Published online Sep 6, 2023. doi: 10.12998/wjcc.v11.i25.6012
Peer-review started: July 6, 2023
First decision: July 18, 2023
Revised: July 26, 2023
Accepted: August 3, 2023
Article in press: August 3, 2023
Published online: September 6, 2023
Processing time: 56 Days and 20.7 Hours
Since its initial detection in 2019, coronavirus disease 2019 (COVID-19) pneu
Here, we report the case of a 39-year-old patient recovering from kidney trans
Given the rapid spread of severe acute respiratory syndrome coronavirus 2 infections, clinicians should be aware of the potential for more cases of COVID-19 among patients following kidney transplantation and be familiar with appro
Core Tip: Here, we report the case of a 39-year-old patient recovering from kidney transplantation who contracted perioperative coronavirus disease 2019 (COVID-19) pneumonia that was successfully controlled with oral paxlovid and a single intravenous drip infusion of tocilizumab following the discontinuation of immunosuppressive drugs. Given the rapid spread of severe acute respiratory syndrome coronavirus 2 infections, clinicians should be aware of the potential for more cases of COVID-19 among patients following kindey transplantation and be familiar with appropriate treatment options and likely clinical outcomes.
- Citation: Chen Q, Niu YL. Successful treatment of a case of COVID-19 pneumonia following kidney transplantation using paxlovid and tocilizumab. World J Clin Cases 2023; 11(25): 6012-6018
- URL: https://www.wjgnet.com/2307-8960/full/v11/i25/6012.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i25.6012
The coronavirus disease 2019 (COVID-19) pandemic was first detected as an outbreak of pneumonia in the Wuhan region of China in December 2019[1,2], and it the causative severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has remained highly transmissible[3-5]. The progression of the pandemic has varied markedly among countries throughout the world, with differences in circulating viral strains and control efforts in different regions. For example, several devastating mutant strains in Italy have rapidly emerged and caused critical illness, particularly among older adults and immunocompromised individuals[6]. Prolonged immunosuppressive treatment is essential among transplant recipients in order to prevent rejection, thus inevitably suppressing the immune status of these individuals as compared to the general population. At present, there is a lack of reported cases of COVID-19 pneumonia in kidney transplant patients, and there are no uniform treatment standards for these individuals[7]. The efficacy of different treatments, the optimal symptomatic management strategies, and the prognostic outcomes in kidney transplant recipients suffering from COVID-19 are also not well understood[8,9]. Here, we describe the case of a 39-year-old male that contracted and was successfully treated for COVID-19 pneumonia after the completion of a kidney transplant procedure. Given the current challenges associated with controlling and preventing the spread of COVID-19, clinicians performing kidney transplantation should be aware of the risk of perioperative infection. By publishing this case report, we hope to provide a reference for kidney transplant teams seeking to effectively manage post-transplant patient recovery.
Elevated creatinine detected for 3 + years, regular hemodialysis admitted for 2 + years.
Three years before, the patient had a cough with no obvious cause. The cough was intermittent, with no sputum, and was accompanied by a loss of appetite, vomiting, and shortness of breath, although no nausea or chest tightness. Examination at the local hospital showed a creatinine level of 800 µmol/L and blood pressure of 180/90 mmHg. The patient was diagnosed with renal failure and was discharged from the hospital after symptomatic treatment. The patient wished to have a kidney transplant and was waiting for a suitable kidney donor after matching. After finding a suitable kidney donor, the patient was admitted to our department with a diagnosis of chronic renal failure, and surgery was scheduled. Since the onset of the disease, the patients’ spirit, appetite, and sleep were fine, the stool was normal, the urine volume had decreased to 800 mL/d, and there was no significant change in body weight.
He had a prior history of hypertension with blood pressure levels of up to 180/90 mmHg managed through the long-term use of controlled-relates nifedipine tablets. He had no history of diabetes mellitus, hepatitis, or tuberculosis.
The patient was a non-smoker and did not drink alcohol. He was a public servant, lived in an area with no endemic diseases, and had not been vaccinated against SARS-CoV-2. He had been born, raised, and lived in his native region and had no history of foreign travel. Additionally, he was not aware of any hereditary diseases in his family.
The patient had been free of nausea, vomiting, and diarrhea since the onset of the disease, and did not exhibit any masses or positive signs, including abdominal pressure, rebound pain, or abdominal muscle tone upon examination.
On admission, his nucleic acid test results indicated that he was negative for COVID-19 pneumonia. On day two following admission, the patients’ creatinine was 320 µmol/L, his estimated glomerular filtration rate was 25 mL/min, and his hemoglobin level was 108 g/L.
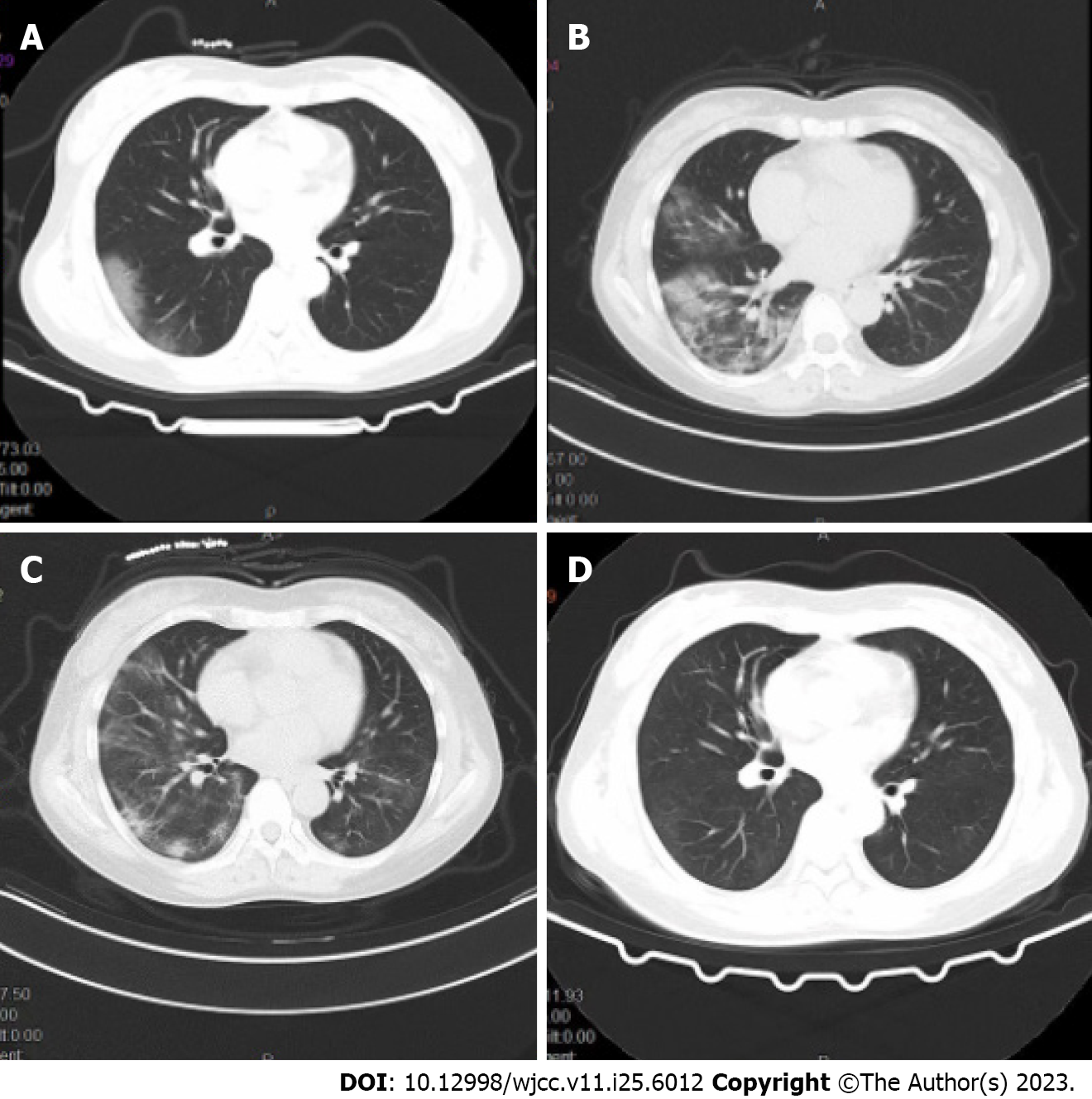
Repeated chest computed tomography (CT) scans revealed patchy shadows in both lungs consistent with the possibility of viral pneumonia (Figure 1A).
A pharyngeal swab was collected for SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) testing, which was positive for the presence of the virus. In conjunction with the patients’ clinical presentation, etiologic evidence, and imaging findings, a diagnosis of COVID-19 pneumonia was made.
A 39-year-old male was admitted to the Affiliated Hospital of Guizhou Medical University (Guiyang, China) on December 19, 2022, due to a three-year history of elevated creatinine levels and 2 years of regular hemodialysis. He had expressed interest in receiving a transplanted kidney and was awaiting a suitable donor following a matching test. On admission, his nucleic acid test results indicated that he was negative for COVID-19 pneumonia.
On the third day, he underwent allogeneic kidney transplantation under general anesthesia, with rabbit anti-human thymocyte immunoglobulin for immune induction and mescaline sodium + prednisone acetate + tacrolimus for immune maintenance. He was administered an empirical antimicrobial treatment consisting of piperacillin sodium/tazobactam sodium (4.5 g/d). Sputum and urine culture results were negative, and serum calcitoninogen was negative and interleukin 164 pg/mL. On day 14 of admission, the patient began exhibiting a persistent future. Given the recent rise in COVID-19 infections in the country, the patient was regarded as possibly being infected with COVID-19 and he was thus placed in an isolated single-occupancy room. A pharyngeal swab was collected for SARS-CoV-2 RT-PCR testing, which was positive for the presence of this virus. Repeated chest CT scans revealed patchy shadows in both lungs consistent with the possibility of viral pneumonia (Figure 1A). On the day of diagnosis, his arterial pO2 had fallen to 74 mmHg and continuous oxygenation was initiated via nasal cannula and a face mask. Oral paxlovid (nirmatrelvir, 150 mg and ritonavir, 100 mg, Pfizer) Q12 h was administered, while the anti-rejection drugs mescaline sodium and tacrolimus were discontinued. The patients’ interleukin-6 (IL-6) levels were rechecked and had risen to 314 pg/mL. After a discussion with our infectious specialist and obtaining written informed consent from the patient, tocilizumab was intravenously administered at a dose of 8 mg/kg diluted in 100 mL of 0.9% saline. After 4 d the patient exhibited no improvements in clinical symptoms and chest CT revealed significant worsening relative to the previous scan (Figure 1B). Following further discussion with our team of physicians, we speculated that reductions in leukocyte counts may be related to the activity of tocilizumab. In an effort to improve the overall immune status of this patient, we administered 5 g/d of intravenous immunoglobulin. Administration was continued for one week. One week following COVID-19 pneumonia, the patients’ pO2 levels gradually rose and were maintained at 90%-95% following the discontinuation of paxlovid and tocilizumab treatment. When IL-16 levels were measured 5 d after r drug administration, they had fallen to 42.42 pg/mL.
On day 7 after onset, a chest CT scan showed some degree of absorption relative to previous scans (Figure 1C). On day 28 following admission, the patient was discharged. At the time of discharge, he was free of fever or sputum production, exhibited a pO2 of 96%, a respiratory rate of 16 breaths/min, and a controlled blood pressure of 136/82 mmHg. Repeat chest CT (Figure 1D) and pharyngeal swab testing for COVID-19 were negative on 10 d after the confirmed diagnosis. Tacrolimus and prednisone acetate were continued, and the patient was advised to undergo repeat outpatient testing after 1 wk.
After a period of hospitalization in our hospital, the patients’ condition gradually recovered.
The ongoing COVID-19 pandemic caused by the SARS-CoV-2 virus has caused over 500 million confirmed infections and 8 million deaths throughout 223 countries and territories[10,11]. The highest number of confirmed cases to date has been reported in the United States. Specific treatment options are lacking, with only symptomatic and routine care for infected individuals. Emerging mutant strains of SARS-CoV-2 represent a particularly substantial threat to post-transplant patients, as these individuals often need to attend outpatient check-up appointments and are more often hospitalized dues to their immunosuppressed status[12,13]. These unique population characteristics can place these individuals at a high risk of severe illness or death.
The incubation period for COVID-19 infections reportedly varies from 1-24 d[14]. The initial presenting symptoms in infected patients are relatively nonspecific and can include fever and an upper respiratory tract infection[15]. These signs and symptoms also vary among patients, and the early detection and management of COVID-19 infections is thus vital to improving therapeutic outcomes and reducing the odds that patients develop severe disease[16,17].
After undergoing organ transplantation, patients face a persistent risk of severe illness and death upon COVID-19 infection owing to their impaired immune function[18]. Most kidney transplant patients have also not been vaccinated against COVID-19, and those that have generally exhibit relatively weak vaccine-induced humoral immunity such that they face very high rates of morbidity and mortality[19]. When they do contract COVID-19, these patients are more likely to progress to severe disease such that they are a particularly important group to provide with therapeutic interventions when possible. However, the precise efficacy of different interventional regimens in COVID-19 patients that are also transplant recipients is likely to vary as the virus continues to mutate.
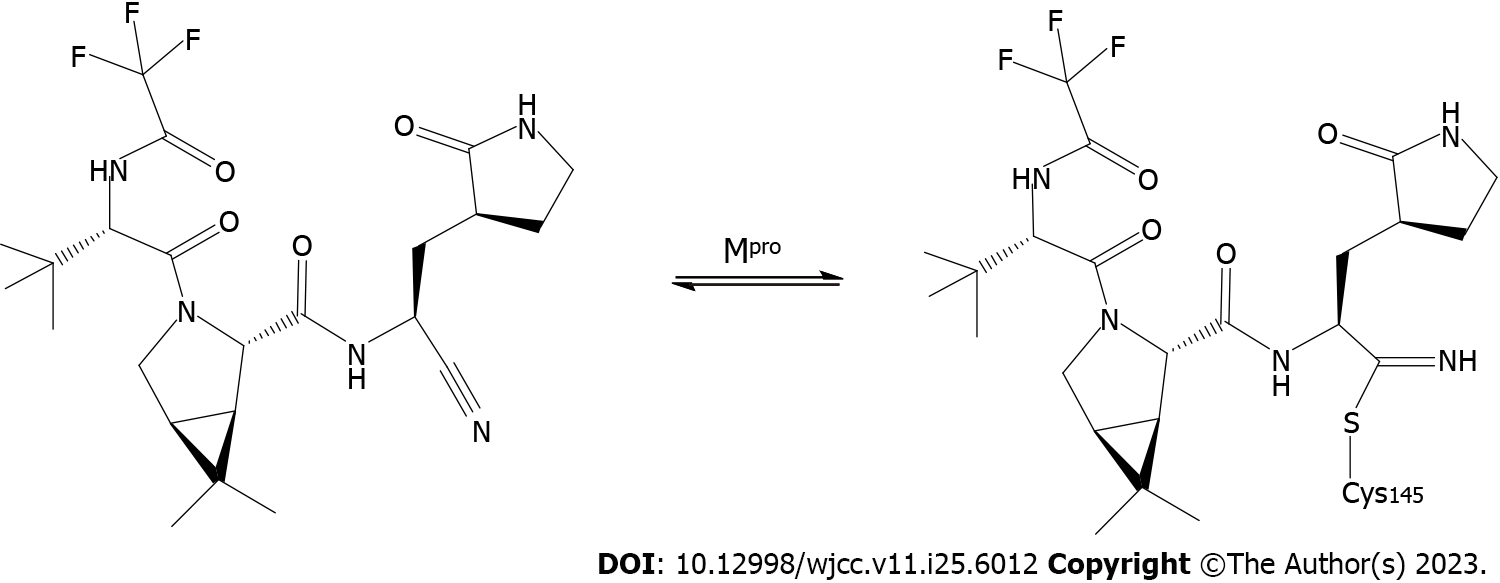
In the present case, when our patient was diagnosed with COVID-19 pneumonia we initially elected to discontinue immunosuppressive treatment with tacrolimus and mycophenolate while maintaining low-dose steroid treatment in light of the high risk of simultaneous bacterial infection[20]. Paxlovid is a recently developed COVID-19 treatment developed by Pfizer that consists of a combination of nirmatrelvir and ritonavir, a peptidomimetic analog of Mpro (3C-like protease)that serves as the primary protease used by SARS-CoV-2[21]. Ritonavir can inhibit the metabolic processing of nirmatrelvir by cytochrome P4503A (CYP3A), thus increasing the circulating levels of the drug (Figure 2). Paxlovid received emergency use authorization from the United States Food and Drug Administration as a treatment for mild-to-moderate COVID-19 in light of its favorable efficacy. Several studies have confirmed that paxlovid administration within the first 5 d after SARS-CoV-2 infection can expedite viral clearance[22]. However, some researchers have questioned the methodology employed in these studies and have suggested that the results are exaggerated. Even so, given the lack of more effective treatment options, paxlovid represents a promising candidate treatment for patients recovering from kidney transplantation who are infected with COVID-19. Accordingly, we elected to use paxlovid to treat our patient following confirmed SARS-CoV-2 infection, with a total paxlovid treatment course lasting 5 d.
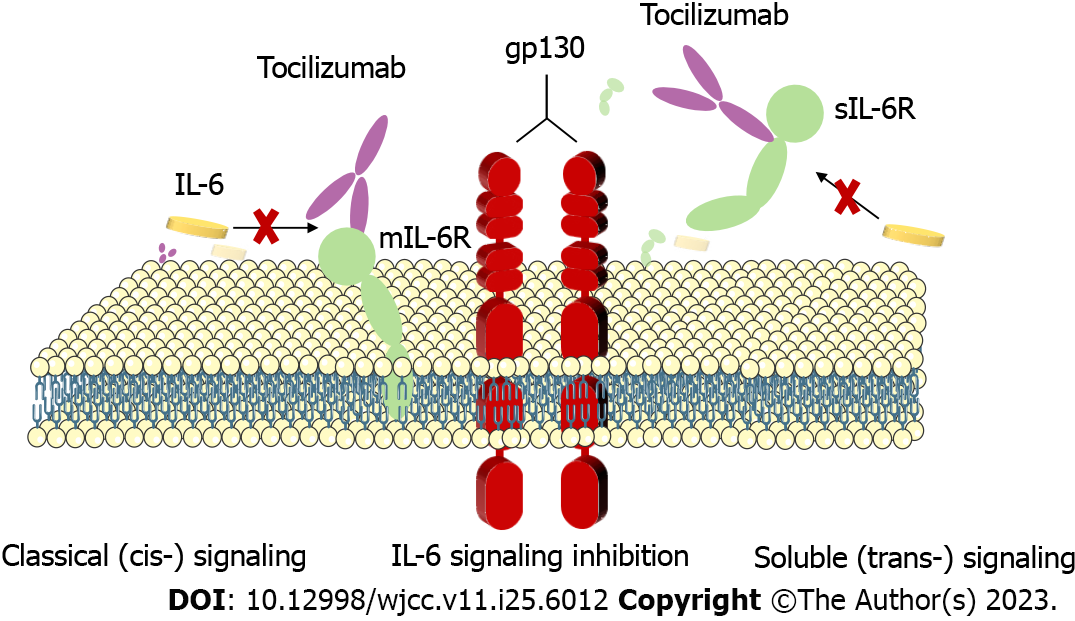
Some reports have indicated that infection with SARS-CoV-2 can result in severe lung damage as a consequence of cytokine release syndrome mediated by an overzealous host immune response to the virus. Those patients with severe disease tend to exhibit higher levels of IL-6 as compared to patients with less severe disease. Tocilizumab is initially used to treat adult patients with moderately severe active rheumatoid arthritis that have failed to satisfactorily respond to disease-modifying anti-rheumatic drug treatment[23]. In a prospective, multicenter, open-label, randomized, controlled trial, investigators found that tocilizumab appeared to be more effective in patients with COVID-19 who had high IL-6 concentrations[24]. Some COVID-19 patients will experience severe disease characterized by dyspnea, respiratory failure, and acute respiratory distress syndrome driven by IL-6 and other components of cytokine release syndrome in critical cases. Blocking of IL-6 production is an attractive means of targeting excessive inflammation during SARS-CoV-2 infection, as IL-6 is known to play a critical role in the COVID-19-induced cytokine storm. Tolizumab is a competitive inhibitor of membrane-bound and soluble IL-6 receptors that blocks the downstream signaling of IL-6 (Figure 3). The use of the IL-6 receptor antagonist tocilizumab as a treatment for severely ill COVID-19 patients thus has the potential to mitigate these severe outcomes[25]. In this case, we treated our patient with tocilizumab given his high circulating IL-6 concentrations in an effort to protect against further lung lesions and associated pulmonary distress. At present, there is no consensus regarding the optimal timing of tocilizumab administration when treating COVID-19, and given that our patient experienced positive clinical outcomes with a single dose of tocilizumab, we did not administer any further doses. It is important to note that IL-6 Levels may remain temporarily elevated following the initiation of tocilizumab treatment as the antibody blocks the IL-6 receptor, whereas the ligand itself remains in circulation and degrades over time. In the present case, the patient was also administered immunoglobulin to enhance his immune system.
The treatment of patients with COVID-19 has forced physicians to choose between trying unproven therapeutic agents and hoping that they work or providing standard supportive care for patients with severe respiratory disease until the identification of an optimal therapeutic agent by randomized controlled clinical trials. The platform trial PEMAP-CAP (randomized, embedded, multi-factorial, adaptive platform trial for community-acquired pneumonia-for critically ill patients) aims to turn the frantic attempts to save lives on the front line into an ongoing international trial with the goal of rapidly identifying the best treatments for terminally ill patients. Because pandemics are unpredictable and can occur suddenly, it is important to have the appropriate infrastructure in place to generate evidence on optimal treatments during a pandemic so that clinicians and policymakers can use this information to improve patient prognosis.
In summary, we have herein detailed a case of COVID-19 pneumonia that developed during the perioperative period in a patient that underwent kidney transplantation to treat end-stage kidney disease. The patient was successfully treated with a series of drugs including paxlovid, tocilizumab, and human immunoglobulin together with the temporary discontinuation of the immunosuppressive therapy given following transplantation. While informative, this case is subject to several limitations. As this is a single case, it is not sufficient to formulate treatment guidelines for these multiple, and the self-healing process in COVID-19 patients following kidney transplantation remains to be fully elucidated. Even so, we believe that this case will provide a valuable reference to clinicians in transplant units, aiding in the recognition and potential treatment of COVID-19 pneumonia among individuals in the perioperative period following transplantation.
Given the sustained high rates of COVID-19 infections throughout the globe, we believe that descriptions of the effective management of exceptional cases are important as a focus of concern for transplant surgeons until sufficiently large volumes of data are available from clinical studies to provide more robust guidance.
Thank you to all the medical staff of the Organ Transplantation Department of the Affiliated Hospital of Guizhou Medical University.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Salvadori M, Italy; Sarier M, Turkey S-Editor: Qu XL L-Editor: A P-Editor: Yuan YY
| 1. | Guan WJ, Zhong NS. Clinical Characteristics of Covid-19 in China. Reply. N Engl J Med. 2020;382:1861-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 259] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 2. | Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, Zhang LJ. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology. 2020;296:E15-E25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1106] [Cited by in RCA: 948] [Article Influence: 189.6] [Reference Citation Analysis (1)] |
| 3. | Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, Xiong Y, Cheng Z, Gao S, Liang K, Luo M, Chen T, Song S, Ma Z, Chen X, Zheng R, Cao Q, Wang F, Zhang Y. Clinical Characteristics of Refractory Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2021;73:e4208-e4213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 486] [Cited by in RCA: 601] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 4. | Arimoto KI, Miyauchi S, Troutman TD, Zhang Y, Liu M, Stoner SA, Davis AG, Fan JB, Huang YJ, Yan M, Glass CK, Zhang DE. Expansion of interferon inducible gene pool via USP18 inhibition promotes cancer cell pyroptosis. Nat Commun. 2023;14:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 5. | Sarier M, Demir M, Emek M, Usta SS, Soylu A, Konuk EY, Turgut H. Comparison of spermiograms of infertile men before and during the COVID-19 pandemic. Rev Assoc Med Bras (1992). 2022;68:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323:1775-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1653] [Cited by in RCA: 2122] [Article Influence: 424.4] [Reference Citation Analysis (0)] |
| 7. | Hippisley-Cox J, Coupland CA, Mehta N, Keogh RH, Diaz-Ordaz K, Khunti K, Lyons RA, Kee F, Sheikh A, Rahman S, Valabhji J, Harrison EM, Sellen P, Haq N, Semple MG, Johnson PWM, Hayward A, Nguyen-Van-Tam JS. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. BMJ. 2021;374:n2244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 201] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 8. | Desai A, Gainor JF, Hegde A, Schram AM, Curigliano G, Pal S, Liu SV, Halmos B, Groisberg R, Grande E, Dragovich T, Matrana M, Agarwal N, Chawla S, Kato S, Morgan G, Kasi PM, Solomon B, Loong HH, Park H, Choueiri TK, Subbiah IM, Pemmaraju N, Subbiah V; COVID19 and Cancer Clinical Trials Working Group. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat Rev Clin Oncol. 2021;18:313-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 9. | Mahalingasivam V, Su G, Iwagami M, Davids MR, Wetmore JB, Nitsch D. COVID-19 and kidney disease: insights from epidemiology to inform clinical practice. Nat Rev Nephrol. 2022;18:485-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 10. | Safiabadi Tali SH, LeBlanc JJ, Sadiq Z, Oyewunmi OD, Camargo C, Nikpour B, Armanfard N, Sagan SM, Jahanshahi-Anbuhi S. Tools and Techniques for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)/COVID-19 Detection. Clin Microbiol Rev. 2021;34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 211] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 11. | Yüce M, Filiztekin E, Özkaya KG. COVID-19 diagnosis -A review of current methods. Biosens Bioelectron. 2021;172:112752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 410] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 12. | Fernandes Q, Inchakalody VP, Merhi M, Mestiri S, Taib N, Moustafa Abo El-Ella D, Bedhiafi T, Raza A, Al-Zaidan L, Mohsen MO, Yousuf Al-Nesf MA, Hssain AA, Yassine HM, Bachmann MF, Uddin S, Dermime S. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann Med. 2022;54:524-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 269] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 13. | Azzi Y, Parides M, Alani O, Loarte-Campos P, Bartash R, Forest S, Colovai A, Ajaimy M, Liriano-Ward L, Pynadath C, Graham J, Le M, Greenstein S, Rocca J, Kinkhabwala M, Akalin E. COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int. 2020;98:1559-1567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 14. | Cravedi P, Mothi SS, Azzi Y, Haverly M, Farouk SS, Pérez-Sáez MJ, Redondo-Pachón MD, Murphy B, Florman S, Cyrino LG, Grafals M, Venkataraman S, Cheng XS, Wang AX, Zaza G, Ranghino A, Furian L, Manrique J, Maggiore U, Gandolfini I, Agrawal N, Patel H, Akalin E, Riella LV. COVID-19 and kidney transplantation: Results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140-3148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 285] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 15. | Nair V, Jandovitz N, Hirsch JS, Nair G, Abate M, Bhaskaran M, Grodstein E, Berlinrut I, Hirschwerk D, Cohen SL, Davidson KW, Dominello AJ, Osorio GA, Richardson S, Teperman LW, Molmenti EP. Covid-19 in kidney transplant recipients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2020;20:1819-1825. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 188] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 16. | Radcliffe C, Palacios CF, Azar MM, Cohen E, Malinis M. Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge. Am J Transplant. 2022;22:2458-2463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 17. | Goffin E, Candellier A, Vart P, Noordzij M, Arnol M, Covic A, Lentini P, Malik S, Reichert LJ, Sever MS, Watschinger B, Jager KJ, Gansevoort RT; ERACODA Collaborators. COVID-19-related mortality in kidney transplant and haemodialysis patients: a comparative, prospective registry-based study. Nephrol Dial Transplant. 2021;36:2094-2105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | Abu Jawdeh BG. COVID-19 in Kidney Transplantation: Outcomes, Immunosuppression Management, and Operational Challenges. Adv Chronic Kidney Dis. 2020;27:383-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Karatas M, Tatar E, Simsek C, Yıldırım AM, Ari A, Zengel B, Uslu A. COVID-19 pneumonia in kidney transplant recipients: A promising treatment algorithm in the absence of a disease-specific drug. J Med Virol. 2021;93:5789-5797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | El Karoui K, De Vriese AS. COVID-19 in dialysis: clinical impact, immune response, prevention, and treatment. Kidney Int. 2022;101:883-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 21. | Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, Goldstein LH, Saliba W. Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients. Clin Infect Dis. 2023;76:e342-e349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 330] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 22. | RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345-1352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 513] [Cited by in RCA: 464] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 23. | Rosas IO, Diaz G, Gottlieb RL, Lobo SM, Robinson P, Hunter BD, Cavalcante AW, Overcash JS, Hanania NA, Skarbnik A, Garcia-Diaz J, Gordeev I, Carratalà J, Gordon O, Graham E, Lewin-Koh N, Tsai L, Tuckwell K, Cao H, Brainard D, Olsson JK. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial. Intensive Care Med. 2021;47:1258-1270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 24. | Declercq J, Van Damme KFA, De Leeuw E, Maes B, Bosteels C, Tavernier SJ, De Buyser S, Colman R, Hites M, Verschelden G, Fivez T, Moerman F, Demedts IK, Dauby N, De Schryver N, Govaerts E, Vandecasteele SJ, Van Laethem J, Anguille S, van der Hilst J, Misset B, Slabbynck H, Wittebole X, Liénart F, Legrand C, Buyse M, Stevens D, Bauters F, Seys LJM, Aegerter H, Smole U, Bosteels V, Hoste L, Naesens L, Haerynck F, Vandekerckhove L, Depuydt P, van Braeckel E, Rottey S, Peene I, Van Der Straeten C, Hulstaert F, Lambrecht BN. Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial. Lancet Respir Med. 2021;9:1427-1438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 25. | Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, Zhou N, Petty LA, Baang JH, Dillman NO, Frame D, Gregg KS, Kaul DR, Nagel J, Patel TS, Zhou S, Lauring AS, Hanauer DA, Martin E, Sharma P, Fung CM, Pogue JM. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |