Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6154
Peer-review started: March 14, 2023
First decision: April 11, 2023
Revised: May 4, 2023
Accepted: May 24, 2023
Article in press: May 24, 2023
Published online: September 16, 2023
Processing time: 177 Days and 10.7 Hours
Atopic dermatitis and asthma are two diseases whose pathogenesis is largely attributable to the activation, at least in the initial stages, of T helper (Th)-2 Lymphocytes, the related cytokine axis, and B lymphocytes with antibody production. Psoriasis is conversely a pathology resulting from a recruitment of Th-17 and Th-1 lymphocytes, after an initial role of innate immunity. Mepoli
We present the case of a 59-year-old patient who developed psoriasiform lesions on the palms after mepolizumab therapy for asthma, for the activation of the parallel cytokine cascade after the blockade of IL-5. We successfully treated the patient with a topical calcipotriol and betamethasone ointment.
We should investigate with further attention the possible impact on the human immunological ecosystem put in place by the inhibition of the activity of individual inflammatory mediators, so as to be able to recognize the initial adverse effects early.
Core Tip: We report the case of a 59-year-old patient with severe asthma who developed palmar psoriasis after 6 mo from the initiation of treatment with a humanized monoclonal antibody directed against interleukin-5 (IL-5), mepolizumab. There are several case reports of psoriasis induced by dupilumab therapy in the literature, but this phenomenon has not yet been recognized with modulating IL-5R signalling. This article reports the pathogenetic hypotheses that may underlie this phenomenon.
- Citation: Artosi F, Diluvio L, Vultaggio M, Campione E, Bianchi L. Mepolizumab induced palmoplantar psoriasis: A case report. World J Clin Cases 2023; 11(26): 6154-6158
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6154.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6154
Despite the lack of a precise overview of the cytokine ecosystem that governs human immune responses, numerous clues suggest that certain types of diseases are more associated with specific cytokine circuits[1]. Extrinsic asthma, atopic dermatitis, and chronic spontaneous urticaria are indeed based on the hyperactivation of the cytokine pathway associated with a predominantly T helper (Th)2-type or humoral response, as confirmed by the efficacy of drugs against inflammatory mediators, including interleukin (IL)-4, IL-5, IL-9, IL-13, IL-31, and thymic stromal lymphopoietin. Often, these conditions are associated with a large production of immunoglobulins E[2-5].
Psoriasis, on the other hand, is a chronic/relapsing inflammatory skin disease characterized, in an initial phase, by activation of innate immunity, followed by the subsequent involvement of the adaptive counterpart, without the involvement of humoral immunity[6]. Specifically, Th1, Th17, and Th22 lymphocytes are activated in response to the differentiation stimulus of the cytokines IL-12 and IL-23 secreted by the activated dermal dendritic cells. In this context, Th1 cells play an important role in producing interferon-γ and tumor necrosis factor-α, amplifying the inflammatory cascade and supplying proliferative stimuli to the keratinocytes. Th17 cells create a positive feedback circuit to increase the production of IL-17 by themselves promoting epidermal hyperplasia and neutrophil recruitment from blood to the psoriasis-affected derma and epidermis[6,7]. Psoriasis affects 2%-5% of the population, and 2.8%-40.9% of individuals with psoriasis have palmoplantar psoriasis (PPP), which is a variant of psoriasis that affects the palms and/or soles and can start at any age[8].
In the literature, there have been several reported cases of patients with atopic dermatitis and/or asthma who, once subjected to treatment with dupilumab, a monoclonal antibody that inhibits IL-4/IL-13 signaling, developed a skin picture of psoriasis for an immunological shift from a Th2/humoral to a Th1/cell-mediated pattern[9-11].
Mepolizumab is a humanized monoclonal antibody directed against IL-5, a central cytokine in the Th-2 axis, therefore involved in the pathogenesis of asthma, is approved by the Food and Drug Administration in 2015. IL-5, secreted by Th2 lymphocytes, has numerous effects on eosinophils, promoting their maturation, activation, survival, and chemotaxis from the bloodstream to the airways[12]. IL-5 acts to increase the likelihood of differentiation of conventional B cells to antibody-secreting plasma cells (ASCs) and synergizes with IL-4[13].
We report the case of a 59-year-old patient with a family history of psoriasis that developed severe psoriasiform lesions on the palms after 6 mo of mepolizumab therapy for severe asthma. We speculate that the appearance of PPP could be explained by a switch of the immune response from the prevalent antibody-mediated immunopathogenesis to a cell-mediated pathology after IL-5 blockage by mepolizumab, in a similar manner to what has occurred in patients after the use of dupilumab.
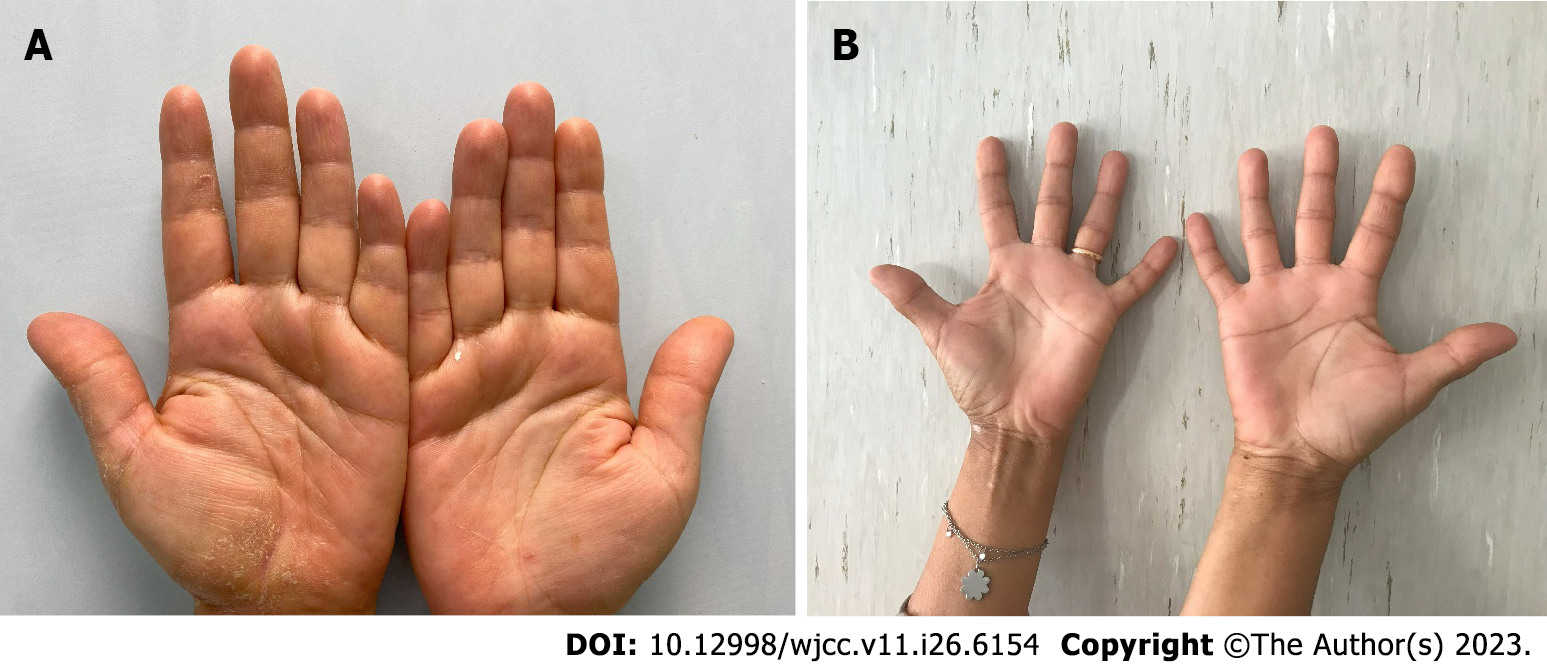
A 59-year-old woman came to the Allergy Dermatology Unit at the Tor Vergata Polyclinic for itchy, scaly erythematous lesions on palmar and plantar skin for 6 mo (Figure 1). She denied any previous exposure to irritating substances.
The patient had a past medical history of severe asthmatic disease, which was diagnosed at her age of 20 and treated with mepolizumab, a humanized monoclonal antibody directed against IL-5[12], for 12 mo with a good clinical response assessed by a pneumologist.
The patient reported that she had no other pathologies.
The patient had a family history of psoriasis, as her father suffered from moderate-severe chronic plaque psoriasis. She had no family history for atopy.
The patient presented itchy, scaly erythematous lesions on palmar and plantar skin for 6 mo; there were no associated lesions on the body, but the nail apparatus showed splinter haemorrhage and pitting. The patient complained of itching and burning, with an itch visual analogue scale (VAS) score of 6. The hand Physician’s Global Assessment (PGA) score was estimated to be 3.
All other physical findings, as well as blood chemistry, urine analysis, and complete blood count, were found to be normal except for the C-reactive protein (CRP) value, slightly above the threshold limit (0.9 mg/dL; normal range: < 0.5 mg/dL).
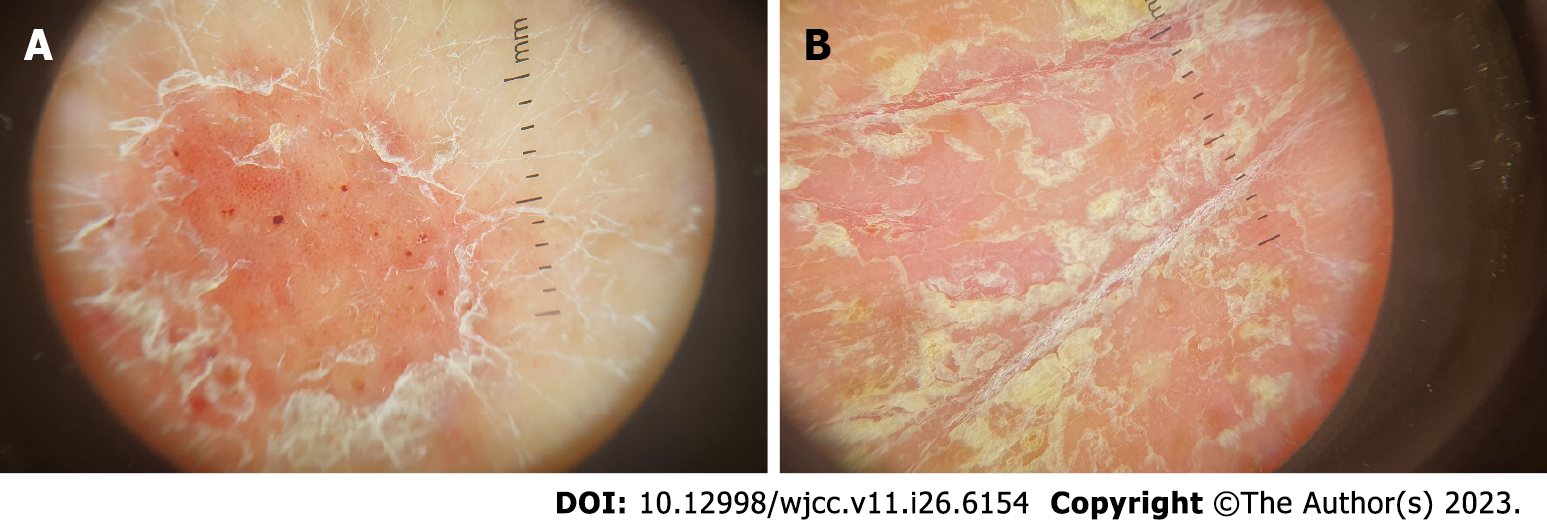
There was no need for imaging examinations for the dermatologic issue. On dermoscopy, characteristic features of psoriasis lesions were diffuse scaling, white scales, and dotted vessels along with a regular distribution of vessels as shown in Figure 2[14].
By clinical evaluation, a diagnosis of PPP was made.
A topical calcipotriol and betamethasone ointment was prescribed to the affected areas once daily for 8 wk.
We prescribed control blood tests and set a date for a new dermatological visit after 12 wk. During the control visit, the patient presented almost complete resolution of the skin features and the symptoms of itch and burning. Itch VAS was assessed and the result was 0. The hand PGA score estimated by the clinician was 0.5. The patient showed us the results of the blood analysis which roughly overlapped with the results shown in the first visit, with a slight increase in CRP (0.8 mg/dL) and a slightly increased cholesterol value (205.0 mg/dL). The patient has continued the treatment with mepolizumab. We prescribed a maintenance treatment with calcipotriol alone three times per week for 8 wk and a new date for a control visit after 12 wk.
IL-4 is a cytokine secreted by Th2 lymphocytes and may serve as an excellent paradigm to shed light on the modulation of the balance that governs the immune response, being fundamental not only for the differentiation of naïve Th lymphocytes to Th2 lymphocytes, but also for stimulating the production of immunoglobulins by B lymphocytes[1,5,15].
IL-4 is also implicated in suppressing the effector functions of Th1 lymphocytes in the context of pathologies connected to a de-regulation of the latter, such as delayed hypersensitivity, certainly inducing a transient cessation of Th1-type pro-inflammatory activity[1]. In this regard, there is some evidence that psoriasis can improve in laboratory animals subjected to the administration of human IL-4 at a precise therapeutic range[16].
In atopic subjects, it is believed that there is an upregulation of the genes that control the release of IL-4 by Th2 Lymphocytes in response to environmental allergens and clinicians have been interfering for years in this altered Th1-Th2 balance through immunotherapy for atopy[17,18]. This therapeutic strategy, consisting in the injection of purified allergens in patients with atopy, can reduce the production of IL-4 by Th-2 Lymphocytes, reducing the reactivity of the memory Th-2 Lymphocytes to these allergens. By the reduction of IL-4 levels, it was possible to find an increase in the mRNA encoding IL-12, secreted by Th1 and antigen-presenting cells. IL-12, in vivo, has been shown to be able to prevent Th2-mediated immune responses and, sometimes, also to convert them into Th1 ones[1,18-20].
To date, the role that IL-5 can play in regulating the Th1/Th2 or humoral-cellular immunity balance is unknown; however, in the light of the different evidence observed with IL-4 inhibitory antibodies, it is possible to hypothesize that a similar link may also exist in the blockade of IL-5, although it must be recognized that different functions are attributable to IL-4 and IL-5 cytokines, both however involved in the first moments of activation of the Th2 axis[21].
Indeed, Il-5 is implicated in activating ASC differentiation and is a co-activator for B cell proliferation, enabling humoral immune responses mostly following the activation of Th2 cells[13], and there is evidence in rats and in vitro studies that IL-5 therapies can treat graft rejection phenomena by reversing autoimmune mechanisms based on Th1 and Th17 activation[22].
At the same time, we think that in our specific case, there was a similar conversion of the immune response from a predominantly antibody-based one, responsible for the patient’s asthma, to one with a strong cell-mediated component, responsible for the patient’s PPP.
Nowadays, given the increasing use of IL inhibitory monoclonal antibodies in numerous pathologies, we should investigate with further attention the possible impact on the human immunological ecosystem put in place by the inhibition of the activity of individual inflammatory mediators, so as to be able to be increasingly aware of the manifestations of pathologies that could arise or be revealed by the treatment itself and therefore recognize the initial lesions early.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dermatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Bai H, China; Lin L, China; Zhang Y, China S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Biedermann T, Röcken M, Carballido JM. TH1 and TH2 lymphocyte development and regulation of TH cell-mediated immune responses of the skin. J Investig Dermatol Symp Proc. 2004;9:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Dubin C, Del Duca E, Guttman-Yassky E. The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev Clin Immunol. 2021;17:835-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 3. | Peebles RS Jr, Aronica MA. Proinflammatory Pathways in the Pathogenesis of Asthma. Clin Chest Med. 2019;40:29-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 4. | Maurer M, Eyerich K, Eyerich S, Ferrer M, Gutermuth J, Hartmann K, Jakob T, Kapp A, Kolkhir P, Larenas-Linnemann D, Park HS, Pejler G, Sánchez-Borges M, Schäkel K, Simon D, Simon HU, Weller K, Zuberbier T, Metz M. Urticaria: Collegium Internationale Allergologicum (CIA) Update 2020. Int Arch Allergy Immunol. 2020;181:321-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 5. | Singh VK, Mehrotra S, Agarwal SS. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol Res. 1999;20:147-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 217] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Zhou X, Chen Y, Cui L, Shi Y, Guo C. Advances in the pathogenesis of psoriasis: from keratinocyte perspective. Cell Death Dis. 2022;13:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 300] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 7. | Vecellio M, Hake VX, Davidson C, Carena MC, Wordsworth BP, Selmi C. The IL-17/IL-23 Axis and Its Genetic Contribution to Psoriatic Arthritis. Front Immunol. 2020;11:596086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Timotijević ZS, Trajković G, Jankovic J, Relić M, Đorić D, Vukićević D, Relić G, Rašić D, Filipović M, Janković S. How frequently does palmoplantar psoriasis affect the palms and/or soles? A systematic review and meta-analysis. Postepy Dermatol Alergol. 2019;36:595-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Safa G, Paumier V. Psoriasis induced by dupilumab therapy. Clin Exp Dermatol. 2019;44:e49-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Kim HS, Yeung J. Psoriasis appearing after dupilumab therapy in atopic dermatitis: A case report. SAGE Open Med Case Rep. 2020;8:2050313X20940458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Fowler E, Silverberg JI, Fox JD, Yosipovitch G. Psoriasiform Dermatitis After Initiation of Treatment with Dupilumab for Atopic Dermatitis. Dermatitis. 2019;30:234-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Nagase H, Ueki S, Fujieda S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol Int. 2020;69:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 13. | Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol. 2008;20:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 250] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 14. | Adabala SS, Doshi BR, Manjunathswamy BS. A Cross-Sectional Study to Assess the Role of Dermoscopy in Differentiating Palmar Psoriasis, Chronic Hand Eczema, and Eczema in Psoriatico. Indian Dermatol Online J. 2022;13:78-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Röcken M, Racke M, Shevach EM. IL-4-induced immune deviation as antigen-specific therapy for inflammatory autoimmune disease. Immunol Today. 1996;17:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 205] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Ghoreschi K, Thomas P, Breit S, Dugas M, Mailhammer R, van Eden W, van der Zee R, Biedermann T, Prinz J, Mack M, Mrowietz U, Christophers E, Schlöndorff D, Plewig G, Sander CA, Röcken M. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat Med. 2003;9:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 328] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 17. | Biedermann T, Röcken M. Th1/Th2 balance in atopy. Springer Semin Immunopathol. 1999;21:295-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Bousquet J, Becker WM, Hejjaoui A, Chanal I, Lebel B, Dhivert H, Michel FB. Differences in clinical and immunologic reactivity of patients allergic to grass pollens and to multiple-pollen species. II. Efficacy of a double-blind, placebo-controlled, specific immunotherapy with standardized extracts. J Allergy Clin Immunol. 1991;88:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Secrist H, Chelen CJ, Wen Y, Marshall JD, Umetsu DT. Allergen immunotherapy decreases interleukin 4 production in CD4+ T cells from allergic individuals. J Exp Med. 1993;178:2123-2130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 376] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Hamid QA, Schotman E, Jacobson MR, Walker SM, Durham SR. Increases in IL-12 messenger RNA+ cells accompany inhibition of allergen-induced late skin responses after successful grass pollen immunotherapy. J Allergy Clin Immunol. 1997;99:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 172] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Larché M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450-63; quiz 464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 399] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | Hall BM, Hall RM, Tran GT, Robinson CM, Wilcox PL, Rakesh PK, Wang C, Sharland AF, Verma ND, Hodgkinson SJ. Interleukin-5 (IL-5) Therapy Prevents Allograft Rejection by Promoting CD4(+)CD25(+) Ts2 Regulatory Cells That Are Antigen-Specific and Express IL-5 Receptor. Front Immunol. 2021;12:714838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |