Published online Sep 6, 2023. doi: 10.12998/wjcc.v11.i25.5988
Peer-review started: May 24, 2023
First decision: June 15, 2023
Revised: July 12, 2023
Accepted: August 8, 2023
Article in press: August 8, 2023
Published online: September 6, 2023
Processing time: 100 Days and 2.1 Hours
Renal tubular acidosis (RTA) is a renal cause of non-anion-gap metabolic acidosis characterized by low urinary ammonia excretion. This condition has a low prevalence, and various congenital and acquired etiologies. To date, only a few cases of idiopathic RTA uncovered during pregnancy have been reported.
A previously healthy 32-year-old Korean woman at 30 wk of gestation was admitted to Pusan National University Hospital with preterm labor. At admi
Herein we describe a case of idiopathic distal RTA discovered during pregnancy. Hypokalemia and metabolic acidosis resolved spontaneously after delivery.
Core Tip: Renal tubular acidosis is a rare disease that presents as non-anion-gap metabolic acidosis. To the best of our knowledge, only a few cases of idiopathic distal renal tubular acidosis uncovered during pregnancy have been reported. To date, the exact pathophysiology by which pregnancy activates this condition has not been established.
- Citation: Seong EY, Kim DW, Kim HJ, Rhee H, Song SH. Incomplete distal renal tubular acidosis uncovered during pregnancy: A case report. World J Clin Cases 2023; 11(25): 5988-5993
- URL: https://www.wjgnet.com/2307-8960/full/v11/i25/5988.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i25.5988
Renal tubular acidosis (RTA) is a rare disease caused by the failure of net acid excretion in the kidneys. Based on its pathophysiology, RTA is divided into four subtypes, three of which are major and related to the mechanisms of renal acid–base handling. Distal (type 1) RTA results from a reduction in net acid secretion in the distal nephron[1], proximal (type 2) RTA is characterized by a decreased capacity of the proximal tubule to reabsorb filtered bicarbonate, and hyperkalemic (type 4) RTA is characterized by impaired renal excretion of both acid and potassium in the distal nephron[2]. Each can be distinguished based on renal function, serum potassium, serum bicarbonate, urine pH, urine citrate, and the presence of Fanconi syndrome. Mixed (type 3) RTA shows features of both distal and proximal RTA and is most often associated with rare autosomal recessive syndromes[1].
RTA has various causes, including primary (inherited or idiopathic) and secondary (acquired) factors. Only a few cases of idiopathic RTA uncovered during pregnancy have been reported[3,4]. Here, we present the case of a healthy pregnant woman in her third trimester who was diagnosed with idiopathic distal RTA and spontaneously recovered from hypokalemia and metabolic acidosis after delivery.
A 32-year-old unemployed Korean woman presented with lower abdominal pain.
The patient was gravida III at 30 wk of gestation. There was lower abdominal pain due to uterine contractions.
The patient had no significant medical history, including kidney stones. She delivered two preterm babies in 2012 and 2016, both by normal spontaneous vaginal delivery at 35 wk of gestation. The babies were healthy and within the normal range of birth weight. She denied any medication history, including herbal medicines, vaccines, depot injections, and nonprescription medications.
The patient reported no significant family history of related illnesses.
On admission, she had a height of 168 cm, weight 82.1 kg, blood pressure 120/70 mmHg, regular heart rate of 97 beats per minute, and temperature 36.4 ℃. The patient complained of lower abdominal pain due to periodic uterine contractions with cervical dilatation and was thus thought to be in preterm labor. The fundus height corresponded to 30 wk of gestation. No notable physical examination findings indicated any volume depletion.
On admission, the patient had moderate hypokalemia with potassium level 2.98 mEq/L. She did not show any symptoms indicative of recent potassium loss from the gastrointestinal tract such as diarrhea, and had a healthy appetite.
Further blood tests revealed the following: White blood cells 13520 cells/uL, hemoglobin 10.9 g/dL, platelets 350000 cells/uL, sodium 136 mEq/L, potassium 2.98 mEq/L, chloride 107 mEq/L, bicarbonate 18 mEq/L, blood urea nitrogen 30 mg/dL, creatinine 0.4 mg/dL, glucose 91 mg/dL, albumin 3.3 mg/dL, calcium 8.5 mg/dL, magnesium 1.7 mEq/L, and serum anion gap of 11 mEq/L. Urine studies revealed a urine pH of 7.0, urine anion gap of 28 mEq/L (urine sodium, 81 mEq/L; urine potassium, 15 mEq/L; and urine chloride, 68 mEq/L), urine osmolar gap of 28 mEq/L, and transtubular potassium gradient of 4.8 (serum osmolality, 282 mOsm/dL/L; urine osmolality, 304 mOsm/dL; urine potassium, 15 mEq/L; and serum potassium 2.98 meq/L). Urine protein and glucose levels were negative and pyuria was absent. Hypercalciuria (440 mg/d) and hypocitraturia (44 mg/d) were also observed.
Ultrasonography of the fetus was performed on the day of admission and showed a biparietal diameter of 8.35 cm, abdominal circumference 26.86 cm, femur length 5.22 cm and estimated body weight 1572 g, which was in the 75th percentile at 30 wk of gestation[5]. The amniotic fluid index was 19.37 cm, calculated by adding the anteroposterior diameters of the largest empty fluid pocket in each quadrant (quadrant 1: 4.50 cm, quadrant 2: 5.74 cm, quadrant 3: 3.92 cm, quadrant 4: 5.21 cm) and the value was in the 95th percentile at 30 wk of gestation[6].
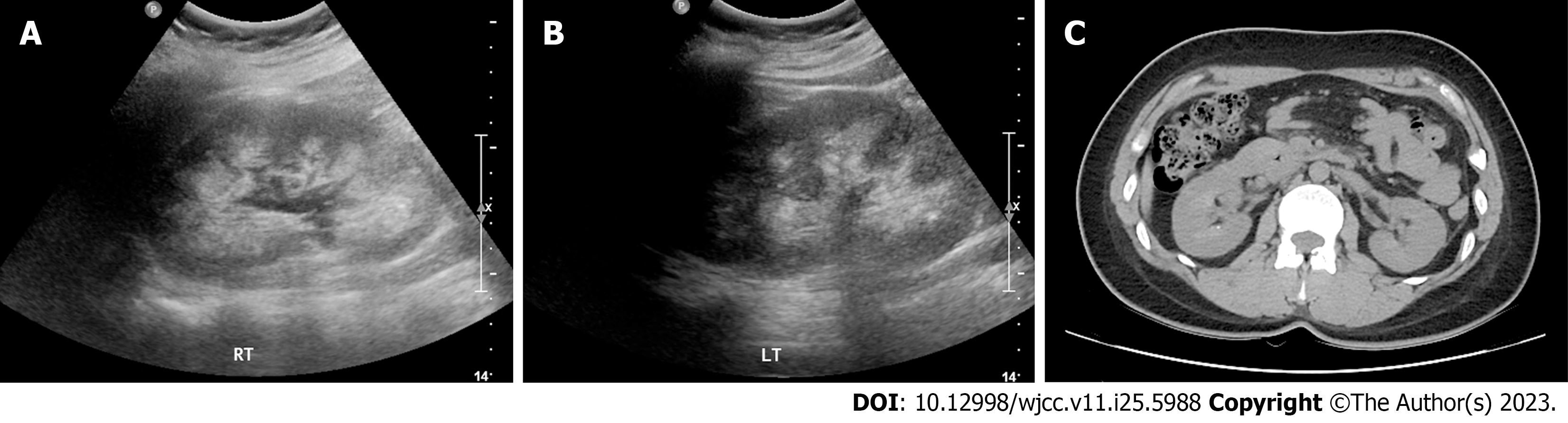
Nephrocalcinosis and tiny renal stones were detected on whole abdominal ultrasonography and computed tomo
Urine and transtubular potassium gradients indicated increased potassium secretion in the distal portions of the kidney. Because the patient had normal blood pressure, non-anion-gap metabolic acidosis, normal aldosterone to renin ratio (1.32 ng/dL per ng/mL per hour), and nephrocalcinosis, incomplete distal RTA was strongly suspected. However, because the patient refused the provocative acid-loading test, the disease was not confirmed. She had no history or clinical indication of autoimmune disease or renal stones and no history of drug use or toxin exposure. Thyroid-stimulating hormone, human immunodeficiency virus, and hepatitis panel results were normal or nonreactive. Additional studies, including next-generation sequencing (NGS) panels of nephropathy-related genes, were performed to determine the cause of distal RTA. The NGS panels consisted of 39 genes, six of which were related to the proximal and distal RTA. The results indicated that none of these genes were significantly altered. Therefore, we concluded that the distal RTA was idiopathic in this case.
After the diagnosis of distal RTA, the patient received a daily dose of 1500 mg sodium bicarbonate to correct metabolic acidosis and 1800 mg potassium chloride supplementation. Blood tests returned to normal 12 days after the start of supplementation, with a serum potassium of 4.2 mEq/L and bicarbonate of 21 mEq/L. After 2 wk, as the shortness of the cervix and the interval between uterine contractions did not progress, the patient was discharged with medications.
One week later, the patient was admitted to the hospital for labor and underwent spontaneous vaginal delivery. As agenesis of the corpus callosum was suspected on prenatal ultrasonography, brain magnetic resonance imaging was performed, which confirmed agenesis of the corpus callosum and colpocephaly. Although the baby experienced a brief period of apnea after birth, it spontaneously recovered with supportive care, and no other brain-related complications were detected.
After delivery, the patient visited the hospital monthly for RTA checkups, and her laboratory findings gradually returned to normal. Four months after the diagnosis, all medications were discontinued.
Distal RTA (type 1) is a relatively common form of RTA. There are various causes of distal RTA, including primary (idiopathic or familial) and secondary (autoimmune disorders, genetic disorders, drugs or toxins, nephrocalcinosis, and tubulointerstitial diseases). Distal RTA should be suspected in all patients with hypokalemia and non-anion-gap metabolic acidosis. Further urine analysis showing a urine pH above 5.5 and a urine anion gap value greater than zero or lack of an increase in the urine osmolar gap helps confirm the disease[1]. Severe hypokalemia (< 2.5 mEq/L) can result in musculoskeletal weakness and rhabdomyolysis. Nephrocalcinosis can be observed using abdominal ultrasonography or radiography.
Administration of an alkali can correct metabolic acidosis in the distal RTA. In patients with severe potassium deficits, potassium replacement should be the first treatment before acidosis is corrected, because serum potassium levels can be lowered to dangerous levels through bicarbonate replacement. Generally, distal RTA treatment includes bicarbonate and potassium supplementation. In patients with recurrent renal stone disease caused by distal RTA, acidosis may lead to stone dissolution by increasing urinary citrate excretion and slowing further stone formation.
Table 1 lists case reports of distal RTA diagnosed during pregnancy. Patients who have been diagnosed with distal RTA before pregnancy generally have confirmed causes such as autoimmune diseases or inherited factors[7,8]. However, in most cases of distal RTA presenting for the first time during pregnancy, the etiologies were unknown. Among cases presenting with unidentified causes of distal RTA, only one case has been reported to be completely cured after pregnancy[9]. Another case of rhabdomyolysis induced by severe hypokalemia in consecutive pregnancies involved a distal RTA of an uncertain etiology. After the successful delivery of healthy babies, the patient required continuous potassium replacement during the follow-up[10]. In a recently reported case of a distal RTA, the patient exhibited severe metabolic acidosis and hypokalemia due to distal RTA. After successful delivery, the distal RTA persisted and only sodium bicarbonate was required during the follow-up[11].
| Ref. | Cause | Clinical course |
| Muthukrishnan et al[10], 2010 | Idiopathic | Recurrent rhabdomyolysis induced by hypokalemia in consecutive pregnancies; successful delivery and potassium replacement was needed on follow-up |
| Mallett et al[14], 2011 | Ibuprofen, codeine abuse | RTA resolved after quitting ibuprofen and codeine; successful delivery and renal function did not fully return to baseline on follow-up |
| Srisuttayasathien[9], 2015 | Idiopathic | Rhabdomyolysis induced by hypokalemia; successful delivery and complete resolution of RTA on follow-up |
| Alkhasoneh et al[11], 2019 | Not identified (patient refusal of autoimmune disease workup) | Severe metabolic acidosis and hypokalemia; successful delivery and only sodium bicarbonate was needed on follow-up |
Renal physiology changes significantly during a normal pregnancy, and is characterized by marked volume expansion and vasodilation. Renal plasma flow increases by approximately 80% and glomerular filtration rate (GFR) by 50%[12]. An elevated GFR indicates an increase in the filtered solute load, although tubular reabsorption is proportionally increased. This imbalance between the filtered solute load and tubular reabsorption causes an increased loss of some electrolytes, leading to a higher need for potassium and bicarbonate. Typically, chronic mild respiratory alkalosis occurs secondary to hyperventilation, which lowers arterial carbon dioxide tension. Owing to these physiological changes, pregnancy has been reported to worsen RTA[1]. In the current case, idiopathic distal RTA was uncovered during pregnancy and spontaneous resolution of hypokalemia and metabolic acidosis occurred after delivery. Since nephrocalcinosis and renal stones were present on imaging at the time of diagnosis, we suspected that a hidden distal RTA might have existed and was uncovered when it worsened during pregnancy.
Theoretically, prolonged maternal metabolic acidosis due to chronic RTA impairs fetal growth and development and causes fetal distress, commonly resulting in preterm labor[11]. There has been a case of distal RTA secondary to possible Sjögren’s syndrome causing multiple pregnancy losses[7,13]. In this case, the patient had a history of preterm delivery of two babies without any congenital brain defects. As she was at 35 wk of gestation, which is near full term, and the babies were healthy, no further evaluations, including laboratory examinations that might suspect RTA, were performed. Since the patient was gravida III diagnosed with RTA, she also had preterm labor, but the gestational age was earlier than that of gravidas I and II. Therefore, further evaluation for the patient was conducted and revealed hypokalemia with metabolic acidosis. The infant also had a congenital brain defect. Although she did not have severe hypokalemia or metabolic acidosis at the time of diagnosis, we cannot rule out the possibility that prolonged maternal metabolic acidosis resulting from an undiagnosed distal RTA was related to consecutive preterm labor and fetal distress.
In conclusion, we report a rare case of idiopathic distal RTA uncovered during pregnancy. The patient had an unremarkable medical history and no symptoms or laboratory findings suggestive of other causes of RTA. The patient was treated accordingly, and 3 wk later delivered an infant with corpus callosum agenesis and colpocephaly. After delivery, the hypokalemia and metabolic acidosis resolved spontaneously and completely, and no further treatment was required.
We thank the patient for her participation and cooperation in this case report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Fabbian F, Italy; Han J, China S-Editor: Yan JP L-Editor: A P-Editor: Cai YX
| 1. | Johnson RJ, Feehally J, Floege J, Tonelli M. Comprehensive Clinical Nephrology. 6th ed. Elsevier, 2018: 150-154, 501-531. |
| 2. | Rodríguez Soriano J. Renal tubular acidosis: the clinical entity. J Am Soc Nephrol. 2002;13:2160-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 277] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 3. | Hardardottir H, Lahiri T, Egan JF. Renal tubular acidosis in pregnancy: case report and literature review. J Matern Fetal Med. 1997;6:16-20. [PubMed] [DOI] [Full Text] |
| 4. | Firmin CJ, Kruger TF, Davids R. Proximal renal tubular acidosis in pregnancy. A case report and literature review. Gynecol Obstet Invest. 2007;63:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Mikolajczyk RT, Zhang J, Betran AP, Souza JP, Mori R, Gülmezoglu AM, Merialdi M. A global reference for fetal-weight and birthweight percentiles. Lancet. 2011;377:1855-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 369] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 6. | Moore TR, Cayle JE. The amniotic fluid index in normal human pregnancy. Am J Obstet Gynecol. 1990;162:1168-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 323] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Yuvaraj A, Ghosh S, Shanmugasundaram L, Abraham G. Sjogren's with distal renal tubular acidosis complicating pregnancy. J Obstet Gynaecol. 2018;38:429-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Seeger H, Salfeld P, Eisel R, Wagner CA, Mohebbi N. Complicated pregnancies in inherited distal renal tubular acidosis: importance of acid-base balance. J Nephrol. 2017;30:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Srisuttayasathien M. Hypokalemia-Induced Rhabdomyolysis as a result of Distal Renal Tubular Acidosis in a Pregnant Woman: A Case Report and Literature Review. Case Rep Obstet Gynecol. 2015;2015:947617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Muthukrishnan J, Harikumar K, Jha R, Modi K. Pregnancy predisposes to rhabdomyolysis due to hypokalemia. Saudi J Kidney Dis Transpl. 2010;21:1127-1128. [PubMed] |
| 11. | Alkhasoneh M, Jacobs J, Kaur G. A case of severe metabolic acidosis during pregnancy. Clin Case Rep. 2019;7:550-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis. 2013;20:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 352] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 13. | Jain SH, Sainarersh VV, Patel HV, Trivedi HL. Renal tubular acidosis: an uncommon cause of bad obstetric history. J Obstet Gynaecol India. 2014;64:34-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Mallett A, Lynch M, John GT, Healy H, Lust K. Ibuprofen-related renal tubular acidosis in pregnancy. Obstet Med. 2011;4:122-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |