Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6147
Peer-review started: April 15, 2023
First decision: July 7, 2023
Revised: July 13, 2023
Accepted: July 19, 2023
Article in press: July 19, 2023
Published online: September 16, 2023
Processing time: 146 Days and 4.8 Hours
Anticoagulation treatment after lower limb surgery is one of the key methods to avoid thrombosis, and low-molecular-weight heparin is the treatment that is most frequently used in clinical practice. But one uncommon side effect of low-molecular-weight heparin is heparin-induced thrombocytopenia (HIT), which can develop into thrombosis if not caught early or managed incorrectly.
We present a case of a patient who underwent hip arthroplasty and experienced thrombocytopenia due to HIT on the 9th d following the application of low-molecular-weight heparin anticoagulation. We did not diagnose HIT in time and applied 1 unit of platelets to the patient, which led to thrombosis. Luckily, the patient recovered following effective and timely surgery and treatment with rivaroxaban.
Patients using low-molecular-weight heparin after lower limb surgery need to have their platelet counts regularly checked. If HIT develops, platelet treatment should be given with caution.
Core Tip: Anticoagulation treatment after lower limb surgery is one of the key methods to avoid thrombosis, and low-molecular-weight heparin is the treatment that is most frequently used in clinical practice. But one uncommon side effect of low-molecular-weight heparin is heparin-induced thrombocytopenia. If heparin-induced thrombocytopenia develops, platelet treatment should be given with caution.
- Citation: Lv FF, Li MY, Qu W, Jiang ZS. Rivaroxaban for the treatment of heparin-induced thrombocytopenia with thrombosis in a patient undergoing artificial hip arthroplasty: A case report. World J Clin Cases 2023; 11(26): 6147-6153
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6147.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6147
Heparin, a commonly used anticoagulant, has significant clinical status, including unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH)[1]. Heparin was first purified by McLean from dog liver in 1918. Heparin has increased in popularity since that time. A smaller-molecular-weight heparin fragment known as LMWH was first discovered in 1976 and was produced by UFH either chemically or through enzymatic depolymerization. The pharmacokinetics of LMWH are more stable; it has a longer half-life, better bioavailability, better absorption after subcutaneous injection, and less adverse effects[2].
Heparin-induced thrombocytopenia (HIT) is a serious adverse medication reaction that is characterized by thrombocytopenia and thrombosis. About 35% of patients worsen to HIT with thrombosis (HITT), which has an incidence rate of 1%-5%[3]. In individuals with HITT, platelet levels rarely drop below 20 × 109/L, and bleeding is uncommon. Furthermore, regular preventive platelet administration is not advised to reduce the chance of thromboembolism. Direct oral anticoagulants should be used preferentially in HIT patients with stable vital signs and a moderate-to-low risk of bleeding according to the 2018 American Society of Hematology (ASH) therapeutic guidelines for venous thromboembolism in HIT[4]. A direct oral anticoagulant with predictable pharmacokinetics and pharmacodynamics is rivaroxaban, which is a factor Xa inhibitor. Specific thromboembolic illnesses can be treated with rivaroxaban based on the findings of a phase III trial[5]. In this case report, a patient with HITT, who was misdiagnosed and improperly treated, was effectively rescued by aggressive surgery and treatment with rivaroxaban.
On October 9, 2022, a 78-year-old female presented with aggravated pain in both hip joints occurring for 1 mo.
Two years prior to admission, the patient had no obvious cause of intermittent pain in both hip joints, which worsened after activity and could be slightly relieved after rest. No diagnosis and treatment were performed. When the pain worsened, she self-administered nonsteroidal anti-inflammatory drugs.
The patient had a 3-year history of diabetes and a 3-year history of coronary artery disease.
The patient had no specific personal and family history.
At admission, the patient’s blood pressure was 131/89 mmHg, and her pulse rate was 68 beats/min. The patient’s lungs auscultated clearly, with normal heart sounds and no murmurs during auscultation.
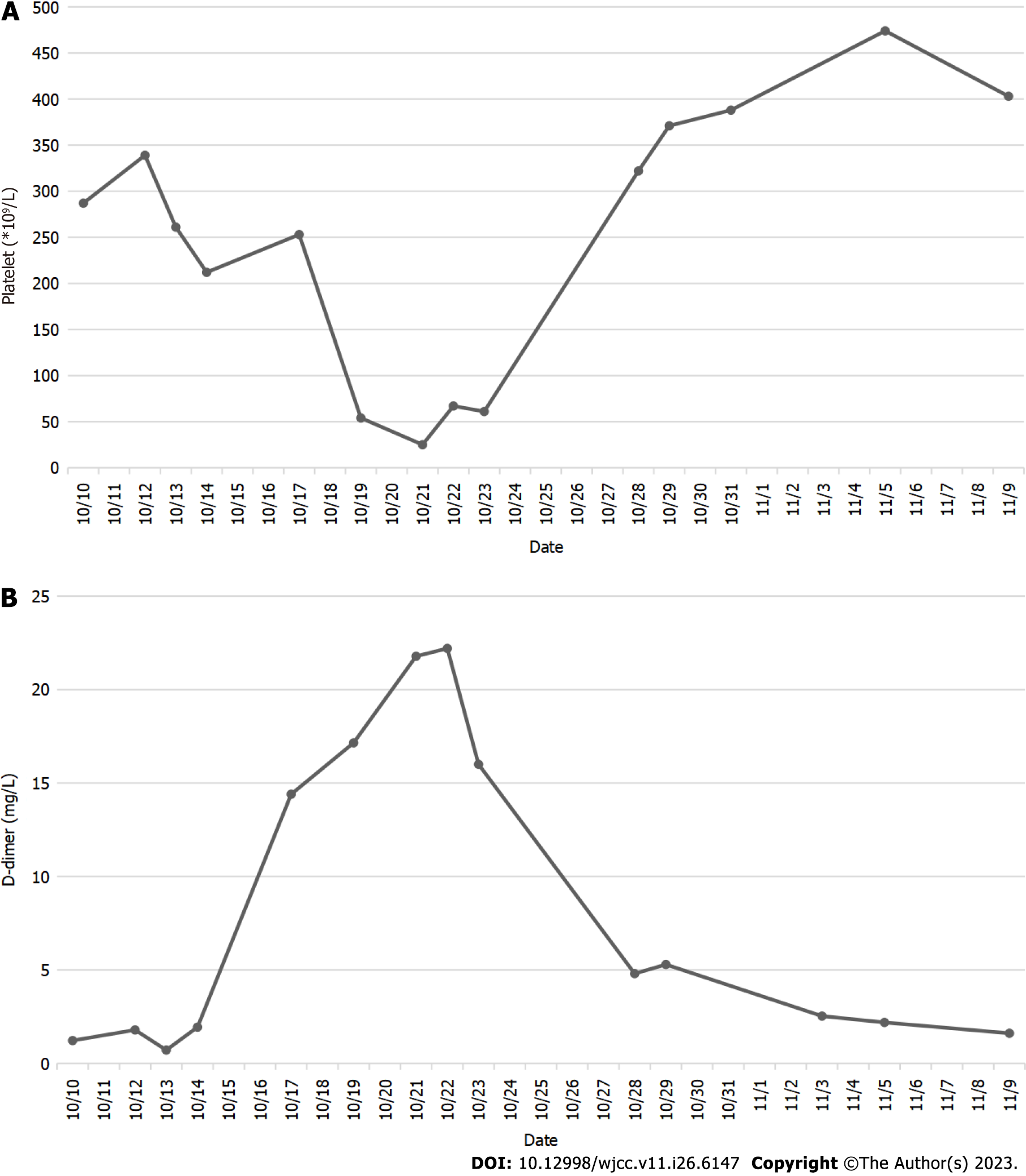
The D-dimer level was 0.71 ng/L (normal range: xxx), and the platelet level was 212 × 109/L (normal range: xxx). The routine blood test, routine coagulation test, and other indicators were frequently checked, taking into account the patient’s health as a whole. The levels of D-dimer and platelets were monitored throughout the patient’s hospital stay (Figure 1).
Osteoarthritis of the hip.
On October 11, 2022, after ruling out surgical contraindication, the patient agreed to hip replacement. Following surgery, the patient received 5000 WU LMWH anticoagulant treatment. Due to diabetic ketoacidosis, the patient was sent to the Department of Intensive Medicine on October 12, 2022. Meanwhile, LMWH was halted, and a common sodium heparin injection was utilized to remove 0.625 WU. Then, the patient was moved to the Department of Endocrinology for additional care on October 14, 2022 when her vital signs stabilized.
The D-dimer level was 14.40 ng/L on October 17, 2022. Therefore, a 5000 WU LMWH injection was given twice a day. The D-dimer and platelet levels were 17.15 ng/L and 54 × 109/L, respectively, on October 19, 2022. Right lower pulmonary artery thrombosis was detected by pulmonary artery computed tomography angiography. Additionally, an ultrasound of the lower extremities revealed no thrombosis in the arteries or veins of either lower extremity. The patient’s platelet count continued to decline with the addition of 5 mg of warfarin sodium tablets once day, reaching 25 × 109/L on October 21, 2022. The Hematology Department recommended administering a 1 unit platelet transfusion.
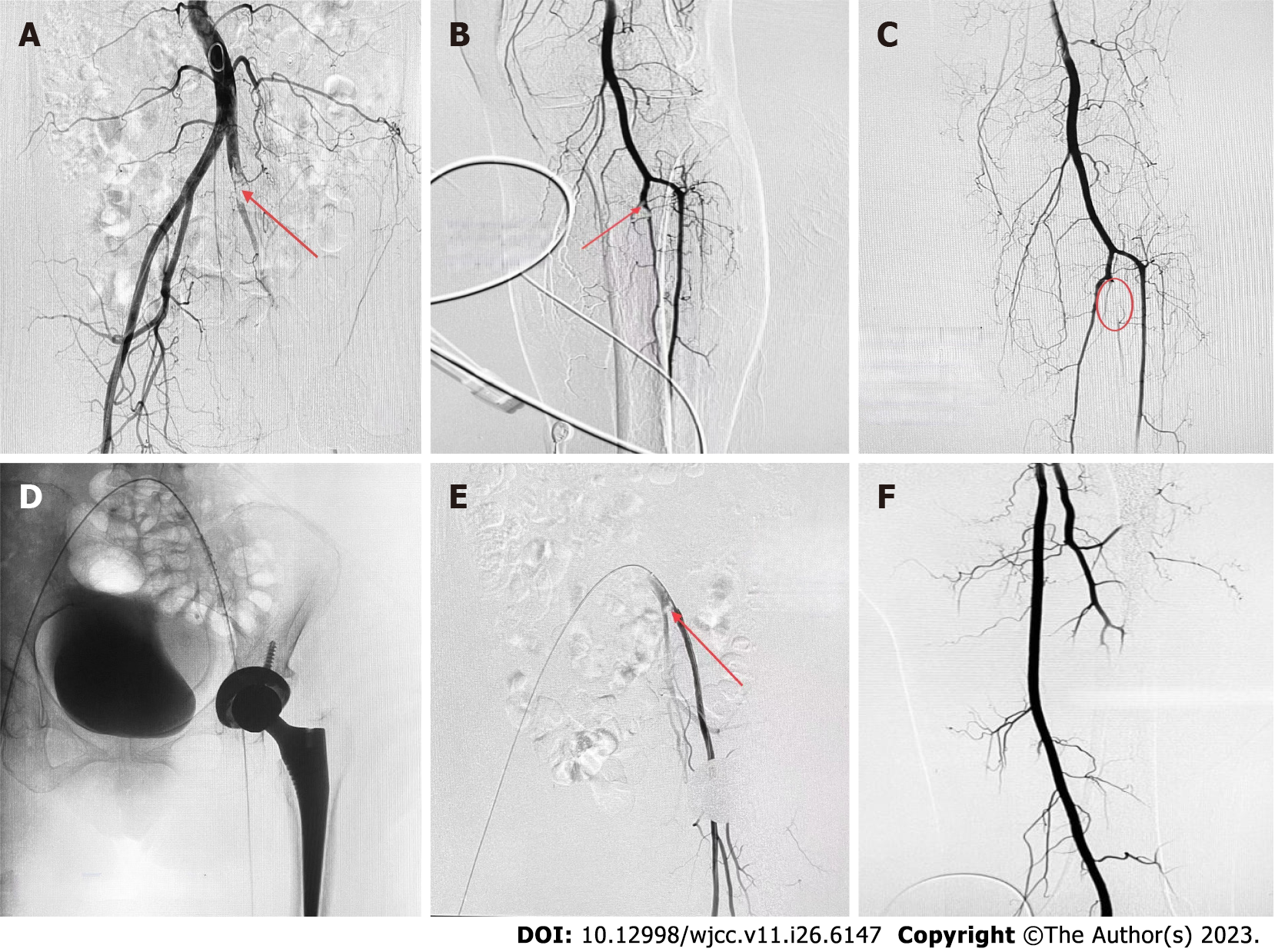
On the evening of October 21, 2022, the patient experienced edema and coldness in the left lower limb, which worsened by the morning. Platelet levels were 67 × 109/L on October 22, 2022. A vascular ultrasound revealed that the local blood flow of the left iliac artery was absent, the blood flow velocity of the left lower limb artery was reduced, and thrombosis was possible. The patient underwent an arteriogram of the left lower limb, which showed thromboses in the tibiofibular trunk and the distal common iliac artery (Figure 2A and B). During the operation, three white thrombi were suctioned out of the common iliac artery, and the tibiofibular trunk was injected with 20 WU urokinase (Figure 2C).
Later, a thrombolytic catheter was placed in the common iliac artery (Figure 2D), and the patient returned to the ward. On October 24, 2022, the iliac artery thromboses became smaller on the repeat angiography (Figure 2E). We then performed a femoral artery thrombectomy, removing two white thrombi, and evaluated the imaging for the left common iliac artery and internal/external iliac arteries (Figure 2F). Thrombocytopenia started on the 9th d after using heparin. The 4T score was 8 points, which indicated a high clinical possibility of HIT. Therefore from October 10, 2022 to October 28, 2022, we stopped administering LMWH and gave rivaroxaban 15 mg every 12 h.
The patient was released on November 11, 2022 when her general health stabilized. After discharge, the patient continued with the current plan of full course anticoagulation therapy, and no significant abnormalities were found in the monitoring of routine blood and coagulation tests. We also informed the patient that she should not use heparin under any circumstances in the future.
HIT is an unfavorable heparin reaction caused by antibodies when heparin medicine is administered. A decline in blood platelet count is the primary clinical sign of HIT, which in extreme situations can result in arteriovenous thrombosis and mortality. Type I and Type II HIT can be distinguished by their mechanisms, onset times, treatments, and prognoses. The incidence of type I HIT is 10%-20% and typically occurs 1-2 d after receiving heparin. Typically, the blood platelet level does not fall below 100 × 109/L, which prevents thrombus and bleeding. Withdrawal or particular care are typically not necessary. Immunity is connected to type II HIT. Its primary characteristic is the considerable reduction in the blood platelet count, which may also be accompanied by a serious risk of thromboembolism[6]. The most common reasons for death and disability in HIT patients are thrombosis and embolism problems. HIT accounts for 20%-30% of amputations and fatalities[7].
Platelet factor IV (PF4) is a protein that the platelets release in response to heparin. PF4 can combine with heparin to create the H-PF4 complex and connect to the platelet membrane simultaneously. The H-PF4 complex possesses immunogenicity in some people, which causes the development of an anti-H-PF4 antibody[8,9]. A larger immune complex can be created by combining the anti-H-PF4 antibody with the H-PF4 complex and the F(ab) 2 fragment. To activate platelets, the immune complex first interacts with the Fc fragment on the surface of the platelet membrane. Numerous substances are released by the active platelets, prompting more platelet activation and aggregation. Additionally, thrombin and platelet-derived microparticle levels increase concurrently, which lowers platelet counts and causes a hypercoagulable state[10]. There are currently no internationally accepted diagnostic standards for HIT. The standard method for diagnosing is to use the 4T score, dynamic platelet monitoring, HIT antibody detection, and/or platelet function test as a basis for the diagnosis[11,12].
The four factors that contribute to thrombocytopenia are reduced platelet production, increased platelet intake and destruction, abnormal platelet dispersion, and hemodilution. Increased platelet breakdown caused by immunological elements leads to HIT. As a result, platelet transfer without discontinuing heparin can worsen the situation and even cause thrombosis. In this case, the patient’s platelet count was 54 × 109/L on the 9th d after starting heparin treatment, and the pulmonary artery computed tomography angiography revealed a pulmonary embolism on the 10th d. However, the patient exhibited no clinical signs. HIT should have been suspected immediately, but the identification was delayed because of our limited knowledge of the disease. On day 11, 1 unit of platelets was administered, and the patient experienced lower extremity arterial thrombosis on day 12. After multiple consultations and literature review, HITT was finally diagnosed, and the patient was successfully treated with surgery and application of direct oral anticoagulants. Rivaroxaban was used in place of heparin for anticoagulation since it had an immediate and potent anticoagulant action and did not worsen the situation.
Rivaroxaban is a new oral anticoagulant. Rivaroxaban contains an FXa inhibitor. A serine protease called FXa is found upstream of the blood agglutination response. It can be found at the intersection of the pathways that connect internal and exterior activation. FXa has the ability to suppress external as well as endogenous coagulation. In blood agglutination reactions, FXA is the principal rate-limiting product of thrombin and has the ability to magnify biological signals. A single FXa inhibitor can inhibit the biological activity of 138 thrombin molecules[13]. As a result, FXa theoretically has a greater anticoagulant impact than a thrombin inhibitor. A recently created tiny molecule with a strong affinity for the Xa factor is called rivaroxaban. It can directly block free FXa activity as well as Xa activity. The action of rivaroxaban is distinct from that of LMWH and does not require the involvement of other components[14]. Additionally, rivaroxaban does not require injection or blood coagulation function monitoring.
Clinical evidence of new oral anticoagulants in HIT patients has not yet been published in a significant prospective trial. The capacity of rivaroxaban to arrest platelet decline after HIT has been substantiated by an increasing number of small studies and case reports. A prospective study conducted in 2016 by Linkins et al[15] revealed that 7 of the 12 HIT patients had had heparin treatment for 1-3 d, and 90% of the patients had steadily increasing blood platelet levels after rivaroxaban therapy. In 2017, Warkentin et al[16] discovered that only 1 of the 16 HIT patients who received rivaroxaban had a worsened condition. The efficiency and safety of rivaroxaban in HIT were further validated by the prospective investigation of Cirbus et al[17]. According to the 2018 ASH management guide on venous thromboembolism in HIT treatment, it is advised to begin strong anticoagulation in patients with acute HITT[4], and rivaroxaban should be administered at a dose of 15 mg twice daily for 3 wk before increasing to 20 mg once per day.
The incidence rate of HIT varies according to the patient population and heparin exposure[16]. In our patient’s case, she was exposed to heparin after hip joint replacement and given LMWH at a therapeutic dose. Additionally, the Department of Intensive Care used the UFH wash pipe during therapy. There was a progressive decrease in platelet levels that was greater than 50% on the 9th d after starting heparin. On the 1st d, a progressive fall in platelet count started, with a decline of more than 50%. A freshly developed common iliac artery thrombosis was found. The 4T score was 8, and the HIT-related IgG antibody was 2.49 optical density units after factors such as thrombotic thrombocytopenic purpura, immune thrombocytopenic purpura, drug and infection-related thrombocytopenia, and immunological thrombocytopenic purpura were disregarded. It was established that HITT existed.
Heparin therapy should be discontinued immediately after HIT has been identified or is strongly suspected, especially in patients who have HITT or who are at risk for subsequent thrombosis. Hemorrhage is uncommon, and the blood platelet count in HIT patients is rarely lower than 20 × 109/L. To reduce the risk of thromboembolism, routine platelet transfusion is not advised. If a platelet transfusion is required, it must be performed after the heparin has been stopped[16]. Our patient did not discontinue LMWH during the early stages of thrombocytopenia due to the possibility of pulmonary artery thrombosis and significant surgery following the initial thrombocytopenia. Arterial thrombosis was found after 9 d. The HITT diagnosis was deemed conclusive following a study of the literature and interdisciplinary consultation. The patient started taking rivaroxaban 15 mg daily 3 d after the arterial thrombus was removed.
LMWH or regular heparin anticoagulation therapy can be administered without risks for medium-sized cases with risk factors for severe disease and quick disease progression as well as heavy and critical cases, according to national and international standards and associated consensus. After excluding thrombotic comorbidities, the ASH and the American College of Chest Physicians both advise using preventive dosages of anticoagulants in patients with newly diagnosed severe and acute coronary disease. However, it is still important to monitor changes in the patient’s platelet counts while they are receiving therapy for pneumonia. When a progressive decline in platelet levels occurs and HIT is highly suspected after other relevant risk factors have been excluded, appropriate treatment should be given promptly, and HIT-related IgG antibody testing should be completed if available.
In the clinic, we should be aware of HIT in patients who have used heparin and have thrombocytopenia. The HIT antibody test should be performed to diagnose HIT when the clinical assessment of the 4T score is moderate or severe. In this instance, HITT and secondary arterial thrombosis also occurred. Heparin was replaced with rivaroxaban for anticoagulation since it had a quick and effective anticoagulation effect and did not worsen the condition. This example offers some clinical proof that the new oral anticoagulants can be used to treat HIT both initially and long-term. However, large-scale clinical investigations are required to produce pertinent data that will help direct the course of treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Substance abuse
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hoffmann M, Germany S-Editor: Liu JH L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Hirsh J, Warkentin TE, Shaughnessy SG, Anand SS, Halperin JL, Raschke R, Granger C, Ohman EM, Dalen JE. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119:64S-94S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 896] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 2. | Kibbe MR, Rhee RY. Heparin-induced thrombocytopenia: pathophysiology. Semin Vasc Surg. 1996;9:284-291. [PubMed] |
| 3. | Kelton JG. Heparin-induced thrombocytopenia: an overview. Blood Rev. 2002;16:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, Linkins LA, Rodner SB, Selleng S, Warkentin TE, Wex A, Mustafa RA, Morgan RL, Santesso N. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360-3392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 428] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 5. | Pudusseri A, Shameem R, Spyropoulos AC. A new paradigm shift in antithrombotic therapy. Front Pharmacol. 2013;4:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106:2710-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 554] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 7. | Smythe MA, Koerber JM, Mattson JC. The incidence of recognized heparin-induced thrombocytopenia in a large, tertiary care teaching hospital. Chest. 2007;131:1644-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Untch B, Ahmad S, Jeske WP, Messmore HL, Hoppensteadt DA, Walenga JM, Lietz H, Fareed J. Prevalence, isotype, and functionality of antiheparin-platelet factor 4 antibodies in patients treated with heparin and clinically suspected for heparin-induced thrombocytopenia. The pathogenic role of IgG. Thromb Res. 2002;105:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Carlsson LE, Santoso S, Baurichter G, Kroll H, Papenberg S, Eichler P, Westerdaal NA, Kiefel V, van de Winkel JG, Greinacher A. Heparin-induced thrombocytopenia: new insights into the impact of the FcgammaRIIa-R-H131 polymorphism. Blood. 1998;92:1526-1531. [PubMed] |
| 10. | Arepally GM, Padmanabhan A. Heparin-Induced Thrombocytopenia: A Focus on Thrombosis. Arterioscler Thromb Vasc Biol. 2021;41:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120:4160-4167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 310] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 12. | Crowther MA, Cook DJ, Albert M, Williamson D, Meade M, Granton J, Skrobik Y, Langevin S, Mehta S, Hebert P, Guyatt GH, Geerts W, Rabbat C, Douketis J, Zytaruk N, Sheppard J, Greinacher A, Warkentin TE; Canadian Critical Care Trials Group. The 4Ts scoring system for heparin-induced thrombocytopenia in medical-surgical intensive care unit patients. J Crit Care. 2010;25:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Rivaroxaban (Xarelto) - a new peripheral artery disease indication. Med Lett Drugs Ther. 2021;63:172-173. [PubMed] |
| 14. | Clinical Review Report: Rivaroxaban (Xarelto): Bayer Inc: Indication: In combination with 75 mg to 100 mg acetylsalicylic acid, for the prevention of stroke, myocardial infarction, and cardiovascular death, and for the prevention of acute limb ischemia and mortality in patients with coronary artery disease with or without peripheral artery disease [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019 Jan- . [PubMed] |
| 15. | Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, Schulman S, Crowther M. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e495S-e530S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 643] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 16. | Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130:1104-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Cirbus K, Simone P, Austin Szwak J. Rivaroxaban and apixaban for the treatment of suspected or confirmed heparin-induced thrombocytopenia. J Clin Pharm Ther. 2022;47:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |