Published online Sep 6, 2023. doi: 10.12998/wjcc.v11.i25.5954
Peer-review started: May 5, 2023
First decision: June 12, 2023
Revised: June 30, 2023
Accepted: August 9, 2023
Article in press: August 9, 2023
Published online: September 6, 2023
Processing time: 118 Days and 19.7 Hours
Male breast cancer (MBC) is an extremely rare condition and accounts for less than 1% of all breast cancers, and malignant tumors occur in less than 1% of the affected men. Mucinous breast cancer is extremely rare and accounts for 2% of all invasive breast cancers. Generally, MBC is accompanied by a retroareolar mass.
Herein, we report a case of male mucinous breast carcinoma (MMBC) without gynecomastia development and with mass localization outside the common retroareolar region, wherein the mass was a painless nodule in the right breast of a 64-year-old man. We also discuss the clinical and pathological characteristics of this unusual tumor. The excised breast specimen showed pure mucinous carci
To the best of our knowledge, this is the first case report of MMBC in the non-retroareolar region of the nipple without gynecomastia development. Mucinous tumors are easily missed during diagnosis, and the incidence of axillary lymph node metastases of chest mucinous tumors has increased.
Core Tip: We not only report a rare case, but also discuss the clinical and pathological characteristics of this unusual tumor. Though a summary of a limited number of cases and a comparison with female breast cancer, we reveal some interesting features of male mucinous breast cancer. It is helpful to distinguish the rare male disease.
- Citation: Sun Q, Liu XY, Zhang Q, Jiang H. Non-retroareolar male mucinous breast cancer without gynecomastia development in an elderly man: A case report. World J Clin Cases 2023; 11(25): 5954-5961
- URL: https://www.wjgnet.com/2307-8960/full/v11/i25/5954.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i25.5954
Male breast cancer (MBC) is particularly uncommon, accounting for approximately 1% of all breast cancer cases[1-3]. Known risk factors for MBC include old age, high estradiol levels, Klinefelter’s syndrome, radiation exposure, gynecomastia, family history of breast cancer, and Breast Cancer 1 protein (BRCA1) and BRCA2 mutations[4]. The most common malignancy in MBC patients is invasive ductal carcinoma, and mucinous carcinoma is extremely rare[5,6]. Anatomically, the male breast comprises the external nipple, areola, internal lymph nodes, adipose tissue, and ducts. The glandular portion of the male breast is located mainly posterior to the nipple-areola complex[7]. MBC occurrence most often involves remarkable gynecomastia development and a retroareolar mass[8-10]. We herein report a case of non-retro
A 64-year-old male presented at the hospital with a painless mass in the right chest for 4 mo.
The patient had a mass over the right lateral nipple for 4 mo, which grew from the size of a pea to the size of an egg yolk, without pain.
The patient with no history of smoking or drinking, no history of hepatitis, no history of cardiovascular disease, and no history of long-term drug use.
There was no significant personal or family history.
The patient was 178 cm in height and 58 kg in weight, with body mass index (BMI): 18.3 kg/m2. Physical examination indicated an approximately 2.0 cm × 1.5 cm-sized nodule present 2.0 cm above the right lateral nipple.
Serum six-sex hormone test revealed the following findings: (1) Follicle-stimulating hormone: 12.60 IU/L (normal: 1.27-19.26 IU/L); (2) Luteinizing hormone: 7.66 IU/L (normal: 1.24-8.62 IU/L); (3) Estradiol: 0.00 pg/mL (normal: < 25-38.95 pg/mL); (4) Testosterone: 3.37 ng/mL (normal: 1.75-7.81 ng/mL); (5) Prolactin: 16.83 ng/mL (normal: 2.64-13.13 ng/mL); and (6) Progesterone: 0.00 ng/mL (normal: 0.14-2.06 ng/mL).
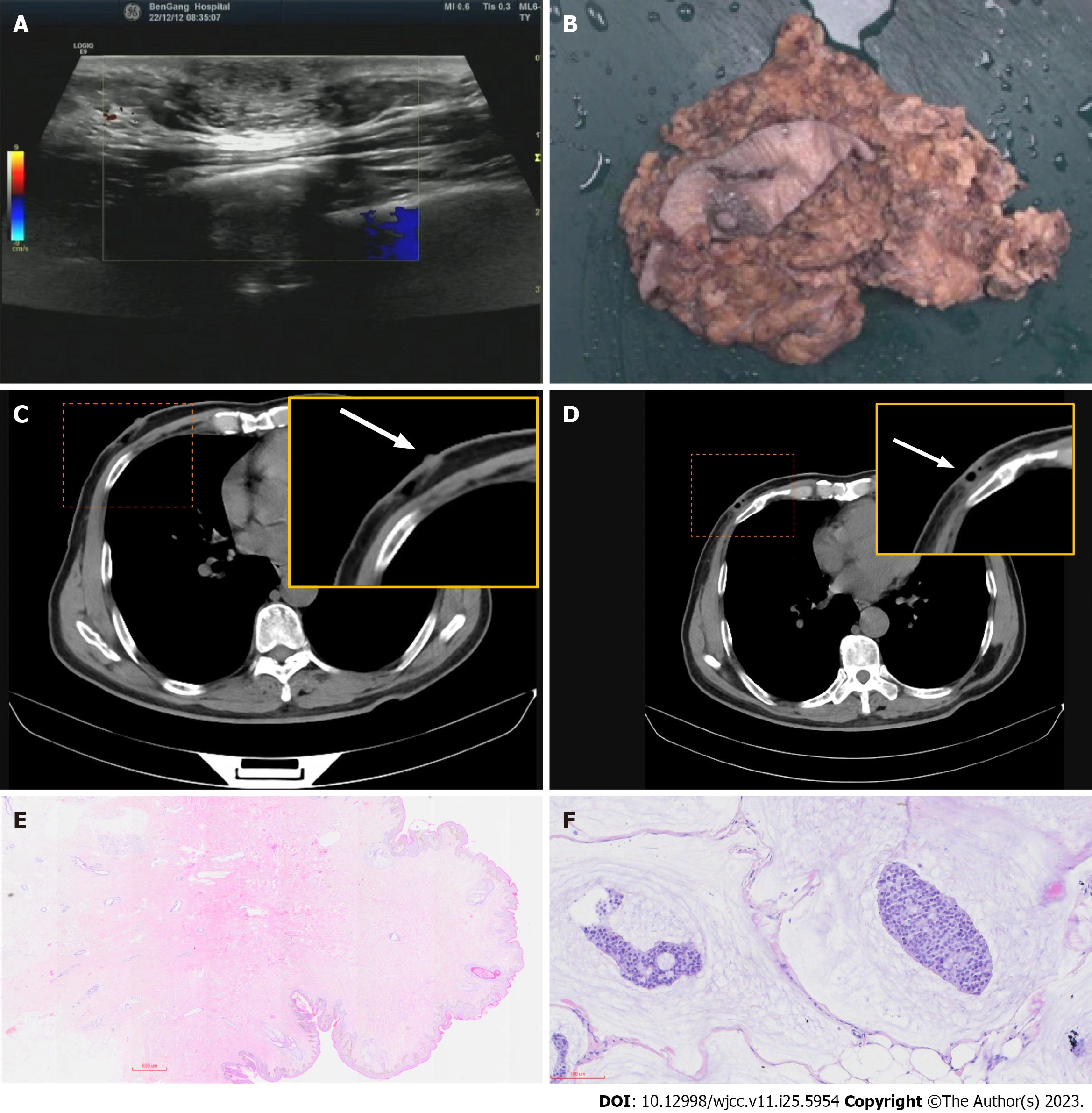
Ultrasound examination revealed the following information: A subcutaneous hypoechoic lesion was located 2.0 cm from the nipple edge on the lateral side of the right nipple and had a size of approximately 1.88 cm × 1.13 cm, with clear borders and no blood flow signal within it. Computerized tomography (CT) reveals posterior nipple glands and post
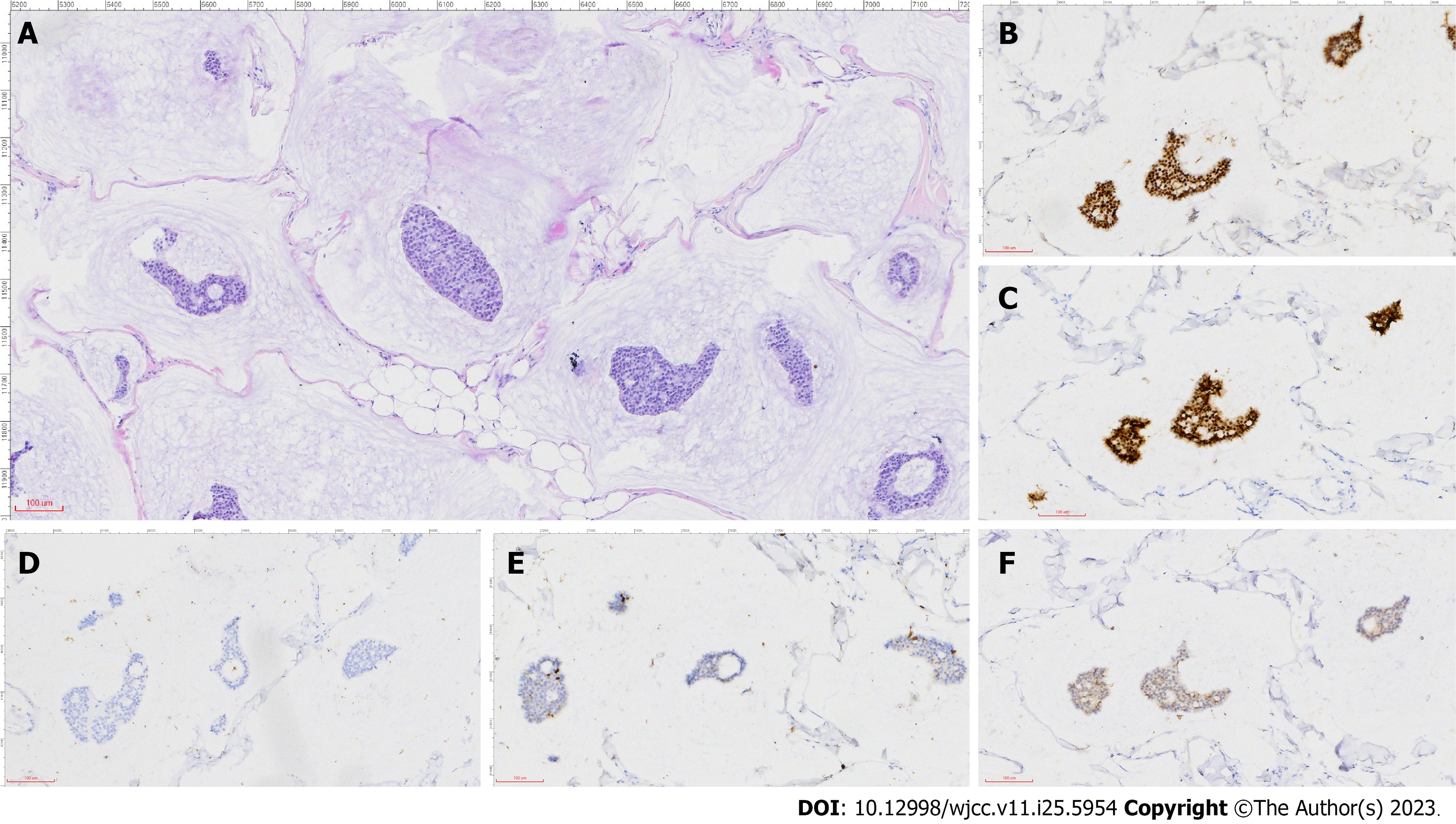
According to histopathological examination and immunohistochemistry analysis of the excised tissue, multidisciplinary consultation by the Department of Breast Surgery, Oncology, Ultrasound, and Pathology made the diagnosis - non-retroareolar MMBC without gynecomastia (Figure 2).
The patient was given the diagnosis of male mucinous breast carcinoma.
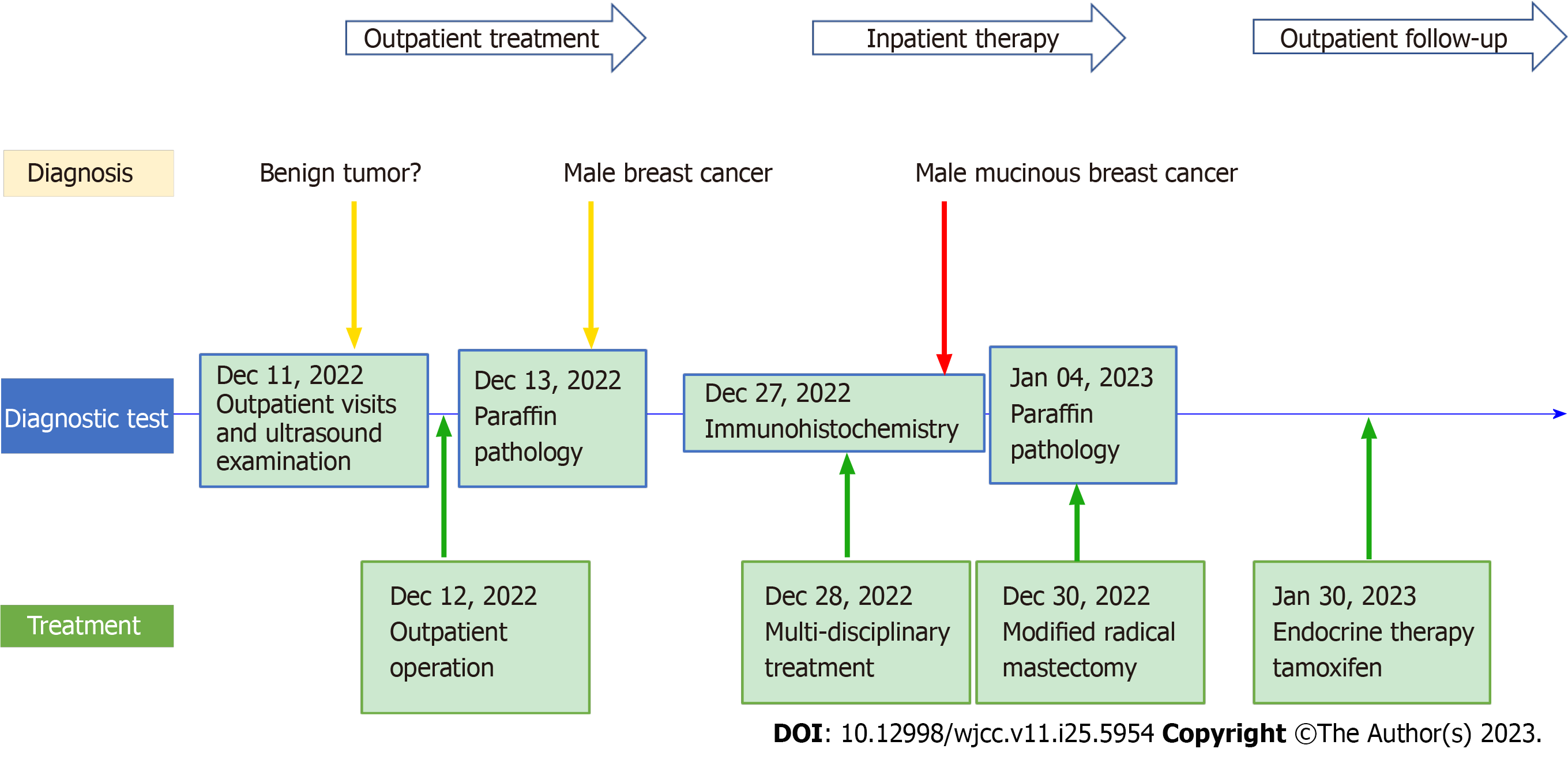
Examination and ultrasound revealed a mass located anterior to the pectoralis major fascia that can be differentiated from a muscle-derived mass. Subcutaneous fibroadenomas are slow growing and occasionally painful. In gynecomastia, the masses are mostly located behind the nipple and are not very well defined with a soft texture. Lymphomas, which are located in the chest 2.0 cm from the nipple in a non-normal lymphatic anatomical location, are more difficult to distinguish and require pathological analysis for identification. The outpatient surgeon diagnosed a possible benign fibroadenoma, which was surgically removed on December 12, and found the tumor lacked a capsule and the specimen was brittle. According to histopathological examination and immunohistochemistry analysis of the excised tissue, multidisciplinary consultation by the Department of Breast Surgery, Oncology, Ultrasound, and Pathology made the diagnosis - non-retroareolar MMBC without gynecomastia. The patient was hospitalized and underwent modified radical mastectomy (MRM) for breast cancer on December 30, 2022, Intraoperatively, the gynecomastia was seen to be very thin, but the area was equivalent to that of a female. Pathological return: No cancerous tissue invasion of the nipple and pectoralis major fascia, peri-cancerous breast: Granulomatous inflammation, postoperative lymph nodes: 0/20 (extra-pectoralis minor 0/19; post-pectoralis minor 0/1), pT2N0M0.
Postoperative axillary and supracostal arch drains were less than 20 mL for three consecutive days starting on postoperative day 6 and were removed on day 9. The patient had no adverse complaints. The flap hemorrhage in the operative area was normal. Perioperative care was equivalent to that of female breast cancer (FBC) and no significant discomfort for the patient during treatment. Tamoxifen (20 mg/d) was administered as adjuvant therapy (Figure 3). The patient was followed up every three months in the outpatient clinic. The follow-up tumor marker carbohydrate antigen 153 fluctuated in the fifth month, and CT and ultrasound showed no abnormality.
MBC occurrence in men accounts for less than 1% of all breast cancer cases, and malignant tumors develop in less than 1% of affected men[11]. Different risk factors concurrently impact MBC development, including clinical disorders related to hormonal imbalance, such as obesity, testicular disease, and radiation exposure; suspected epidemiological risk factors are prostate cancer, prostate cancer treatment, occupational exposure, dietary factors, and alcohol intake[12]. By contrast, carrying a BRCA2 mutation confers a lifetime risk for MBC to the male carrier, which is approximately 80-100 times higher than that in the general population[13]. The rate of mucinous adenocarcinoma of all invasive breast drops from 4% to 2%[2,14]. Mucinous breast cancer rarely occurs in men. A single-center trial retrospectively assessed 16868 breast cancer patients over 10 years, with 72 cases of MBC accounting for 0.42% of the total patient population and six cases of mucinous adenocarcinoma accounting for 8.33% of the total MBC patient population[15].
The presence of extracellular mucin on microscopic observation is the basis for the microscopic diagnosis of mucinous breast cancer, defined as pure mucinous cancer and mixed mucinous cancer (MMC) at a cutoff of 90%. MMC can be further differentiated into partially mixed mucinous adenocarcinoma and mixed mucinous adenocarcinoma at a cutoff of 50%. Immunohistochemistry analysis revealed that the expression of estrogen and progesterone receptors was positive, and that of the androgen receptor was negative.
A 71-year-old MBC patient was treated by a physician in Virginia in the 1970s[16]. Although he had bilateral breast cancer, the pathology was not fully documented. However, searches for PubMed, several cases of male mucinous breast cancer cases have been successfully treated in recent decades. We have summarized and analyzed mucinous adenocarcinoma cases among men (Table 1). As assessed using hematoxylin-eosin staining, all patients had mucinous adenocarcinoma[8-10,17-22]. In one case, the lesion type was mixed invasive ductal carcinoma (IDC) component[17], and in another case, the lesion type was concurrent breast mucinous carcinoma and extramammary lymphoma[8]. The patient’s age ranged from 35 to 83 years, with a mean age of 69.3 years. The history of onset ranged from 2-96 mo, with a median time of nearly 12 mo, and swelling of the retroareolar region is the main factor for tumor development. No significant difference was noted in the location of the masses to the left and right (four left and five right). All of them were located posterior to the nipple-areolar region, except for one case wherein the mass was present in the axillary position. The mass diameter was used as a statistical criterion, with a minimum value of 13 mm, a maximum value of 95 mm, and a mean diameter length of 44.5 mm. Four of the eight patients who reported lymph node metastasis had combined lymph node metastasis (50%). HER-2/CerbB-2 was tested in four patients with non-amplified or negative expressions. BRCA testing was negative in two patients with combined diffuse large B-cell tumors and those with an IDC component. All patients had MRM at the correct location. A Japanese patient underwent MRM after two resections of enlarged masses within a period of 3 years until the fifth year, which was when the mass recurred and showed ipsilateral axillary lymph node enlargement[22]. Postoperative adjuvant therapy revealed the following findings: All patients were treated with tamoxi
| Ref. | Country | Age (yr) | Case history (yr) | Laterality | Past history | Tumor max-diameter | Tumor location | Pathology characteristic | Lymphatic metastasis | ER | PR | HER2/CerbB-2 | KI67 % | BRCA | Surgery | Adjuvant therapy | DFS (mo) |
| Fujikawa et al[20], 1998 | Japan | 35 | 2 | Right | Negative | 80 | Areola | M | Negative | Positive | Positive | None | None | None | MRM | C + TAM | 30 |
| Peschos et al[19], 2008 | Greece | 83 | None | Left | None | 70 | Areola | M | None | Positive | Positive | None | None | None | MRM | C + TAM | None |
| Hammedi et al[10], 2010 | Tunisia | 75 | 2 mo | Right | Negative | 40 | Areola | M | Positive + 2 | Negative | Positive | Negative | None | None | MRM | C + R + TAM | 36 |
| Dragoumis et al[21], 2012 | Greece | 59 | 1 | Right | Negative | 30 | Areola | M | Positive | Positive | Positive | Negative | None | None | MRM | C + R + TAM | 74 |
| Imakado and Masuda[22], 2012 | Japan | 72 | 3 | Left | Negative | 20 | Areola-aside | M | Positive | Positive | Positive | None | None | None | MRM | TAM | 60 |
| Gupta et al[18], 2015 | India | 73 | 8 | Right | Negative | 95 | Areola | M | Negative | Positive | Positive | Middle 2 | 11 | None | MRM | TAM | 8 |
| Kim and Lee[8], 2021 | Korean | 78 | 1 | Left | Negative | 35 | Areola | M + B-cell | Positive + 2 | None | None | None | None | Negative | MRM | C + TAM | 11 |
| Takahashi et al[17], 2021 | Japan | 75 | None | Right | Sister breast cancer | 13 | Axilla | M + IDC | Negative | Positive | Positive | Negative | 33 | Negative | MRM | TAM | 24 |
| Ahmed et al[9], 2022 | United States | 74 | 1 | Left | Thyroid cancer | 18 | Areola | M | Negative | None | None | None | None | None | MRM | TAM | 48 |
Having presented the case, we now move on to discuss the broader implications and findings. In summary, the median age of onset of mucinous adenocarcinoma in men is above 70 years. The history of onset of MMBC patients is longer than a year, and the average mass size is greater than 40 mm; however, MMBC is frequently overlooked. The chief complaint and the primary clinical manifestation of patients are swelling behind the nipple region or skin changes in the nipple region. Patients with axillary metastatic lymph nodes had a predominantly 1-year history and a smaller average mass size than the other patients; hence, it cannot be speculated that the length of history and mass size are the key factors attributed to the occurrence of lymph node metastasis. Generally, the trend in postoperative adjuvant therapy has switched from chemotherapy combined with endocrine therapy before 2010 to predominantly hormone therapy alone after 2010, considering that it was influenced by the treatment of luminal-type FBC, while the treatment strategy changed the same. All patients had better prognoses than men with nonspecific breast cancer. Previous case reports have shown that the retroareolar mass usually occurs with gynecomastia and is in the inner margin of the nipple[22]. Another case of mucinous breast cancer combined with Paget’s disease also had a nipple-centered clinical presentation[17]. We present the first case of MMBC in the non-retroareolar region of the nipple. The patient had no evident signs of gynecomastia, and the mass was located on the upper outer nipple at an easily misdiagnosed distance. The absence of cancerous tissue in the nipple and posterior glands confirmed this finding. Similar to this finding, Mizutani et al[23] reported a rare case of cutaneous mucinous adenocarcinoma in a 78-year-old man. In the fourth year of follow-up review after prostate cancer treatment, an abnormally enlarged axillary lymph node (10 mm) was found, and a whole-body examination revealed the presence of a 30-mm-sized mass in the patient’s inner upper right chest. For several years, the patient complained of a mass in the right chest but did not pay close attention to it; eventually, the mass was surgically excised as a benign 1-cm margin plus axillary lymph node dissection, and no breast tissue was found in the pathological specimen, but the enlar
In our case, the patient’s gynecomastia symptoms are not visible because of the patient’s low BMI and the absence of fat encapsulation in the gland beneath the skin. The patient was considered to have an atypical clinical presentation of MMBC, and MRM is essential. Because of the strong expression of the hormone receptors (estrogen and progesterone receptors), low tumor cell proliferation index indicated by Ki67, and negative pathological axillary lymph nodes, the patient would have a well-predicted prognosis. A 5-year outpatient follow-up with endocrine treatment is required.
We encountered a rare case of MMBC. It was difficult to differentiate from MBC because the patient had no representative clinical indications of gynecomastia, and the mass was located distant from the nipple region. A diagnosis of male mucinous breast cancer should be considered, even when the body surface mass on the male chest is not located in the retroareolar region, and MRM and endocrine treatment is necessary.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kang SY, South Korea; Wu K, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Fox S, Speirs V, Shaaban AM. Male breast cancer: an update. Virchows Arch. 2022;480:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | Limaiem F, Ahmad F. Mucinous Breast Carcinoma. 2023 Feb 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. [PubMed] |
| 3. | McClurg DP, Urquhart G, McGoldrick T, Chatterji S, Miedzybrodzka Z, Speirs V, Elsberger B. Analysis of the Clinical Advancements for BRCA-Related Malignancies Highlights the Lack of Treatment Evidence for BRCA-Positive Male Breast Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Gucalp A, Traina TA, Eisner JR, Parker JS, Selitsky SR, Park BH, Elias AD, Baskin-Bey ES, Cardoso F. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res Treat. 2019;173:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 5. | Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006;367:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 403] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 6. | Cardoso F, Bartlett JMS, Slaets L, van Deurzen CHM, van Leeuwen-Stok E, Porter P, Linderholm B, Hedenfalk I, Schröder C, Martens J, Bayani J, van Asperen C, Murray M, Hudis C, Middleton L, Vermeij J, Punie K, Fraser J, Nowaczyk M, Rubio IT, Aebi S, Kelly C, Ruddy KJ, Winer E, Nilsson C, Lago LD, Korde L, Benstead K, Bogler O, Goulioti T, Peric A, Litière S, Aalders KC, Poncet C, Tryfonidis K, Giordano SH. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol. 2018;29:405-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 251] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 7. | Ruddy KJ, Winer EP. Male breast cancer: risk factors, biology, diagnosis, treatment, and survivorship. Ann Oncol. 2013;24:1434-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 8. | Kim SY, Lee JY. Multiple Primary Malignant Neoplasms: A Case Report of Breast Mucinous Carcinoma and Extramammary Diffuse Large B-Cell Lymphoma in a Male Patient. Taehan Yongsang Uihakhoe Chi. 2021;82:729-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Ahmed U, Wagner S, Jordan S. Mucinous carcinoma in a male patient: Diagnosis and management of breast cancer in male patients. Radiol Case Rep. 2022;17:124-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 10. | Hammedi F, Trabelsi A, Abdelkrim SB, Abid LB, Jomaa W, Bdioui A, Beizig N, Mokni M. Mucinous carcinoma with axillary lymph node metastasis in a male breast: A case report. N Am J Med Sci. 2010;2:111-113. [PubMed] |
| 11. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15255] [Article Influence: 3051.0] [Reference Citation Analysis (4)] |
| 12. | Khan NAJ, Tirona M. An updated review of epidemiology, risk factors, and management of male breast cancer. Med Oncol. 2021;38:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Rizzolo P, Silvestri V, Tommasi S, Pinto R, Danza K, Falchetti M, Gulino M, Frati P, Ottini L. Male breast cancer: genetics, epigenetics, and ethical aspects. Ann Oncol. 2013;24 Suppl 8:viii75-viii82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Anderson WF, Chu KC, Chang S, Sherman ME. Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:1128-1135. [PubMed] |
| 15. | Liu DY, Xie GR, Chen M. [Analysis on the clinical and prognostic features of 71 male patients with breast cancer]. Zhonghua Liu Xing Bing Xue Za Zhi. 2013;34:187-190. [PubMed] |
| 16. | Brodie EM, King ER. Histologically different, synchronous, bilateral carcinoma of the male breast (a case report). Cancer. 1974;34:1276-1277. [PubMed] [DOI] [Full Text] |
| 17. | Takahashi E, Terata K, Nanjo H, Ishiyama K, Hiroshima Y, Yamaguchi A, Yatsuyanagi M, Kudo C, Wakita A, Takashima S, Sato Y, Imai K, Motoyama S, Minamiya Y. A male with primary accessory breast carcinoma in an axilla is strongly suspected of having hereditary breast cancer. Int Cancer Conf J. 2021;10:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Gupta K, Sharma S, Kudva R, Kumar S. Mixed Mucinous and Infiltrating Carcinoma Occurring in Male Breast- Study of Clinico-Pathological Features: A Rare Case Report. J Clin Diagn Res. 2015;9:ED07-ED08. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Peschos D, Tsanou E, Dallas P, Charalabopoulos K, Kanaris C, Batistatou A. Mucinous breast carcinoma presenting as Paget's disease of the nipple in a man: a case report. Diagn Pathol. 2008;3:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Fujikawa T, Sonobe M, Nishimura S, Matsusue S, Takeda H, Nakamura Y. A case of Mucinous Carcinoma of the Male Breast with Unusual Ultrasonographic Findings Mimicking Phyllodes Tumor. Breast Cancer. 1998;5:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Dragoumis DM, Assimaki AS, Tsiftsoglou AP. Pure mucinous carcinoma with axillary lymph node metastasis in a male breast. Breast Cancer. 2012;19:365-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Imakado S, Masuda R. A case of carcinoma of the male breast mimicking a mucinous carcinoma of the skin. Clin Pract. 2012;2:e61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Mizutani K, Taira M, Akiyama M. Primary mucinous carcinoma of the skin on the breast with lymph node metastasis. J Dermatol. 2014;41:760-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Yadav S, Karam D, Bin Riaz I, Xie H, Durani U, Duma N, Giridhar KV, Hieken TJ, Boughey JC, Mutter RW, Hawse JR, Jimenez RE, Couch FJ, Leon-Ferre RA, Ruddy KJ. Male breast cancer in the United States: Treatment patterns and prognostic factors in the 21st century. Cancer. 2020;126:26-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 25. | Zheng G, Leone JP. Male Breast Cancer: An Updated Review of Epidemiology, Clinicopathology, and Treatment. J Oncol. 2022;2022:1734049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |