Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6105
Peer-review started: May 16, 2023
First decision: July 18, 2023
Revised: July 18, 2023
Accepted: August 17, 2023
Article in press: August 17, 2023
Published online: September 16, 2023
Processing time: 114 Days and 21.2 Hours
Coronavirus disease 2019 (COVID-19) pandemic stimulates research works to find a solution to this crisis from starting 2020 year up to now. With ending of the 2021-year, various advances in pharmacotherapy against COVID-19 have emerged. Regarding antiviral therapy, casirivimab and imdevimab antibody combination is a type of new immunotherapy against COVID-19. Standard antiviral therapy against COVID-19 includes Remdesivir and Favipiravir.
To evaluate the efficacy of antibodies cocktail (casirivimab and imdevimab) compared to standard antiviral therapy in reducing the need for invasive mechanical ventilation (IMV).
265 COVID-19 polymerase chain reaction confirmed patients with indication for antiviral therapy were included in this study and were divided into 3 groups (1: 2: 2): Group A: REGN3048-3051 antibodies cocktail (casirivimab and imdevimab), group B: Remdesivir, group C: Favipiravir. The study design is a single-blind non-randomized controlled trial Mansoura University Hospital owns the study’s drugs. The duration of the study was about 6 mo after ethical approval.
Casirivimab and imdevimab achieve less need for O2 therapy and IMV, with less duration of this need than remdesivir and favipiravir.
Group A (casirivimab and imdevimab) achieve better clinical outcomes than groups B (remdesivir) and C (favipiravir) intervention groups.
Core Tip: This research can benefit the coronavirus disease 2019 (COVID-19) patients by determining the most appropriate antiviral drug according to the case. This study may change the protocol of treatment of COVID-19 patients. Casirivimab and imdevimab achieve better clinical outcomes than remdesivir and favipiravir.
- Citation: Hegazy SK, Tharwat S, Hassan AH. Comparing the efficacy of regen-cov, remdesivir, and favipiravir in reducing invasive mechanical ventilation need in hospitalized COVID-19 patients. World J Clin Cases 2023; 11(26): 6105-6121
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6105.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6105
Coronavirus disease 2019 (COVID-19) is a viral disease triggered coronavirus 2 that killed a considerable number of individuals worldwide[1]. COVID-19 infection is graded as mild, moderate, severe, or critical[2]. From the beginning of the 2020 year until the present, the COVID-19 pandemic pushes research efforts to discover a solution to this catastrophe. Various improvements in pharmacotherapy against COVID-19 have surfaced as the year 2021 draws to a close[3].
Remdesivir is a conventional antiviral against COVID-19 that has been licensed by the Food and Drug Administration (FDA) for the treatment of mild, moderate, severe, and critical hospitalized COVID-19 patients[4]. Favipiravir, ivermectin, nitazoxanide, hydroxychloroquine, and ribavirin are among other medicines that have exhibited controversial antiviral activity. Favipiravir has become a routine antiviral treatment for mild and moderate COVID-19 outpatients[5].
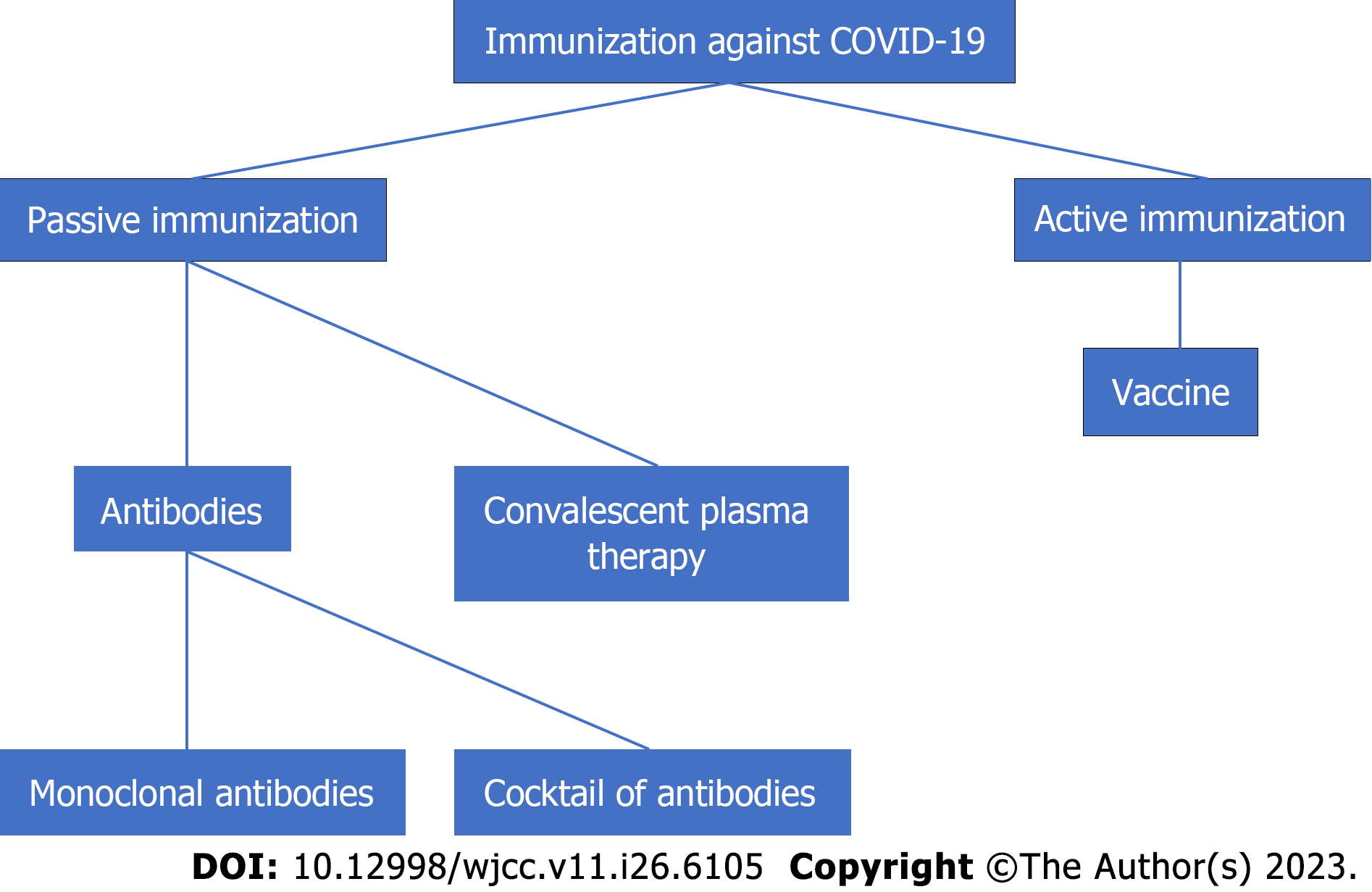
Immunotherapy to target virus antigens has just emerged, with a goal date of the end of 2020[6]. Figure 1 depicts two types of immunotherapies: Passive immunotherapy and active immunotherapy. Passive immunotherapy involves either the direct infusion of produced antibodies directed specifically at viruses or the administration of products containing antibodies, such as plasma. Active immunotherapy, like vaccination, helps the body generate antibodies against viruses[6].
These antibodies have three antiviral targets: antibodies that block virus attachment and entrance, antibodies that inhibit various aspects of the immune system response, and antibodies that limit virus multiplication and transcription. Table 1 lists many groups of antibodies under research for COVID-19 therapy, as well as their targets[6].
| Antibody | Mechanism | Company | Stage of study/identification method |
| Canakinumab | IL-1β inhibitor | Novartis | In clinical stage for several inflammatory diseases including arthritis, periodic fever and lung cancer; repurposed by novartis for COVID-19 |
| Secukinumab (cosentyx®) | IL-17 inhibitor | Novartis | In clinical stage for several autoimmune diseases including psoriasis; repurpose by novartis for COVID-19 |
| TZLS-501 | Fully human monoclonal antibody targeting the receptor of IL-6, it binds to both membrane-bound and soluble forms of IL-6R, and rapidly depletes the circulating levels of IL-6 in blood | Tiziana Life Sciences and Novimmune | Preclinical stage |
| Pritumumab | Fully human IgG antibody targeting vimentin | Nascent Biotech Inc. | Received FDA approve for several carcinoma; Research began for COVID-19 |
| COVID-HIG and COVID-EIG | Hyperimmune polyclonal antibody derived from human plasma or immunized horse | Emergent BioSolutions | Enter clinical trial within 4-5mo |
| Rcig | Recombinant anti SARS-CoV-2 hyperimmune gamma globulin, polyclonal antibodies | GigaGen | Preclinical stage-Aimed for COVID19 hospitalized patients and prophylaxis in high-risk individuals |
| Antibody cocktail including REGN3048-3051 | Fully human multivalent antibodies against the spike protein isolated from genetically modified mice or recovered COVID-19 patients | Regeneron | Phase 1 clinical trial for Middle East Respiratory Syndrome completed last year; clinical trial for SARS-CoV-2 starts by early summer |
This study focuses on an antibody cocktail that includes casirivimab and imdevimab (REGN3048-3051). REGN3048 and REGN3051 are monoclonal antibodies that target the spike glycoprotein on the surface of viral particles, blocking viral entry into human cells via the angiotensin-converting enzyme 2 receptor[7,8]. They show promising antiviral activity, but more research is needed to investigate their benefit in COVID patients[9].
Previous research[9] on casirivimab and imdevimab had shown that the efficacy of this antibody cocktail is proven in treatment of outpatients with COVID-19 in both high (8.0 g of REGN3048-3051), and low (2.4 g of REGN3048-3051) doses when compared to placebo. Time-weighted average change in viral load from baseline to day 7 (log10 scale) in patient, and clinical efficacy: Percentage of patients with symptoms offset on day 7, and one or more medically related visits.
According to a recent study[9], effectiveness is higher in outpatients whose immune response has not yet matured to make antibodies against virus (seronegative outpatients) and in outpatients with a high baseline viral load.
Data for REGN3048-3051 are now available. The FDA has allowed an Emergency Use Authorization (EUA) for REGN3048-3051 in the post-exposure prophylaxis and treatment of moderate and mild COVID-19 in pediatric and adults outpatients (weighing < 40 kg, and >12 years of age) who have positive polymerase chain reaction (PCR) results of COVID-19 and are at high risk of progressing to severe COVID-19 that needs hospital admission or leads to death[10].
Casirivimab and imdevimab, on the other hand, are still not approved for use in patients[10] who require an increase in baseline flow rate of oxygen due to COVID-19 in those on chronic oxygen therapy due to non-COVID-19 related comorbidity, require oxygen therapy due to COVID-19, or hospitalized due to COVID-19.
Casirivimab and imdevimab are now licensed experimental antibodies; however, unexpected and serious side events have been recorded with their use[10]. After a single intravenous injection, this antibody combination exhibits linear pharmacokinetics, with half-lives ranging from 25 to 37 d. Casirivimab and imdevimab are not metabolized by liver cytochrome enzymes and are not eliminated by the kidneys[10]. The importance of this study came from that it is the only study that has discussed the use of casirivimab and imdevimab in COVID-19 patients.
The gap of knowledge comes from the limitations of the previous studies including short duration of follow up, non-using much clinically focused outcomes, non-studying antiviral efficacy on long-term in lowering the level of inflammatory markers, and these studies had been conducted on outpatients only and not included inpatients. This research is an extension of published paper that has written by the same authors[11].
The purpose of this study is to compare the efficacy of a cocktail of antibodies (casirivimab and imdevimab) to standard antiviral medication (remdesivir and Favipiravir) in minimizing the requirement for invasive mechanical ventilation (IMV) in hospitalized patients with moderate, severe, or critical COVID19.
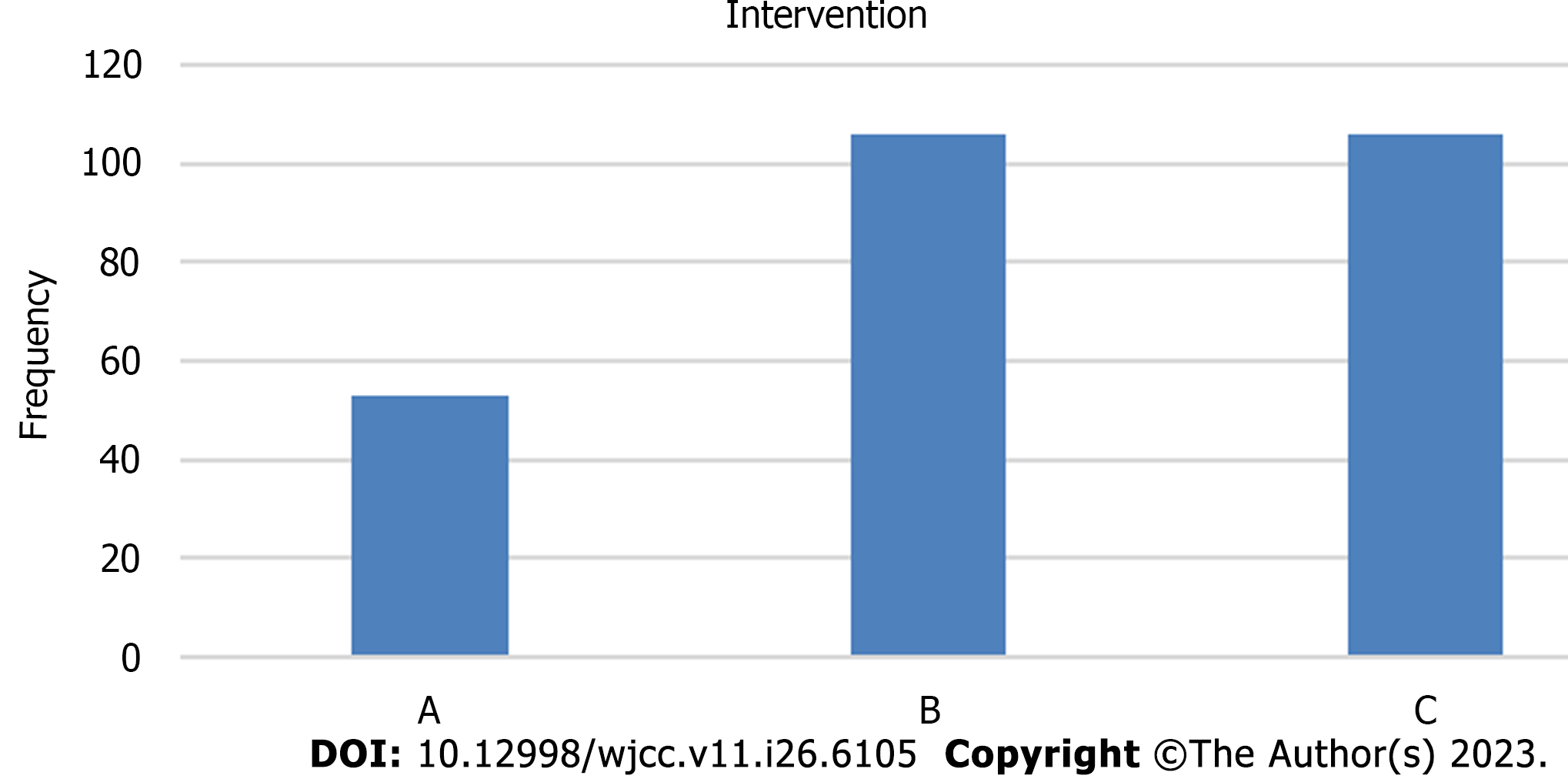
265 COVID-19 PCR confirmed patients with indication for antiviral therapy were included in this study and were randomized (1: 2: 2) into 3 groups: Group A: Antibodies cocktail (casirivimab and imdevimab), group B: Remdesivir, group C: Favipiravir[11]. A ratio of (1: 2: 2) was chosen as this ratio is the closest to reality according to number of patients who received each drug, and also antibodies cocktail product was available for only about 50 COVID-19 patients. Population in this study was COVID-19 patients hospitalized in isolation hospital-Mansoura university[11]. A computer file containing a written informed consent from included patients was provided[11].
Patient should fulfill all these characteristics to be included: weight not less than 40 kg, age more than 12 years old, PCR-confirmed patients to be positive before inclusion, and moderate, severe or critical COVID-19 disease as defined by WHO[11].
Patient should not have any of the following to be included: Prior use of standard antiviral therapy (remdesivir or favipiravir), history of infusion related reactions or hypersensitivity due to monoclonal antibodies administration, patients expected to die within 48 h, and current use of non-standard antiviral therapy (oseltamivir, hydroxychloroquine, azithromycin, ivermectin, nitazoxanide, acyclovir, ribavirin, semipirvir, lopinavir/ritonavir, sofosbuvir, daclatasvir)[11].
Population included in this study was assigned into 3 groups with 1: 2: 2 ratios to receive either casirivimab and imdevimab or standard antiviral therapy (remdesivir or favipiravir) as shown Figures 2 and 3[11].
Group A patients received casirivimab and imdevimab in low-dose 1.2 gm dissolved in 250 mL normal saline administered as single I.V infusion dose within 30-60 min. Group B patients received remdesivir : Loading dose (day 1): 200 mg dissolved in 500 mL normal saline administered by I.V infusion over 60 min. Maintenance dose (day 2-5 or day 2-10): 100 mg dissolved in 250 mL normal saline administered by I.V infusion over 30 min. Group C patients received Favipiravir : Loading dose (day 1): 1800 or 1600 mg administered enterally every12 h. Maintenance dose (day 2-5 or day 2-10): 800 or 600 mg administered enterally every 12 h. standard of care had been given to all patients as guided by Egyptian COVID-19 treatment protocol[11].
The type of this study is single blind non-randomized controlled trial (non-RCT) and is considered a phase IV clinical trial (post-marketing study) to report efficacy of new medicine[11]. Another resource used to obtain information about casirivimab and imdevimab is fact sheet for health care providers-EUA of casirivimab and imdevimab which provides clinical data about the use of this antibodies cocktail. Endnote citation software was used for references citation[11].
The research protocol was approved by Institutional review board, faculty of medicine, Mansoura University, MS21.11.1737, research ethics committee, faculty of medicine, Tanta University, 35039/11/21, and research ethics committee, ministry of health, Egypt, 10-2022/18. Registry name and registration number: Clinicaltrials.gov, NCT
Outcomes include need for IMV, and IMV and oxygen support duration (days). In addition to clinical outcomes measured before and during intervention, patients’ characteristics (age, gender) and relevant medical and medication history and current COVID-19 treatment drugs were recorded on admission. Duration of research was about 6 mo from November 2021 to April 2022.
Statistical analysis: Categorical variables were presented as proportion. Continuous variables were presented as mean ± SD. Intention-to-treat strategy was used in this study. Statistical analysis was achieved with SPSS, version 26. ANOVA or Kruskal-Walli’s test was used for comparison between groups, as comparison was performed between three groups. We reported the P-value for our statistical tests with level of statistical significance is P value ≤ 0.05[11].
Regarding baseline characteristics, Kruskal-Wallis or ANOVA test (depending on type of data and the continuous data distribution (normal or not)) was used to compare these characteristics between the study groups. We reported the P value for our statistical tests. The level of statistical significance was P value ≤ 0.05[11]. In case of existing differences in some baseline characteristics, logistic regression was performed. This allowed studying the effect of these variables on the primary outcomes of the study to exclude the effect of these confounding variables and to ensure the effect on the outcomes is due to antiviral drugs[11]. Regarding the outcomes, we compared the need for IMV and duration of this need using the Kruskal-Walli’s test with reporting the P value.
Sample size: A total sample sizes of 246 patients would achieve at least 80 % power to detect a risk difference of 0.2 (20%) in the need for IMV with a significance level (α) of 0.05 and 95% confidence level using the ANOVA or Kruskal-Walli’s test of independent proportion in G*Power software. To compensate for the estimated loss-to-follow-up and to increase the study power, we increased the sample size to 53 patients in Antibodies cocktail group compared to 106 patients in both remdesivir and favipiravir groups. As antibodies cocktail product was available for only about 50 COVID-19 patients, a ratio of (1: 2: 2) was used. In addition, the ratio (1: 2: 2) is the closest to reality according to number of patients who received each drug[11]. The online system had been used to obtain mortality rate in these three months[11]. The admission rate at Isolation Hospital-Mansoura University was 250 cases per month on average; our needed sample was about 250 cases[11].
All continuous data revealed no normal distribution after statistical analysis with SPSS software. As a result, the Kruskal-Wallis test was used to compare categorical, nominal, and non-normally distributed continuous data between the three groups. Figure 4 represent a flow chart showing the flow of patients in the trial.
Table 2 shows the statistical significance of the differences between the three groups (the first P value) in baseline characteristics, as well as a comparison of each two groups (the following three P values) in baseline characteristics if there is a statistically significant difference between the three groups. Supplementary Figures 1-9 show the frequencies and distributions of baseline characteristics in the three groups[11].
| Variables | Intervention | |||
| Casirivimab/Imdevimab (A) | Remdesivir (B) | Favipiravir (C) | P valuea | |
| Age | 58.34 ± 16.096 | 59.30 ± 15.985 | 65.02 ± 14.261 | 0.006 |
| B and C | 0.07 | |||
| A and C | 0.07 | |||
| A and B | 0.63 | |||
| Gender | 0.03 | |||
| Male | 24/53 | 42/106 | 61/106 | |
| Female | 29/53 | 64/106 | 45/106 | |
| B and C | 0.09 | |||
| A and C | 0.145 | |||
| A and B | 0.501 | |||
| Number of co-morbidities | 0.022 | |||
| 0 | 10/53 | 32/106 | 22/106 | |
| 1 | 16/53 | 27/106 | 19/106 | |
| 2 | 14/53 | 28/106 | 33/106 | |
| 3 | 11/53 | 16/106 | 18/106 | |
| 4 | 2/53 | 2/106 | 10/106 | |
| 5 | 0/53 | 1/106 | 3/106 | |
| 6 | 0/53 | 0/106 | 1/106 | |
| B and C | 0.06 | |||
| A and C | 0.32 | |||
| A and B | 0.207 | |||
| Method of diagnosis | 1 | |||
| Symptoms only | 0/53 | 0/106 | 0/106 | |
| Labs and radiology | 0/53 | 0/106 | 0/106 | |
| PCR confirmed | 53/53 | 106/106 | 106/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| Severity of COVID | 0.024 | |||
| Moderate | 18/53 | 20/106 | 20/106 | |
| Sever | 27/53 | 60/106 | 53/106 | |
| Critical | 8/53 | 26/106 | 33/106 | |
| B and C | 0.475 | |||
| A and C | 0.07 | |||
| A and B | 0.035 | |||
| Number of symptoms | 0.001 | |||
| 2 | 4/53 | 2/106 | 2/106 | |
| 3 | 13/53 | 6/106 | 4/106 | |
| 4 | 32/53 | 97/106 | 97/106 | |
| 5 | 4/53 | 1/106 | 3/106 | |
| B and C | 0.482 | |||
| A and C | 0 | |||
| A and B | 0.003 | |||
| Antibiotics use | 1 | |||
| Yes | 53/53 | 106/106 | 106/106 | |
| No | 0/53 | 0/106 | 0/106 | |
| B and C | 0.102 | |||
| A and C | 0.002 | |||
| A and B | 0.075 | |||
| Macrolide use | 0.007 | |||
| Yes | 8/53 | 8/106 | 2/106 | |
| No | 45/53 | 98/106 | 104/106 | |
| B and C | 0.102 | |||
| A and C | 0.002 | |||
| A and B | 0.075 | |||
| Fluroquinolones use | 0.106 | |||
| Yes | 41/53 | 92/106 | 95/106 | |
| No | 12/53 | 14/106 | 11/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| 3rd and 4th generation cephalosporin use | 0.551 | |||
| Yes | 39/53 | 86/106 | 83/106 | |
| No | 14/53 | 20/106 | 23/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| Carbapenems use | 0.168 | |||
| Yes | 10/53 | 32/106 | 22/106 | |
| No | 43/53 | 74/106 | 84/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| Piperacillin/tazobactam use | 1 | |||
| Yes | 0/53 | 0/106 | 0/106 | |
| No | 53/53 | 106/106 | 106/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| Amoxicillin/clavulanate use | 0.472 | |||
| Yes | 0/53 | 0/106 | 0/106 | |
| No | 53/53 | 106/106 | 106/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| Cotrimoxazole use | 1 | |||
| Yes | 0/53 | 0/106 | 0/106 | |
| No | 53/53 | 106/106 | 106/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| Linezolid use | 0.115 | |||
| Yes | 5/53 | 12/106 | 4/106 | |
| No | 48/53 | 94/106 | 102/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| Teicoplanin use | 0.365 | |||
| Yes | 1/53 | 0/106 | 2/106 | |
| No | 52/53 | 106/106 | 104/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| Anticoagulant use | 0.411 | |||
| Yes | 49/53 | 101/106 | 96/106 | |
| No | 4/53 | 5/106 | 10/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| Dose of anticoagulant | 0.088 | |||
| Prophylactic | 39/53 | 80/106 | 81/106 | |
| Therapeutic | 14/53 | 26/106 | 25/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| Antiplatelet use | 0.012 | |||
| Yes | 5/53 | 6/106 | 0 | |
| No | 48/53 | 100/106 | 106/106 | |
| B and C | 0.039 | |||
| A and C | 0.005 | |||
| A and B | 0.262 | |||
| Steroids use | 0.002 | |||
| Yes | 45/53 | 105/106 | 98/106 | |
| No | 8/53 | 1/106 | 8/106 | |
| B and C | 0.5 | |||
| A and C | 0.068 | |||
| A and B | 0.001 | |||
| Additive-therapy use | 0.104 | |||
| Yes | 51/53 | 106/106 | 105/106 | |
| No | 2/53 | 0/106 | 1/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| Paracetamol use | 0.019 | |||
| Yes | 50/53 | 105/106 | 106/106 | |
| No | 3/53 | 1/106 | 0/106 | |
| B and C | 0.574 | |||
| A and C | 0.006 | |||
| A and B | 0.022 | |||
| Zinc use | 0.003 | |||
| Yes | 4/53 | 0/106 | 1/106 | |
| No | 49/53 | 106/106 | 105/106 | |
| B and C | 0.614 | |||
| A and C | 0.004 | |||
| A and B | 0.001 | |||
| Acetyl cysteine use | 0.135 | |||
| Yes | 52/53 | 106/106 | 106/106 | |
| No | 1/53 | 0/106 | 0/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| Lactoferrin use | 0.135 | |||
| Yes | 1/53 | 0/106 | 0/106 | |
| No | 52/53 | 106/106 | 106/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| Vitamin C use | 0.07 | |||
| Yes | 4/53 | 7/106 | 1/106 | |
| No | 49/53 | 99/106 | 105/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| O2 therapy use | 0 | |||
| Yes | 37/53 | 99/106 | 102/106 | |
| No | 16/53 | 7/106 | 4/106 | |
| B and C | 0.497 | |||
| A and C | 0 | |||
| A and B | 0 | |||
| NP use | 0.84 | |||
| Yes | 18/53 | 35/106 | 39/106 | |
| No | 35/53 | 71/106 | 67/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| SFM use | 0.002 | |||
| Yes | 30/53 | 82/106 | 87/106 | |
| No | 23/53 | 24/106 | 19/106 | |
| B and C | 0.428 | |||
| A and C | 0 | |||
| A and B | 0.004 | |||
| MR use | 0.003 | |||
| Yes | 8/53 | 33/106 | 14/106 | |
| No | 45/53 | 73/106 | 92/106 | |
| B and C | 0.001 | |||
| A and C | 0.783 | |||
| A and B | 0.019 | |||
| HFNC use | 0.202 | |||
| Yes | 5/53 | 22/106 | 18/106 | |
| No | 48/53 | 84/106 | 88/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| CPAP use | 0 | |||
| Yes | 4/53 | 39/106 | 36/106 | |
| No | 49/53 | 67/106 | 70/106 | |
| B and C | 0.635 | |||
| A and C | 0.001 | |||
| A and B | 0 | |||
| IMV use | 0 | |||
| Yes | 1/53 | 29/106 | 29/106 | |
| No | 52/53 | 77/106 | 77/106 | |
| B and C | 1 | |||
| A and C | 0 | |||
| A and B | 0 | |||
| Vasopressor use | 0.002 | |||
| Yes | 0/53 | 23/106 | 18/106 | |
| No | 53/53 | 83/106 | 88/106 | |
| B and C | 1 | |||
| A and C | 0.016 | |||
| A and B | 0.001 | |||
| Prone positioning | 0.75 | |||
| Yes | 0/53 | 5/106 | 9/106 | |
| No | 53/53 | 101/106 | 97/106 | |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| O2 saturation on O2 therapy | 96.26 ± 2.391 | 95.86 ± 3.795 | 96.01 ± 3.130 | 0.942 |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| O2 saturation on RA | 92.36 ± 4.816 | 87.62 ± 7.171 | 88.35 ± 7.006 | 0 |
| B and C | 0.448 | |||
| A and C | 0 | |||
| A and B | 0 | |||
| PaO2 | 77.868 ± 41.79 | 56.432 ± 35.30 | 63.294 ± 39.45 | 0.005 |
| B and C | 0.252 | |||
| A and C | 0.2 | |||
| A and B | 0.001 | |||
| PaCO2 | 36.689 ± 12.59 | 37.325 ± 14.60 | 37.603 ± 12.08 | 0.891 |
| B and C | NA | |||
| A and C | NA | |||
| A and B | NA | |||
| PaO2/FiO2 | 233.5057 ± 207 | 156.7358 ± 171 | 164.142 ± 138 | 0.01 |
| B and C | 0.136 | |||
| A and C | 0.69 | |||
| A and B | 0.002 | |||
Age: A-C and B-C have a statistically significant difference, but A-B has a statistically non-significant difference[12].
Gender: There is a statistically significant difference between B and C, but not between A and B or A and C[11].
The total number of comorbidities: There is a statistically significant difference between B and C, but not between A and B or A and C[12].
Diagnosis method: The three groups differ in a statistically insignificant way[12].
COVID-19 severity: There are statistically significant differences between A-B and A-C, but no difference exists between C-B. Group A has statistically considerably fewer severe cases than groups B and C[12].
Number of symptoms: There is a statistically significant difference between A-B and A-C and a statistically non-significant difference between C-B[12].
Antibiotics use: In general, there is no statistically significant difference in antibiotic use across the three groups. In the case of macrolides (azithromycin and clarithromycin), there is only a statistically significant difference between A and C.
Use of anticoagulants (enoxaparin, heparin, rivaroxaban): In terms of anticoagulant use, whether preventive or therapeutic dose, there is a statistically insignificant difference between the three groups.
Antiplatelet therapy (aspirin, clopidogrel): There is a statistically significant difference between A and C, but not between A and B and C and B.
Steroids (dexamethasone, prednisolone, and methylprednisolone) usage: A statistically significant difference exists between A and B, but no differences exist between A and C and C and B.
Uses of adjunct therapy (paracetamol, vitamin C, zinc, acetyl cysteine, lactoferrin): Among the three groups, there is a statistically insignificant difference in additive treatment use. Regarding paracetamol and zinc consumption, there is only a statistically significant difference between A-C and A-B.
Use of oxygen therapy: In general, the differences between A-B and A-C are statistically significant, while the difference between C-B is statistically non-significant. In terms of nasal prongs and high-flow nasal cannula use, there is a statistically insignificant difference between the three groups. Regarding the use of a simple face mask (SFM), continuous positive airway pressure, or non-invasive ventilation (NIV), and IMV, there is a statistically significant difference between A-B and A-C. In the use of mask reservoirs (MR), there is a statistically significant difference between B and C.
Use of vasopressors: In terms of vasopressor use, A-B and A-C comparisons show statistically significant differences and C-B comparison shows a statistically non-significant difference. The use of vasopressors is statistically significantly lower in group A than in groups B and C.
Prone positioning: The three groups have a statistically insignificant difference in prone positioning.
Blood gases: There is a statistically significant difference in PaO2 between A-B and A-C, as well as A-B in PaO2/FiO2. In PaCO2, there is a statistically insignificant difference between the three groups.
Table 3[11] shows how regression analysis was used to investigate the effect of baseline parameters-which reveal a statistically significant difference between groups-on the study outcomes and the likelihood of confounding variables.
| Unstandardized coefficients | Standardized coefficients | t-value | P value | |||
| B | Std. Error | Beta | Std. Error | |||
| (Constant) | 0.806 | 1.297 | 0.621 | 0.535 | ||
| Age | 0.003 | 0.001 | 0.098 | 0.053 | 1.835 | 0.068 |
| Gender | 0.029 | 0.044 | 0.038 | 0.058 | 0.652 | 0.515 |
| No of co-morbidities | -0 | 0.015 | -0.01 | 0.048 | -0.144 | 0.885 |
| Severity of COVID | -0 | 0.036 | -0.01 | 0.059 | -0.123 | 0.903 |
| No of symptoms | 0.029 | 0.033 | 0.049 | 0.057 | 0.854 | 0.394 |
| Macrolide | -0.03 | 0.083 | -0.03 | 0.074 | -0.362 | 0.718 |
| Fluroquinolones | 0.001 | 0.068 | 0.001 | 0.073 | 0.02 | 0.984 |
| Cephalosporin | 0.046 | 0.071 | 0.052 | 0.081 | 0.646 | 0.519 |
| Carbapenems | 0.057 | 0.07 | 0.065 | 0.08 | 0.818 | 0.415 |
| Amox/calv | -0.19 | 0.398 | -0.02 | 0.039 | -0.479 | 0.632 |
| Linezolid | -0.01 | 0.072 | -0.01 | 0.058 | -0.097 | 0.923 |
| Teicoplanin | -0.2 | 0.316 | -0.03 | 0.044 | -0.636 | 0.526 |
| Other Antibiotics | -0.03 | 0.105 | -0.01 | 0.043 | -0.274 | 0.784 |
| Anticoagulant | -0.1 | 0.099 | -0.07 | 0.067 | -1.033 | 0.303 |
| Prophylaxis/therapeutic | -0.01 | 0.048 | -0.02 | 0.063 | -0.298 | 0.766 |
| Antiplatelet | -0.03 | 0.083 | -0.02 | 0.06 | -0.34 | 0.734 |
| Steroids | -0.04 | 0.078 | -0.04 | 0.065 | -0.561 | 0.576 |
| Additive therapy | 0.096 | 0.12 | 0.041 | 0.051 | 0.803 | 0.423 |
| Paracetamol | -0.05 | 0.095 | -0.02 | 0.05 | -0.475 | 0.635 |
| Zinc | -0.1 | 0.084 | -0.06 | 0.049 | -1.21 | 0.228 |
| Acetylcysteine | -0.03 | 0.176 | -0.01 | 0.052 | -0.151 | 0.88 |
| Lactoferrin | 0.312 | 0.237 | 0.092 | 0.07 | 1.315 | 0.19 |
| Vitamin C | 0.044 | 0.072 | 0.031 | 0.05 | 0.615 | 0.539 |
| Nasal prongs use | -0 | 0.043 | -0.01 | 0.055 | -0.083 | 0.934 |
| FM use | 0.04 | 0.053 | 0.046 | 0.06 | 0.764 | 0.446 |
| MR use | -0.03 | 0.05 | -0.03 | 0.054 | -0.538 | 0.591 |
| HFNC use | 0.004 | 0.05 | 0.003 | 0.048 | 0.071 | 0.944 |
| CPAP | 0.003 | 0.092 | 0.004 | 0.101 | 0.035 | 0.972 |
| vasopressor | 0.054 | 0.093 | 0.042 | 0.072 | 0.579 | 0.563 |
| Prone position | -0.18 | 0.105 | -0.08 | 0.046 | -1.74 | 0.084 |
| PaO2 | -0 | 0.001 | -0.19 | 0.067 | -2.841 | 0.005 |
| PaCO2 | -0.01 | 0.002 | -0.15 | 0.052 | -2.852 | 0.005 |
| PaO2/Fio2 | 0.001 | 0 | 0.175 | 0.065 | 2.695 | 0.008 |
Table 4 displays the significance of differences in clinical outcomes between the three groups and also includes a pairwise comparison of clinical outcomes between each two groups if there is a statistically significant difference between the three groups[11]. The distributions and frequency of these outcomes across the three groups are depicted in Supple
| Variables | Intervention | |||
| Casirivimab/Imdevimab(A) | Remdesivir (B) | Favipiravir (C) | P valuea | |
| PaO2/FiO2 on day 3 | 298.57 ± 211.3 | 154.14 ± 138.9 | 166.96 ± 130 | 0 |
| B and C | 0.478 | |||
| A and C | 0 | |||
| A and B | 0 | |||
| PaO2/FiO2 on day 7 | 320.62 ± 93.64 | 163.55 ± 172.6 | 178.59 ± 138 | 0 |
| B and C | 0.413 | |||
| A and C | 0 | |||
| A and B | 0 | |||
| PaO2/FiO2 on day 14 | 389.75 ± 51.93 | 154.67 ± 174 | 165.2 ± 98.87 | 0.005 |
| B and C | 0.155 | |||
| A and C | 0.022 | |||
| A and B | 0.001 | |||
| PaO2/FiO2 on day 28 | 172.75 ± 181 | 53 ± 0 | 0.48 | |
| B and C | NA | |||
| Need for IMV | 0.005 | |||
| Yes | 1/53 | 22/106 | 22/106 | |
| No | 52/53 | 84/106 | 84/106 | |
| B and C | 1 | |||
| A and C | 0.003 | |||
| A and B | 0.003 | |||
| Duration of need for O2 therapy and IMV | 3.72 ± 3.527 | 9.2 ± 7.107 | 7.46 ± 5.077 | 0 |
| B and C | 0.119 | |||
| A and C | 0 | |||
| A and B | 0 | |||
Influence on blood oxygen pressure: On days 3, 7, and 14, statistically significant differences in PaO2/FiO2 exist between A-B and A-C.
IMV need during hospitalization: There is a statistically significant difference in IMV need between A-B and A-C.
Influence on the number of days requiring IMV or O2 therapy: There is a statistically significant difference in number of days with need for IMV or oxygen therapy between A-B and A-C.
In this study, casirivimab and imdevimab were compared to remdesivir and favipiravir for treatment in COVID-19 hospitalized patients. There are no comparable treatment comparisons or relevant studies to compare this research to for similarities and differences[11].
The age difference between groups A and B is statistically significant. Group B has statistically considerably more females than group C. The co-morbidities number in group C is statistically considerably higher than in group B. A statistically significant less severe cases exist in group A than groups B and C. Group A has a statistically significantly lower number of symptoms than groups B and C. PaO2 and PaO2/FiO2 values are statistically considerably higher in group A than in group B, and PaO2 values are statistically significantly higher in group A than in group C. In terms of antibiotic use, there is a statistically insignificant difference between the three groups. Antiplatelet (aspirin) use is statistically significant higher in group A than in group C, while steroid use is statistically significant higher in group B than in group A. The use of O2 therapy in group A is statistically significant less than groups B and C and O2 therapy using SFM, NIV, IMV in group A is statistically significant less than groups B and C, while the use of MR as O2 source is more in group B than group C. the use of vasopressors in group A is statistically significant less than groups B and C. Finally, there is statistically significant more cases in group A who not need O2 therapy with statistically significant higher O2 saturation on room air than groups B and C.
Following a statistical analysis of the baseline characteristics of the three groups’ cases, some baseline parameters show statistically significant differences between the three groups. Gender, age, severity of COVID, number of co-morbidities, number of symptoms, usage of antiplatelets, steroids, and zinc upon admission differ between the three groups[11].
As a result, it is vital to rule out the effect of these variables on the study’s outcomes, which are indicated by the necessity for invasive mechanical breathing. As a result, regression analysis was used to investigate the influence of these variables on the study’s outcome (need for IMV). Regression analysis showed that all baseline differences between the three groups did not affect the research outcome (IMV need).
Effect on oxygen pressure in blood: PaO2/FiO2 values on day 3, 7, 14 are statistically significant lower in groups B and C than group A. From these results, it is concluded that group A has more favorable oxygen level in blood than groups B and C.
Need for IMV during hospitalization: Group A has statistically significant lower need for IMV than groups B and C.
Effect on number of days in which there is need for IMV or oxygen therapy: Group A has statistically significant less duration with need for O2 therapy or IMV than groups B and C.
The study’s limitations include non-blinding of drugs, non-randomization of antiviral agents between included patients, applicable only on hospitalized COVID-19 patients (not outpatients), and baseline characteristics differences across the groups. This study’s generalizability is limited to COVID-19 hospitalized patients and does not include COVID-19 outpatients.
Casirivimab and imdevimab achieve less need for O2 therapy and IMV, less duration of this need than remdesivir and favipiravir. So, Casirivimab and imdevimab achieve better outcomes than remdesivir and favipiravir.
Various advances in immunotherapy against coronavirus disease 2019 (COVID-19) have emerged. Casirivimab and imdevimab antibody combination is a type of new immunotherapy against COVID-19. Other antiviral therapy against COVID-19 includes remdesivir and favipiravir.
This study may change the protocol of treatment of COVID-19 patients.
The objectives are to compare the efficacy of antibodies cocktail (casirivimab and imdevimab), remdesivir, and favipravir in reducing the need for invasive mechanical ventilation.
The study design is a single-blind non-randomized controlled trial Mansoura University Hospital owns the study’s drugs. The duration of the study was about 6 mo after ethical approval.
Casirivimab and imdevimab cause less need for O2 therapy, and invasive mechanical ventilation, also they achieve less duration of this need than remdesivir and favipiravir.
Casirivimab and imdevimab achieve better clinical outcomes than remdesivir, and favipravir.
COVID-19 catastrophe causes progress in research works to find an end to this crisis. With ending of 2021 year.
This study is a part of big research that was divided into five parts to enable their publication as it discusses several outcomes (size limitations in journal publication)and one part of this research has been published which is clinical study to compare the efficacy and safety of casirivimab and imdevimab, remdesivir, and favipiravir in hospitalized COVID-19 patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: He Z, China; Karim HMR, India S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | Okonji EF, Okonji OC, Mukumbang FC, Van Wyk B. Understanding varying COVID-19 mortality rates reported in Africa compared to Europe, Americas and Asia. Trop Med Int Health. 2021;26:716-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [Internet]. Bethesda (MD): National Institutes of Health (US); 2021-Apr-21 . [PubMed] |
| 3. | Umakanthan S, Chattu VK, Ranade AV, Das D, Basavarajegowda A, Bukelo M. A rapid review of recent advances in diagnosis, treatment and vaccination for COVID-19. AIMS Public Health. 2021;8:137-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Aleem A, Kothadia JP. Remdesivir. StatPearls. Sep 8, 2022. [cited 8 September 2022]. Available from https://www.ncbi.nlm.nih.gov/books/NBK563261/. |
| 5. | de Almeida SMV, Santos Soares JC, Dos Santos KL, Alves JEF, Ribeiro AG, Jacob ÍTT, da Silva Ferreira CJ, Dos Santos JC, de Oliveira JF, de Carvalho Junior LB, de Lima MDCA. COVID-19 therapy: What weapons do we bring into battle? Bioorg Med Chem. 2020;28:115757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Owji H, Negahdaripour M, Hajighahramani N. Immunotherapeutic approaches to curtail COVID-19. Int Immunopharmacol. 2020;88:106924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Baum A, Fulton BO, Wloga E, Copin R, Pascal KE, Russo V, Giordano S, Lanza K, Negron N, Ni M, Wei Y, Atwal GS, Murphy AJ, Stahl N, Yancopoulos GD, Kyratsous CA. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369:1014-1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1116] [Cited by in RCA: 1017] [Article Influence: 203.4] [Reference Citation Analysis (0)] |
| 8. | Hansen J, Baum A, Pascal KE, Russo V, Giordano S, Wloga E, Fulton BO, Yan Y, Koon K, Patel K, Chung KM, Hermann A, Ullman E, Cruz J, Rafique A, Huang T, Fairhurst J, Libertiny C, Malbec M, Lee WY, Welsh R, Farr G, Pennington S, Deshpande D, Cheng J, Watty A, Bouffard P, Babb R, Levenkova N, Chen C, Zhang B, Romero Hernandez A, Saotome K, Zhou Y, Franklin M, Sivapalasingam S, Lye DC, Weston S, Logue J, Haupt R, Frieman M, Chen G, Olson W, Murphy AJ, Stahl N, Yancopoulos GD, Kyratsous CA. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369:1010-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 994] [Article Influence: 198.8] [Reference Citation Analysis (0)] |
| 9. | Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, Perry C, Pan C, Hosain R, Mahmood A, Davis JD, Turner KC, Hooper AT, Hamilton JD, Baum A, Kyratsous CA, Kim Y, Cook A, Kampman W, Kohli A, Sachdeva Y, Graber X, Kowal B, DiCioccio T, Stahl N, Lipsich L, Braunstein N, Herman G, Yancopoulos GD; Trial Investigators. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med. 2021;384:238-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1296] [Article Influence: 324.0] [Reference Citation Analysis (0)] |
| 10. | Food and drug administration. Emergency use authorization (EUA) of regen-cov (casirivimab and imdevimab): Food and Drug Administration (FDA). Sep 9, 2021. [cited 9 September 2021]. Available from: https://www.fda.gov/media/145611/download. |
| 11. | Hegazy SK, Tharwat S, Hassan AH. Clinical study to compare the efficacy and safety of casirivimab & imdevimab, remdesivir, and favipravir in hospitalized COVID-19 patients. J Clin Virol Plus. 2023;3:100151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Hegazy SK, Tharwat S, Hassan AH. Study to compare the effect of casirivimab & imdevimab, remdesivir, and favipiravir on progression and multi-organ function of hospitalized COVID-19 patients. Open Medicine. 2023;18:20230768. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |