Published online Sep 16, 2023. doi: 10.12998/wjcc.v11.i26.6040
Peer-review started: June 30, 2023
First decision: July 18, 2023
Revised: July 19, 2023
Accepted: August 18, 2023
Article in press: August 18, 2023
Published online: September 16, 2023
Processing time: 70 Days and 0.3 Hours
At present, understanding of the most effective ventilation methods for treating chronic obstructive pulmonary disease (COPD) patients experiencing acute worsening symptoms and respiratory failure remains relatively limited. This report analyzed the efficiency and side effects of various ventilation techniques used for individuals experiencing an acute COPD exacerbation.
To determine whether pressure-controlled ventilation (PCV) can lower peak airway pressures (PAPs) and reduce the incidence of barotrauma compared to volume-controlled ventilation (VCV), without compromising clinical outcomes and oxygenation parameters.
We have evaluated 600 patients who were hospitalized due to a severe COPD exacerbation, with 400 receiving mechanical ventilation for the respiratory failure. The participants were divided into two different groups, who were administered either VCV or PCV, along with appropriate management. We thereafter observed patients' attributes, clinical factors, and laboratory, radiographic, and arterial blood gas evaluations at the start and during their stay in the intensive care unit (ICU). We have also employed appropriate statistical methods for the data analysis.
Both the VCV and PCV groups experienced significant enhancements in the respiratory rate, tidal volume, and arterial blood gas values during their time in the ICU. However, no significant distinctions were detected between the groups in terms of oxygenation indices (partial pressures of oxygen/raction of inspired oxygen ratio) and partial pressures of carbon dioxide improvements. There was no considerable disparity observed between the VCV and PCV groups in the hospital mortality (32% vs 28%, P = 0.53), the number of days of ICU stay [median interquartile range (IQR): 9 (6-14) d vs 8 (5-13) d, P = 0.41], or the duration of the mechanical ventilation [median (IQR): 6 (4-10) d vs 5 (3-9) d, P = 0.47]. The PCV group displayed lower PAPs compared to the VCV group (P < 0.05) from the beginning of mechanical ventilation until extubation or ICU departure. The occurrence of barotrauma was considerably lower in the PCV group in comparison to the VCV group (6% vs 16%, P = 0.03).
Both VCV and PCV were found to be effective in treating patients with acute COPD exacerbation. However, PCV was associated with lower PAPs and a significant decrease in barotrauma, thus indicating that it might be a safer ventilation method for this group of patients. However, further large-scale study is necessary to confirm these findings and to identify the best ventilation approach for patients experiencing an acute COPD exacerbation.
Core Tip: Mechanical ventilation is a life-saving intervention for patients with acute exacerbation of chronic obstructive pulmonary disease (COPD) and respiratory failure. Volume-controlled ventilation (VCV) and pressure-controlled ventilation (PCV) are two common modes of mechanical ventilation with different advantages and disadvantages. This study compared the efficacy and safety of VCV and PCV in patients with acute COPD exacerbation and respiratory failure. The results showed that both VCV and PCV were effective in treating patients with acute COPD exacerbation, but PCV was associated with lower peak airway pressures and a significant decrease in barotrauma, indicating that it might be a safer ventilation method for this group of patients. However, further large-scale studies are necessary to confirm these findings and identify the best ventilation approach for patients experiencing an acute COPD exacerbation. Clinicians should weigh the benefits and risks of each mode of mechanical ventilation when selecting a ventilation strategy.
- Citation: Wang JJ, Zhou Z, Zhang LY. Clinical evaluation of ventilation mode on acute exacerbation of chronic obstructive pulmonary disease with respiratory failure. World J Clin Cases 2023; 11(26): 6040-6050
- URL: https://www.wjgnet.com/2307-8960/full/v11/i26/6040.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i26.6040
Chronic obstructive pulmonary disease (COPD) is a progressive and debilitating respiratory condition which is characterized by permanent airflow restriction and persistent inflammation in the lungs[1]. COPD presents a considerable global health challenge as it is the third leading cause of mortality worldwide and incurs significant healthcare costs for global populations[2]. The development of COPD has been linked to numerous risk factors, including tobacco smoke, air pollution, occupational exposures, and genetic predisposition, which can contribute to both onset and exacerbation of the disease[3,4]. As a chronic condition, COPD has been associated with significant morbidity and diminished quality of life for the affected individuals. A number of aggravating factors have been identified during the disease process, these factors can culminate in acute exacerbations of COPD (AECOPD)[5]. Some factors that cause AECOPD have been identified, including respiratory tract infections, smoking, environmental pollution, prior exacerbations, non-adherence to treatment and associated comorbidities[6-8]. These factors may affect the condition and prognosis of COPD patients through different mechanisms, such as increasing inflammation in the airways and lungs, damaging airway epithelial cells, stimulating airway smooth muscle contraction, reducing lung function and immunity, increasing cardiovascular risk and systemic inflammation levels, etc.[9]. Therefore, timely identification and management of these factors are very important for preventing and reducing the occurrence and severity of AECOPD.
These acute exacerbations typically involve increased symptoms, such as deteriorating dyspnea, heightened sputum production, and more frequent coughing, thus causing a significant decline in the patient's clinical status.
AECOPD represents a significant event in the natural course of COPD which poses a substantial clinical and economic burden to the patients, healthcare providers, and healthcare systems worldwide. It is often marked by persistent and progressive airflow restriction in the lungs and accompanied by chronic inflammatory responses[10]. Acute exacerbations can rapidly result in the deterioration of lung function, thereby elevating the risk of hospitalization and, in severe cases, leading to respiratory failure requiring mechanical ventilation[11]. Indeed, AECOPD with respiratory failure has been found to be a major contributor of death among COPD patients[12]. Invasive mechanical ventilation is often regarded as the last resort for management of COPD patients with respiratory failure; however, when appropriately administered, it can be life-saving in several cases[13].
The significance of effective and timely mechanical ventilation cannot be underestimated, because it has been reported to minimize the complications and enhance clinical outcomes in several critically ill patients[14].
However, a number of factors must be considered when selecting the optimal ventilation mode and settings for the patients with respiratory failure due to AECOPD, including lung mechanics, gas exchange, and hemodynamic consequences[15]. Additionally, in recent years, various mechanical ventilation modes have been developed and employed in the clinical practice, with primary aim to optimize patient-ventilator interaction and minimize ventilator-induced lung injury (VILI). Among these, volume-controlled ventilation (VCV) and pressure-controlled ventilation (PCV) are the two commonly used modes in treating critically ill patients with respiratory failure[16].
VCV, the conventional ventilation mode is frequently used to deliver a pre-set tidal volume to the patient within a specific time, thus resulting in a consistently delivered volume but variable airway pressures. Moreover, VCV has been reported to improve the gas exchange and respiratory mechanics in patients with respiratory failure. It has also been associated with higher airway pressures, thereby potentially inducing barotrauma and hemodynamic side effects, such as reduced venous return as well as impaired cardiac output[17]. On the other hand, in PCV, the ventilator can deliver a pre-set inspiratory pressure, and the resulting tidal volume can fluctuate according to the patient's lung mechanics. PCV has been reported to offer relatively better control of peak and plateau airway pressures, thus resulting in improved patient safety and reduced barotrauma risk[18]. In general, the VCV can ensure the minute ventilation and oxygenation level, but it may cause higher airway pressure and barotrauma risk, as well as negative effects on the cardiovascular system[19]. However, the PCV can control the peak and plateau airway pressure, thus improving patient safety and reducing barotrauma risk, but it may cause fluctuations in tidal volume and minute ventilation, and decrease respiratory synchrony[17]. Therefore, when choosing a mechanical ventilation mode, factors such as patient condition, lung mechanics, airway resistance, airway leakage, cardiovascular function, etc. should be considered comprehensively to achieve the best ventilation effect and the least complications. However, the debate over superiority of either of the two modes in managing the patients with respiratory failure due to AECOPD remains a significant point of discussion among the clinicians and researchers[20]. It has been established that selecting an appropriate ventilation method can significantly influence the results of AECOPD patients needing mechanical ventilation, thereby leading to improved survival rates, quicker liberation from ventilatory assistance, shorter stays in intensive care units (ICU), and fewer complications[21,22]. Overall, given these important considerations, it is crucial to identify the most efficient and secure ventilation strategy for clinically managing the patients with respiratory failure due to AECOPD.
This study aimed to compare the clinical outcomes and examine the possible effects on patient outcomes associated with the two prevalent modes of mechanical ventilation, VCV and PCV, in patients with respiratory failure due to AECOPD. The research also examined the occurrence of VILI and barotrauma and investigated the prevalence of ventilator-associated pneumonia (VAP) as well as other complications linked with VCV and PCV application. The novelty and significance of this study lie in the fact that there is no clear evidence to indicate which ventilation mode is more suitable for patients with respiratory failure due to AECOPD, who often have special problems such as lung heterogeneity, increased airway resistance, airway leakage, etc., requiring individualized ventilation strategies. This study may help to provide more evidence-based guidance for clinicians to choose the optimal ventilation mode for these patients, in order to improve their survival and quality of life, and reduce mechanical ventilation-related complications.
This research employed a prospective, observational cohort design to assess the effectiveness and side effects related to application of the different ventilation techniques in patients experiencing acute COPD exacerbation accompanied by respiratory failure. This study was conducted for two years and received ethical approval from the Institutional Ethics Committee (Approval Number: 20190034), in line with the principles of the Declaration of Helsinki. Before enrollment, written informed consent was collected from the patients or their legal representatives.
Throughout the study, 500 patients suffering an acute COPD exacerbation were admitted. The inclusion criteria were as following: (1) Be aged 18 years or older; (2) Have a confirmed diagnosis of COPD according to the Global Initiative for COPD (GOLD) guidelines; (3) Experiencing a respiratory failure, requiring mechanical ventilation; and (4) Provided informed consent to participate in the study. Exclusion criteria included patients who (1) Had concurrent lung disorders; (2) Experienced respiratory failure due to reasons other than acute COPD exacerbation; or (3) Were unable to provide informed consent. Among the 500 admissions, 400 patients met these requirements and were included in the study. These patients were allowed to receive therapies according to the standard guidelines for managing AECOPD, such as antibiotics, bronchodilators, corticosteroids, and oxygen therapy.
The participants were divided into two distinct clusters based on the ventilation technique utilized during their stay in ICU: A VCV group and a PCV group. The assignment of the patients to either group was based on the physicians’ suggestions and individual patient needs, with a deliberate attempt to maintain similar demographic and clinical features between the two groups.
Both the cohorts received standardized treatments based on the GOLD guidelines for AECOPD management, in conjunction with the mechanical ventilation. The ventilator settings were initially set based on ideal body weight and clinical condition of each patient, thus mainly targeting a tidal volume of 6-8 mL/kg, a respiratory rate of 12-20 breaths per minute, and a positive end-expiratory pressure (PEEP) of 5-12 cm H2O.
The ventilator settings and management were then adjusted based on the patients' clinical and physiological parameters, with a focus on minimizing lung injury and providing adequate oxygenation as well as ventilation. For VCV patients, various adjustments were made in tidal volume, respiratory rate, and PEEP based on the measured airway pressures and gas exchange status. On the contrary, for PCV patients, the inspiratory pressure was adjusted according to oxygenation targets as well as airway pressure limitations. The respiratory rate and PEEP were also modified based on clinical findings and gas exchange parameters.
Additionally, in both the groups, optimal attention was given to minimizing excessive sedation, thus ensuring adequate pain control, and providing appropriate patient-ventilator synchrony. The use of adjunct therapies, such as inhaled bronchodilators, corticosteroids, antibiotics, and diuretics, was tailored to meet the requirements of individual patients based on their specific clinical and laboratory findings.
All the patients were closely monitored for their readiness to wean from the mechanical ventilation. The weaning process was initiated once the patients have demonstrated significant clinical improvement, acceptable gas exchange parameters, and adequate airway protection. A structured weaning protocol was followed, starting with a spontaneous breathing trial using a T-piece, followed by proper assessment of the patients' tolerance and performance during the trial. The successful completion of the spontaneous breathing trial was followed by the extubation of the patient. However, patients who failed to complete the trial successfully were reconnected to the mechanical ventilation and reassessed regularly for their readiness to wean.
Upon admission, relevant information about different demographic characteristics (age, sex, and body mass index), co-existing morbidities (hypertension, diabetes mellitus, coronary artery disease), smoking status, and severity scores (Acute Physiology and Chronic Health Evaluation II and Modified Medical Research Council dyspnea scale) were noted before starting the treatment.
At the start of the mechanical ventilation, various baseline clinical factors such as vital signs, respiratory rate, tidal volume, arterial blood gas metrics [pH, partial pressures of oxygen (PaO2), fraction of inspired oxygen (FiO2), as well as partial pressures of carbon dioxide (PaCO2)], and hemodynamic indicators (heart rate, blood pressure) were documented. The Primary outcomes assessed included in-hospital mortality, ICU duration, and length of mechanical ventilation. The secondary outcomes that were measured included changes in the respiratory and oxygenation parameters, complications (VILI, VAP, and hemodynamic complications like arrhythmias and low blood pressure), as well as the need for additional interventions during ICU stay.
Descriptive statistics were employed to analyze the obtained data. The continuous variables were expressed as mean ± SD or median with interquartile range, depending on the data distribution. The various categorical variables were reported as absolute numbers and percentages. The differences between the two groups were analyzed using Student's t-test or Mann-Whitney U test for the continuous variables, based on the data distribution. The chi-square test or Fisher's exact test, as suitable, was applied to compare the categorical variables. Kaplan-Meier curves were constructed for comparison of the cumulative probabilities of survival between the two groups. A Cox proportional hazards regression model was utilized to assess the potential effect of ventilation mode on in-hospital mortality, controlling for potential confounders. All tests conducted were two-sided, and a P value below 0.05 was considered as statistically significant. Statistical analyses were conducted with Statistical Package for the Social Sciences (SPSS), version 22.0 (IBM Corp., Armonk, NY, United States).
Among the 400 participants enrolled in this study, 200 patients were allocated to the VCV group, whereas the remaining 200 were assigned to the PCV group. At baseline, there were no significant differences observed in the demographic and clinical attributes between the patients included in the two groups, including age, sex, body mass index, co-existing diseases, smoking status, and severity scores (P > 0.05) (Table 1).
| Characteristic | VCV group (n = 200) | PCV group (n = 200) | P value |
| Age (mean ± SD, years) | 67.6 ± 10.3 | 68.4 ± 11.1 | 0.60 |
| Sex (male, %) | 58 | 60 | 0.81 |
| Body mass index (mean ± SD, kg/m2) | 25.3 ± 3.6 | 24.8 ± 3.4 | 0.23 |
| Hypertension (%) | 38 | 42 | 0.62 |
| Diabetes mellitus (%) | 28 | 32 | 0.57 |
| Coronary artery disease (%) | 22 | 19 | 0.68 |
| Smoking history (%) | 76 | 78 | 0.85 |
| APACHE II score (mean ± SD) | 18.4 ± 5.8 | 17.9 ± 6.3 | 0.53 |
| MMRC dyspnea scale [median (IQR)] | 3 (2-4) | 3 (2-4) | 0.78 |
Both the groups exhibited significant increase in the respiratory rate, tidal volume, and arterial blood gas parameters during their ICU stay (P < 0.05). However, no significant disparities were observed between the VCV and PCV groups regarding oxygenation indices and PaCO2 improvement throughout the study (P > 0.05). In addition, with respect to the primary clinical outcomes (Table 2), no significant differences emerged between the VCV and PCV groups related to in-hospital mortality, ICU duration, or mechanical ventilation duration (P > 0.05 for each comparison).
| Parameter | VCV group (initial/final) | PCV group (initial/final) | P value |
| Respiratory rate | 22/16 | 24/16 | < 0.05 |
| Tidal volume | 500/480 | 520/470 | < 0.05 |
| pH | 7.35/7.45 | 7.30/7.43 | < 0.05 |
| PaO2 | 75/85 | 80/85 | < 0.05 |
| PaCO2 | 45/38 | 47/39 | < 0.05 |
| PaO2/FiO2 ratio | 300/350 | 315/345 | > 0.05 |
Nonetheless, the PCV group exhibited significantly lower peak airway pressures (PAPs) in comparison to the VCV group (P < 0.05), and the incidence of barotrauma was also markedly reduced (6% vs 16%, P = 0.03) (Table 3). Besides, we adjusted for the potential confounding factors of other drugs that administered to manage AECOPD (including antibiotics, bronchodilators, corticosteroids, and oxygen therapy) in our statistical analysis, which were found to be not affect the comparison of VCV and PCV in terms of clinical outcomes and safety.
| Outcome | VCV group (n = 200) | PCV group (n = 200) | P value |
| In-hospital mortality (%) | 32 | 28 | 0.53 |
| ICU length of stay [median (IQR), days] | 9 (6-14) | 8 (5-13) | 0.41 |
| Duration of mechanical ventilation [median (IQR), days] | 6 (4-10) | 5 (3-9) | 0.47 |
| Lower Peak Airway Pressures | N/A | N/A | < 0.05 |
| Barotrauma (%) | 16 | 6 | 0.03 |
There were no significant disparities found in the occurrence of VAP or multiple organ dysfunction syndrome (MODS) between the two distinct groups (P > 0.05), but cardiovascular complications were slightly more common in the VCV group in comparison to the PCV group (22% vs 12%, P = 0.05). These complications consisted of arrhythmias, low blood pressure and myocardial ischemia, which required additional interventions.
The demand for the different rescue therapies [prone positioning, recruitment maneuvers, and extracorporeal membrane oxygenation (ECMO)] was also evaluated and no significant differences were observed between the VCV and PCV groups (P > 0.05) (Table 4).
| Complication | VCV group (n = 200) | PCV group (n = 200) | P value |
| Ventilator-associated pneumonia (%) | 29 | 27 | 0.85 |
| Multiple organ dysfunction syndrome (%) | 16 | 14 | 0.75 |
| Cardiovascular complications (%) | 22 | 12 | 0.05 |
| Rescue therapies (%) | N/A | N/A | > 0.05 |
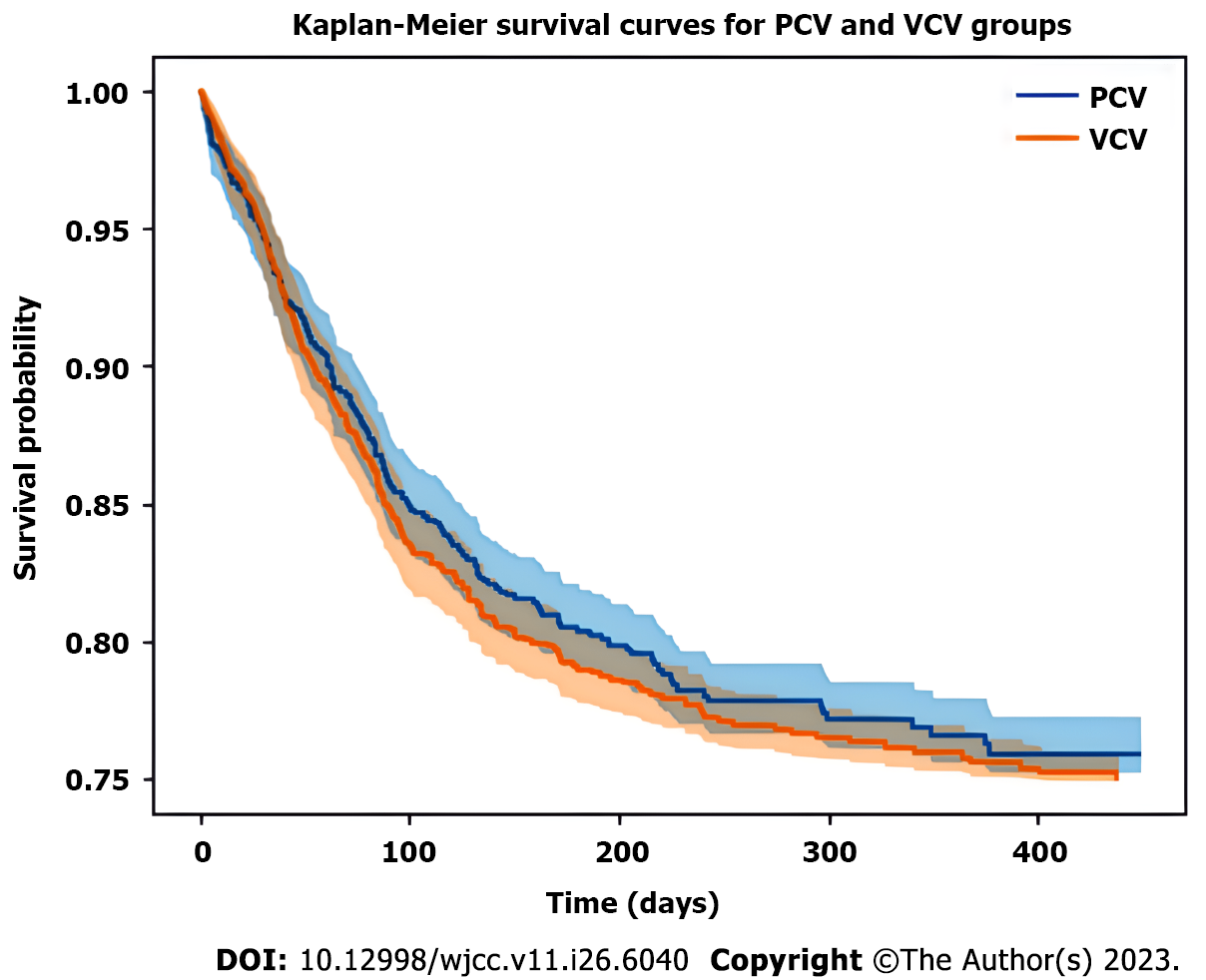
The results of Kaplan-Meier survival analysis indicated no significant disparities in the cumulative probabilities of overall survival between the two groups (log-rank test, P = 0.87) (Figure 1). Furthermore, the Cox proportional hazards regression model showed no significant effect of ventilation mode on in-hospital mortality after accounting for potential the various confounding variables (log hazard ratio for PCV group vs VCV group: -0.16, 95% confidence interval: -0.35-0.04, P = 0.82) (Figure 2).
The objective of our study was to evaluate and compare the efficacy as well as safety of VCV and PCV in patients with acute exacerbation of AECOPD who were experiencing respiratory failure. Our results indicated that no significant differences were observed in the primary outcomes, including in-hospital mortality, ICU length of stay, and duration of mechanical ventilation, between the VCV and PCV groups. However, we did notice some differences in respiratory and oxygenation parameters, complications, and the requirement for additional interventions.
The initial characteristics of patients in both the groups were similar, thereby ensuring that the study groups were balanced with respect to the demographic and clinical variables. Both VCV and PCV were able to effectively manage patients with AECOPD and respiratory failure, as demonstrated by improvements in the respiratory rate, tidal volume, arterial blood gas values (including pH, PaO2, and PaCO2), and oxygenation indices (PaO2/FiO2 ratio) during their ICU stay. This observation was consistent with a previous studies, thus suggesting that both ventilation modes can be used successfully to treat patients with AECOPD suffering from the respiratory failure[23,24].
Although both VCV and PCV were able to effectively manage patients with AECOPD and respiratory failure in our study, consistent with previous reports, our study also revealed some novel and significant differences between the two ventilation modes in terms of respiratory mechanics, oxygenation parameters, complications, and additional interventions. As an important complications of mechanical ventilation that can affect patient prognosis and quality of life, the occurrence of VILI and barotrauma was essentially analyzed[25]. The requirement for additional interventions was also evaluated in this study, such as noninvasive ventilation, prone positioning, or ECMO, which can indicate the severity and complexity of the patient’s condition and the adequacy of the ventilation mode[26]. These differences may have important implications for the choice of ventilation mode for this specific and challenging subgroup of COPD patients. To our knowledge, this is the first study to compare VCV and PCV in such a comprehensive and detailed manner in patients with AECOPD and respiratory failure.
Interestingly, we found that the PCV group had significantly PAPs in comparison to the VCV group. This difference was observed from the initiation of mechanical ventilation until extubation or ICU discharge. Lower PAPs have been reported to be beneficial for patients with respiratory failure, as they can help minimize the risk of VILI and alveolar overdistension[27]. Increased airway pressures can lead to barotrauma, manifesting as pneumothorax, pneumomediastinum, or subcutaneous emphysema[28,29]. In our study, the incidence of barotrauma was noted to be significantly lower in the PCV group compared to the VCV group. This could be possibly attributed to the lower PAPs achieved with PCV.
A noteworthy feature of PCV is its ability to provide better control of airway pressures, thereby significantly reducing the risk of various complications related to high airway pressures, such as VILI and barotrauma. These complications can further impair the respiratory function and lead to increased mortality in patients experiencing respiratory failure[30,31]. Thus, it is crucial to assess the potential advantages and disadvantages associated with each ventilation mode concerning the specific patient populations.
Another significant finding in this study was the marginally higher incidence of cardiovascular complications diagnosed in the VCV group compared to the PCV group. These complications encompassed arrhythmias, hypotension, and myocardial ischemia, necessitating supplemental interventions like inotropic support and anti-arrhythmic medications. This disparity could be partially attributed to the hemodynamic impact of the two ventilation modes[32,33]. Interestingly, In comparison to VCV, PCV has been reported to exert more favorable effects on cardiac output, arterial blood pressure, and venous return[34]. The underlying mechanisms for these differences may probably involve changes in intrathoracic pressure, along with the sequential activation of respiratory muscles, which could directly or indirectly affect the cardiac function[35,36]. VCV may also cause lung overdistension and injury, which can induce inflammatory responses and impair cardiac function[37]. Therefore, PCV may be more advantageous than VCV for patients with AECOPD and respiratory failure who have underlying cardiovascular diseases or risk factors.
Regarding complications and additional interventions, our study discovered that no significant differences could be found between the two groups in the incidence VAP or MODS. The need for rescue therapies, such as prone positioning, recruitment maneuvers, and ECMO, was also found to be identical between the two groups. These results further highlighted the similar efficacy of VCV and PCV in managing AECOPD patients with respiratory failure, as reported in several previous studies[17], with a slight advantage favoring use of PCV in terms of preventing barotrauma and cardiovascular complications.
It is essential to recognize that there is currently no universal approach when selecting a ventilation mode for the respiratory failure in AECOPD. The choice of an appropriate ventilation strategy should be tailored based on the clinical status of affected patient, underlying lung mechanics, and tolerance to the various ventilation modes[38]. In addition, emerging reports have suggested that a more personalized approach to mechanical ventilation can yield significantly better outcomes and fewer complications for patients with respiratory failure. For example, Pelosi et al[39] suggested that ventilatory parameters should be titrated according to individualized goals and close monitoring of targeted physiologic variables through reviewing the current evidence and challenges for personalized mechanical ventilation in acute respiratory distress sydrome (ARDS), based on lung physiology and morphology, ARDS etiology, lung imaging, and biological phenotypes[39]. Similarly, Gattinoni et al[40] proposed a personalized approach to mechanical ventilation in ARDS, based on the concept of lung recruitability and the distribution of aeration within the lung. They argued that different ventilation modes and settings should be applied according to the degree of lung recruitability and the presence of non-aerated or overdistended regions in the lung[40]. Furthermore, Sinha and Calfee[41] advocated for a personalized approach to mechanical ventilation in ARDS and pointed out the potential of precision medicine to improve the diagnosis and treatment, based on the identification of biological phenotypes using biomarkers and gene expression profiles[41].
There are several limitations associated with our study. The sample size was relatively small, and all patients were recruited from a single center, which may effectively limit the generalizability of our findings. Furthermore, patient allocation to the VCV or PCV groups was primarily based on the discretion of physician, as opposed to randomization, and thus may be subject to some selection bias. Future studies should be designed to address these limitations by utilizing larger sample sizes and implementing a randomized controlled design to further elucidate the role of various ventilation modes in patients affected with AECOPD and respiratory failure.
Our findings demonstrated that both VCV and PCV were effective in managing AECOPD patients with the respiratory failure. However, the utilization of PCV was associated with lower PAPs, reduced incidence of barotrauma, and slightly fewer cardiovascular complications, which suggested that PCV may provide some marginal benefits over VCV in this patient population. The possible mechanisms for these benefits may be related to the lower airway pressures, better oxygenation, and more favorable hemodynamics achieved by PCV compared to VCV. However, Further investigation is needed to confirm these findings and to identify the most effective ventilation strategies for patients suffering with respiratory failure due to AECOPD.
When Respiratory failure occurs in patients with acute exacerbation of chronic obstructive pulmonary disease (COPD), the most effective ventilation method is still unclear. This study analyzed the effects and side effects of different ventilation techniques on the deterioration of acute COPD. Mechanical ventilation is a life-saving measure for patients with exacerbation of acute COPD and Respiratory failure, but it may also lead to adverse reactions. This study compared the efficacy and safety of volume control ventilation (VCV) and pressure control ventilation (PCV) in patients with exacerbation of acute COPD and Respiratory failure. The results showed that PCV can reduce peak airway pressure (PAP) and the incidence of barotrauma, which may be a safer ventilation mode.
At present, there is limited understanding of the most effective ventilation methods for treating patients with acute exacerbation of COPD. The purpose of this study was to compare the efficacy and safety of VCV and PCV in patients with exacerbation of acute COPD and Respiratory failure. We studied 600 hospitalized patients with severe exacerbation of COPD, of whom 400 received mechanical ventilation. The results showed that the PCV group performed better in terms of PAP and the incidence of barotrauma compared to the VCV group.
The primary objective of this study is to compare the efficacy and safety of VCV and PCV in patients experiencing an acute exacerbation of COPD and respiratory failure. We aim to determine whether PCV can lower PAPs and reduce the incidence of barotrauma compared to VCV, without compromising clinical outcomes and oxygenation parameters. Additionally, we seek to explore the factors influencing the choice of ventilation mode and the prognosis of these patients, including lung mechanics, gas exchange, hemodynamics, and complications.
This study evaluated 600 hospitalized patients with severe COPD exacerbation, of which 400 required mechanical ventilation for respiratory failure. The participants were divided into two groups, receiving either VCV or PCV with appropriate management. Patient characteristics, clinical factors, and laboratory, radiographic, and arterial blood gas evaluations were observed at the start and during their intensive care unit (ICU) stay. Statistical methods were employed for data analysis. Both VCV and PCV groups showed significant improvements in respiratory rate, tidal volume, and arterial blood gas values during ICU stay. No significant differences were found between the groups in terms of oxygenation indices and partial pressures of carbon dioxide (PaCO2) improvements. PCV demonstrated lower PAPs throughout mechanical ventilation and a significantly lower incidence of barotrauma compared to VCV.
Both VCV and PCV were effective in treating patients with acute exacerbation of COPD. Significant improvements were observed in respiratory rate, tidal volume, and arterial blood gas values for both groups during ICU stay. There were no significant differences between VCV and PCV in terms of oxygenation indices and PaCO2 improvements. Hospital mortality, duration of ICU stay, and duration of mechanical ventilation did not show significant differences between the two groups. However, the PCV group exhibited lower PAPs throughout mechanical ventilation and a significantly lower incidence of barotrauma compared to the VCV group. These findings suggest that PCV may be a safer ventilation method for patients with acute COPD exacerbation.
This study compared VCV and PCV in patients with acute exacerbation of COPD and respiratory failure. Both VCV and PCV were found to be effective in improving respiratory parameters and arterial blood gas values during ICU stay. However, PCV demonstrated advantages over VCV, with lower PAPs and a significantly reduced incidence of barotrauma. There were no significant differences between the two groups in terms of oxygenation indices, hospital mortality, duration of ICU stay, or duration of mechanical ventilation. These findings suggest that PCV may be a safer ventilation method for patients with acute COPD exacerbation.
This study highlights the need for a better understanding of effective ventilation methods for acute COPD exacerbation and respiratory failure. The comparison between VCV and PCV provides insights into their efficacy and safety, with PCV showing advantages such as lower PAPs and reduced barotrauma incidence. However, further large-scale studies are required to validate these findings and determine the optimal ventilation approach. Future research should focus on identifying factors that influence ventilation mode selection and patient prognosis, including lung mechanics, gas exchange, hemodynamics, and complications. Additionally, personalized ventilation strategies tailored to individual characteristics and needs should be explored to enhance patient outcomes. These efforts aim to improve the survival and quality of life for individuals experiencing acute COPD exacerbation.
I would like to express my gratitude to all those who participated in the manuscript research together.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Klapper W, Germany; Leis JA, Canada S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 1026] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 2. | Lashari BH, Criner GJ. Advances in Surgical and Mechanical Management of Chronic Obstructive Pulmonary Disease. Med Clin North Am. 2022;106:1013-1025. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Brandsma CA, Van den Berge M, Hackett TL, Brusselle G, Timens W. Recent advances in chronic obstructive pulmonary disease pathogenesis: from disease mechanisms to precision medicine. J Pathol. 2020;250:624-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 4. | Eapen MS, Hansbro PM, Larsson-Callerfelt AK, Jolly MK, Myers S, Sharma P, Jones B, Rahman MA, Markos J, Chia C, Larby J, Haug G, Hardikar A, Weber HC, Mabeza G, Cavalheri V, Khor YH, McDonald CF, Sohal SS. Chronic Obstructive Pulmonary Disease and Lung Cancer: Underlying Pathophysiology and New Therapeutic Modalities. Drugs. 2018;78:1717-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Deep A, Behera PR, Subhankar S, Rajendran A, Rao CM. Serum Electrolytes in Patients Presenting With Acute Exacerbation of Chronic Obstructive Pulmonary Disease (COPD) and Their Comparison With Stable COPD Patients. Cureus. 2023;15:e38080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Viniol C, Vogelmeier CF. Exacerbations of COPD. Eur Respir Rev. 2018;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 7. | Dixit D, Bridgeman MB, Andrews LB, Narayanan N, Radbel J, Parikh A, Sunderram J. Acute exacerbations of chronic obstructive pulmonary disease: diagnosis, management, and prevention in critically ill patients. Pharmacotherapy. 2015;35:631-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Viniol C, Vogelmeier CF. Risk factors of chronic obstructive pulmonary disease exacerbations. Clin Respir J. 2020;14:323-334. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 9. | Wedzicha JA. Mechanisms of Chronic Obstructive Pulmonary Disease Exacerbations. Ann Am Thorac Soc. 2015;12 Suppl 2:S157-S159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Srivastava K, Thakur D, Sharma S, Punekar YS. Systematic review of humanistic and economic burden of symptomatic chronic obstructive pulmonary disease. Pharmacoeconomics. 2015;33:467-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Crisafulli E, Barbeta E, Ielpo A, Torres A. Management of severe acute exacerbations of COPD: an updated narrative review. Multidiscip Respir Med. 2018;13:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Deniz S, Şengül A, Aydemir Y, Çeldir Emre J, Özhan MH. Clinical factors and comorbidities affecting the cost of hospital-treated COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:3023-3030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | McCurdy BR. Noninvasive positive pressure ventilation for acute respiratory failure patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser. 2012;12:1-102. [PubMed] |
| 14. | Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A; COVID-19 Lombardy ICU Network. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3537] [Cited by in RCA: 3826] [Article Influence: 765.2] [Reference Citation Analysis (0)] |
| 15. | Abbas A, Embarak S, Walaa M, Lutfy SM. Role of diaphragmatic rapid shallow breathing index in predicting weaning outcome in patients with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:1655-1661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Jo YY, Chang YJ, Lee D, Kim YB, Jung J, Kwak HJ. Comparisons of Mechanical Power and Respiratory Mechanics in Pressure-Controlled Ventilation and Volume-Controlled Ventilation during Laparoscopic Cholecystectomy in Elderly Patients. J Pers Med. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Kim KN, Kim DW, Jeong MA, Sin YH, Lee SK. Comparison of pressure-controlled ventilation with volume-controlled ventilation during one-lung ventilation: a systematic review and meta-analysis. BMC Anesthesiol. 2016;16:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Schick V, Dusse F, Eckardt R, Kerkhoff S, Commotio S, Hinkelbein J, Mathes A. Comparison of Volume-Guaranteed or -Targeted, Pressure-Controlled Ventilation with Volume-Controlled Ventilation during Elective Surgery: A Systematic Review and Meta-Analysis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (2)] |
| 19. | Ugurlucan M, Basaran M, Erdim F, Selimoglu O, Caglar IM, Zencirci E, Filizcan U, Ogus NT, Yildiz Y, Tireli E, Isik O, Dayioglu E. Pressure-controlled mechanical ventilation is more advantageous in the follow-up of patients with chronic obstructive pulmonary disease after open heart surgery. Heart Surg Forum. 2014;17:E1-E6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Keenan SP, Sinuff T, Burns KE, Muscedere J, Kutsogiannis J, Mehta S, Cook DJ, Ayas N, Adhikari NK, Hand L, Scales DC, Pagnotta R, Lazosky L, Rocker G, Dial S, Laupland K, Sanders K, Dodek P; Canadian Critical Care Trials Group/Canadian Critical Care Society Noninvasive Ventilation Guidelines Group. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ. 2011;183:E195-E214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 21. | Antenora F, Fantini R, Iattoni A, Castaniere I, Sdanganelli A, Livrieri F, Tonelli R, Zona S, Monelli M, Clini EM, Marchioni A. Prevalence and outcomes of diaphragmatic dysfunction assessed by ultrasound technology during acute exacerbation of COPD: A pilot study. Respirology. 2017;22:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Bongiovanni F, Michi T, Natalini D, Grieco DL, Antonelli M. Advantages and drawbacks of helmet noninvasive support in acute respiratory failure. Expert Rev Respir Med. 2023;17:27-39. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Liu X, Du C, Hu F, Zhao Y, Zhou J, Wang Q, Mu Y, Lu J, Gao L, Cui B, Ma Y, Sun T, Qian F, Chen Z. Management of acute exacerbation of chronic obstructive pulmonary disease under a tiered medical system in China. Ther Adv Respir Dis. 2022;16:17534666221075499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Jun J, Sun L, Wang Y, Liu FZ, Yang GR, Han BX. Invasive mechanical ventilation with high concentration oxygen therapy for aecopd patients with acute myocardial infarction. Chest. 2019;156:A886. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Madhuvu A, Endacott R, Plummer V, Morphet J. Ventilation bundle compliance in two Australian intensive care units: An observational study. Aust Crit Care. 2021;34:327-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Han J, Hu Y, Liu S, Hu Z, Liu W, Wang H. Volume-controlled ventilation versus pressure-controlled ventilation during spine surgery in the prone position: A meta-analysis. Ann Med Surg (Lond). 2022;78:103878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med. 2017;195:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 773] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 28. | Rohrs EC, Bassi TG, Nicholas M, Wittmann J, Ornowska M, Fernandez KC, Gani M, Reynolds SC. Risk factors for ventilator-induced-lung injury develop three to five times faster after a single episode of lung injury. Can J Respir Ther. 2023;59:103-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 29. | Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1169] [Cited by in RCA: 1354] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 30. | Kacmarek RM, Villar J, Sulemanji D, Montiel R, Ferrando C, Blanco J, Koh Y, Soler JA, Martínez D, Hernández M, Tucci M, Borges JB, Lubillo S, Santos A, Araujo JB, Amato MB, Suárez-Sipmann F; Open Lung Approach Network. Open Lung Approach for the Acute Respiratory Distress Syndrome: A Pilot, Randomized Controlled Trial. Crit Care Med. 2016;44:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 188] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 31. | Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard JC, Lamontagne F, Bhatnagar N, Stewart TE, Guyatt G. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 937] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 32. | Bernasconi M, Ploysongsang Y, Gottfried SB, Milic-Emili J, Rossi A. Respiratory compliance and resistance in mechanically ventilated patients with acute respiratory failure. Intensive Care Med. 1988;14:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Dmytriiev D, Melnychenko M, Dobrovanov O, Nazarchuk O, Vidiscak M. Perioperative hemodynamic protective assessment of adaptive support ventilation usage in pediatric surgical patients. Acute Crit Care. 2022;37:636-643. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1115] [Article Influence: 61.9] [Reference Citation Analysis (1)] |
| 35. | Pinsky MR. My paper 20 years later: Effect of positive end-expiratory pressure on right ventricular function in humans. Intensive Care Med. 2014;40:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Wongviriyawong C, Winkler T, Harris RS, Venegas JG. Dynamics of tidal volume and ventilation heterogeneity under pressure-controlled ventilation during bronchoconstriction: a simulation study. J Appl Physiol (1985). 2010;109:1211-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Woods SJ, Waite AA, O'Dea KP, Halford P, Takata M, Wilson MR. Kinetic profiling of in vivo lung cellular inflammatory responses to mechanical ventilation. Am J Physiol Lung Cell Mol Physiol. 2015;308:L912-L921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Qadir N, Bartz RR, Cooter ML, Hough CL, Lanspa MJ, Banner-Goodspeed VM, Chen JT, Giovanni S, Gomaa D, Sjoding MW, Hajizadeh N, Komisarow J, Duggal A, Khanna AK, Kashyap R, Khan A, Chang SY, Tonna JE, Anderson HL 3rd, Liebler JM, Mosier JM, Morris PE, Genthon A, Louh IK, Tidswell M, Stephens RS, Esper AM, Dries DJ, Martinez A, Schreyer KE, Bender W, Tiwari A, Guru PK, Hanna S, Gong MN, Park PK; Severe ARDS: Generating Evidence (SAGE) Study Investigators; Society of Critical Care Medicine's Discovery Network. Variation in Early Management Practices in Moderate-to-Severe ARDS in the United States: The Severe ARDS: Generating Evidence Study. Chest. 2021;160:1304-1315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (2)] |
| 39. | Pelosi P, Ball L, Barbas CSV, Bellomo R, Burns KEA, Einav S, Gattinoni L, Laffey JG, Marini JJ, Myatra SN, Schultz MJ, Teboul JL, Rocco PRM. Personalized mechanical ventilation in acute respiratory distress syndrome. Crit Care. 2021;25:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 40. | Gattinoni L, Tonetti T, Quintel M. Regional physiology of ARDS. Crit Care. 2017;21:312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |