre TA, Kirkwood AA, Gohill S, Follows G, Walewska R, Walter H, Cross M, Forconi F, Shah N, Chasty R, Hart A, Broom A, Marr H, Patten PEM, Dann A, Arumainathan A, Munir T, Shankara P, Bloor A, Johnston R, Orchard K, Schuh AH, Fox CP; the UK CLL Forum. Efficacy of venetoclax monotherapy in patients with relapsed chronic lymphocytic leukaemia in the post-BCR inhibitor setting: a UK wide analysis. Br J Haematol. 2019;185:656-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Das M. Venetoclax with decitabine or azacitidine for AML. Lancet Oncol. 2018;19:e672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Eckstein OS, Wang L, Punia JN, Kornblau SM, Andreef M, Wheeler DA, Goodell MA. Mixed phenotype acute leukemia (MPAL) has a high frequency of mutations in epigenetic regulatory genes: results from whole exome sequencing. Blood. 2014;124:3560. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Kwag D, Cho BS, Bang SY, Lee JH, Min GJ, Park SS, Park S, Yoon JH, Lee SE, Eom KS, Kim YJ, Lee S, Min CK, Cho SG, Lee JW, Kim HJ. Venetoclax with decitabine vs decitabine monotherapy in elderly acute myeloid leukemia: a propensity score-matched analysis. Blood Cancer J. 2022;12:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 13. | Paulson K, Kumar R, Ahsanuddin A, Seftel MD. Azacytidine as a novel agent in the treatment of acute lymphoblastic leukemia. Leuk Lymphoma. 2011;52:134-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Cui JK, Xiao Y, You Y, Shi W, Li Q, Luo Y, Jiang L, Zhong ZD. Decitabine for relapsed acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation. J Huazhong Univ Sci Technolog Med Sci. 2017;37:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Burke MJ, Lamba JK, Pounds S, Cao X, Ghodke-Puranik Y, Lindgren BR, Weigel BJ, Verneris MR, Miller JS. A therapeutic trial of decitabine and vorinostat in combination with chemotherapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2014;89:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Benton CB, Thomas DA, Yang H, Ravandi F, Rytting M, O'Brien S, Franklin AR, Borthakur G, Dara S, Kwari M, Pierce SR, Jabbour E, Kantarjian H, Garcia-Manero G. Safety and clinical activity of 5-aza-2'-deoxycytidine (decitabine) with or without Hyper-CVAD in relapsed/refractory acute lymphocytic leukaemia. Br J Haematol. 2014;167:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Garcia-Manero G, Thomas DA, Rytting ME, O’Brien S, Franklin ARK, Borthakur G, Kwari M, Dara S, Yang H, Kantarjian H. Final report of a phase I trial of decitabine with or without hyperCVAD in relapsed acute lymphocytic leukemia (ALL). Blood. 2010;116:867. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Wu X, Zhang J, Chen Q, Zhou L, Li M, Qiu H, Sun A, Wu D. Efficacy of venetoclax in combination with azacitidine followed by haploidentical transplantation in refractory acute myeloid leukaemia and mixed phenotype acute leukaemia. Br J Haematol. 2020;189:e200-e204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Klocke H, Dong ZM, O'Brien C, Burwick N, Richard RE, Wu DY, Chauncey TR, Graf SA. Venetoclax and Decitabine for T/Myeloid Mixed-Phenotype Acute Leukemia Not Otherwise Specified (MPAL NOS). Case Rep Hematol. 2020;2020:8811673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |