Published online Sep 6, 2023. doi: 10.12998/wjcc.v11.i25.5919
Peer-review started: March 25, 2023
First decision: June 21, 2023
Revised: July 5, 2023
Accepted: August 7, 2023
Article in press: August 7, 2023
Published online: September 6, 2023
Processing time: 160 Days and 0.1 Hours
Synchronous multiple lung cancers are rare and refer to the simultaneous presence of two or more primary lung tumors, which present significant challen
We report a case of multiple synchronous lung cancers with hilar lymph node metastasis of small cell carcinoma of unknown origin in a 73-year-old man. Transbronchial lung biopsy revealed squamous cell carcinoma. Although enlargement of lymph node 12u was detected, no distant metastases were observed. The patient was preoperatively diagnosed with T1cN0M0 and underwent thoracoscopic right upper lobectomy with nodal dissection (ND2a). Based on histopathological findings, the primary lesion was squamous cell carcinoma. A microinvasive adenocarcinoma was also observed on the cranial side of the primary lesion. Tumors were detected in two resected lymph nodes (#12u and #11s). Both tumors were pathologically diagnosed as small cell carcinomas. The primary lesion of the small cell carcinoma could not be identified even by whole-body imaging; however, chemotherapy was initiated for hilar lymph node metastasis of the small cell carcinoma of unknown origin.
Multiple synchronous lung cancers can be accompanied by hilar lymph node metastasis of small cell carcinomas of unknown origin.
Core Tip: Synchronous multiple lung cancer is relatively rare. We report a case of synchronous multiple lung cancers with lymph node metastasis in the hilar region of a small cell carcinoma of unknown origin. Immunohistochemistry was helpful in aiding the diagnosis. As such cases have a poor prognosis, an accurate diagnosis is necessary to determine treatment options.
- Citation: Yoshino R, Yoshida N, Yasuda S, Ito A, Nakatsubo M, Yuzawa S, Kitada M. Synchronous multiple lung cancers with hilar lymph node metastasis of small cell carcinoma: A case report. World J Clin Cases 2023; 11(25): 5919-5925
- URL: https://www.wjgnet.com/2307-8960/full/v11/i25/5919.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i25.5919
Synchronous multiple primary cancerous lesions are rare in patients with primary lung cancer[1]. Such lesions are associated with a poorer prognosis than solitary lung cancer. Moreover, it is necessary to differentiate these lesions from intrapulmonary metastases when these tumors exhibit the same histological type.
Furthermore, hilar and mediastinal lymph node metastases of unknown origin are rare in cases of small cell carcinoma[2]. We report the rare case of a 73-year-old man with a preoperative diagnosis of squamous cell carcinoma of the lung who underwent surgery and was found to have multiple synchronous cancers (squamous cell carcinoma and microinvasive adenocarcinoma) on histopathological examination. Additionally, the histological type of the hilar lymph node metastasis was small cell carcinoma. To the best of our knowledge, a similar case study has not been reported previously. Herein, we describe our case and review the relevant literature.
The patient had no subjective symptoms such as respiratory distress or chest pain. Computed tomography (CT) revealed abnormal shadows in the chest.
The patient had been receiving treatment for diabetes mellitus; during a regular checkup, a mass shadow in the right upper lung was observed on chest radiography. The lesions were closely examined, and the patient was diagnosed with squamous cell carcinoma of the lung using bronchoscopy. Subsequently, the patient was referred to our department for surgical resection.
The patient had a history of diabetes mellitus and hypertension, which were well-controlled. No other cardiac or respiratory disease was observed.
The patient’s family history was unremarkable. The patient had a history of smoking 20 cigarettes per day for 38 years (20–58 years of age).
Physical findings on admission were as follows: Height, 169 cm; weight, 79.3 kg; body mass index, 27.8 kg/m2. Respiratory sounds were evident in both lungs. Superficial lymph nodes were not palpable.
The patient’s blood count was within the normal range. Blood glucose level was 177 mg/dL, and hemoglobin A1c level was slightly elevated at 6.9%. No abnormal findings were observed for any other biochemical or coagulation marker. Levels of tumor markers (carcinoembryonic antigen, cytokeratin 19 fragment, and pro-gastrin-releasing peptide) were not elevated. Respiratory function tests and electrocardiograms revealed no abnormalities.
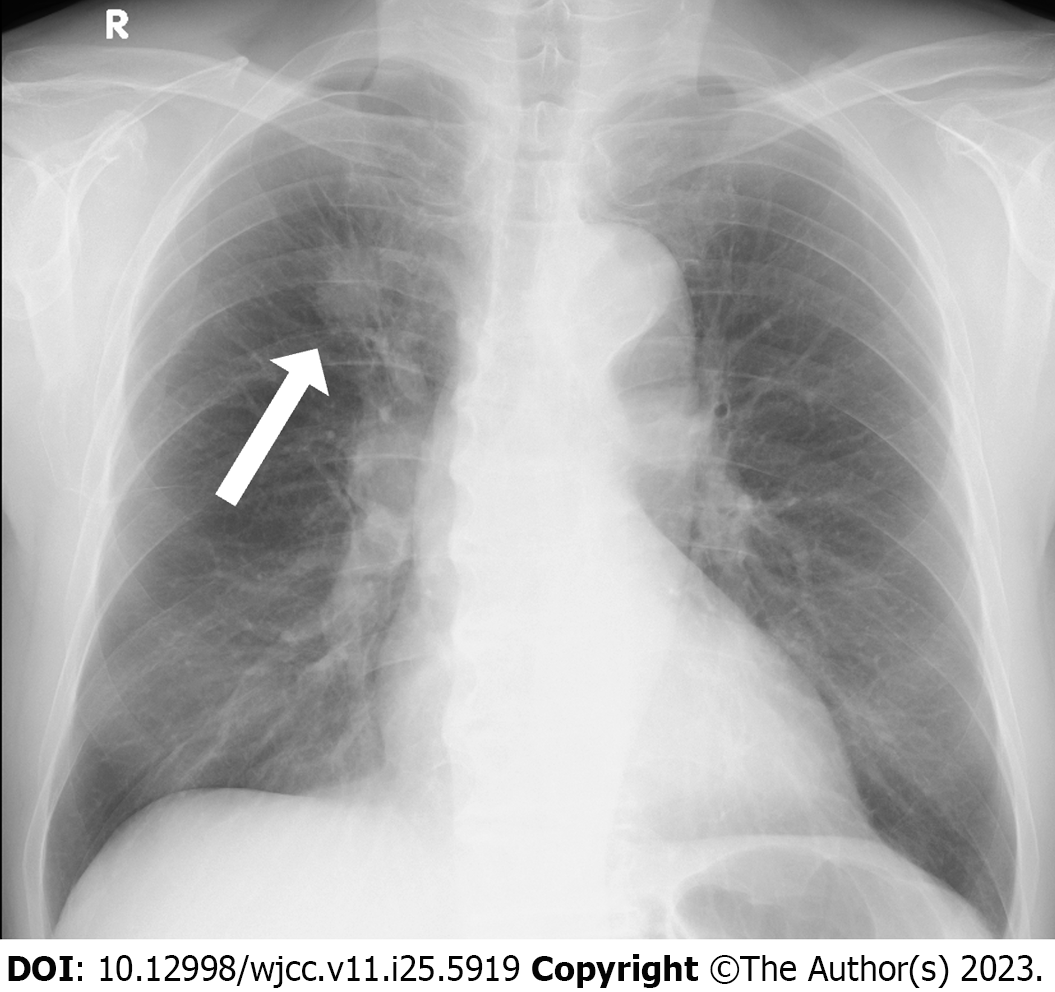
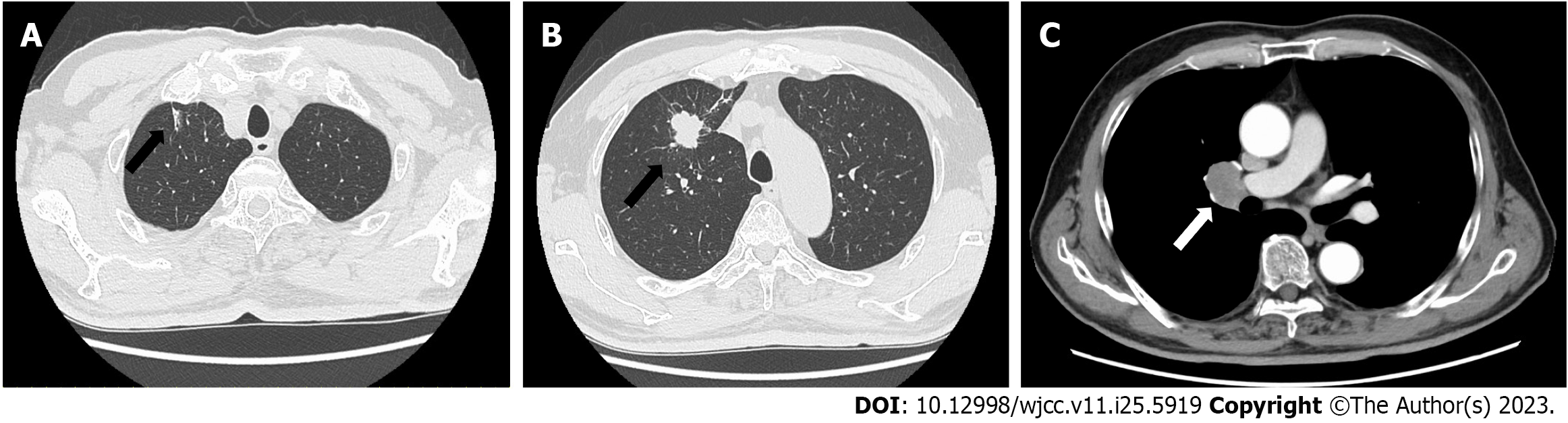
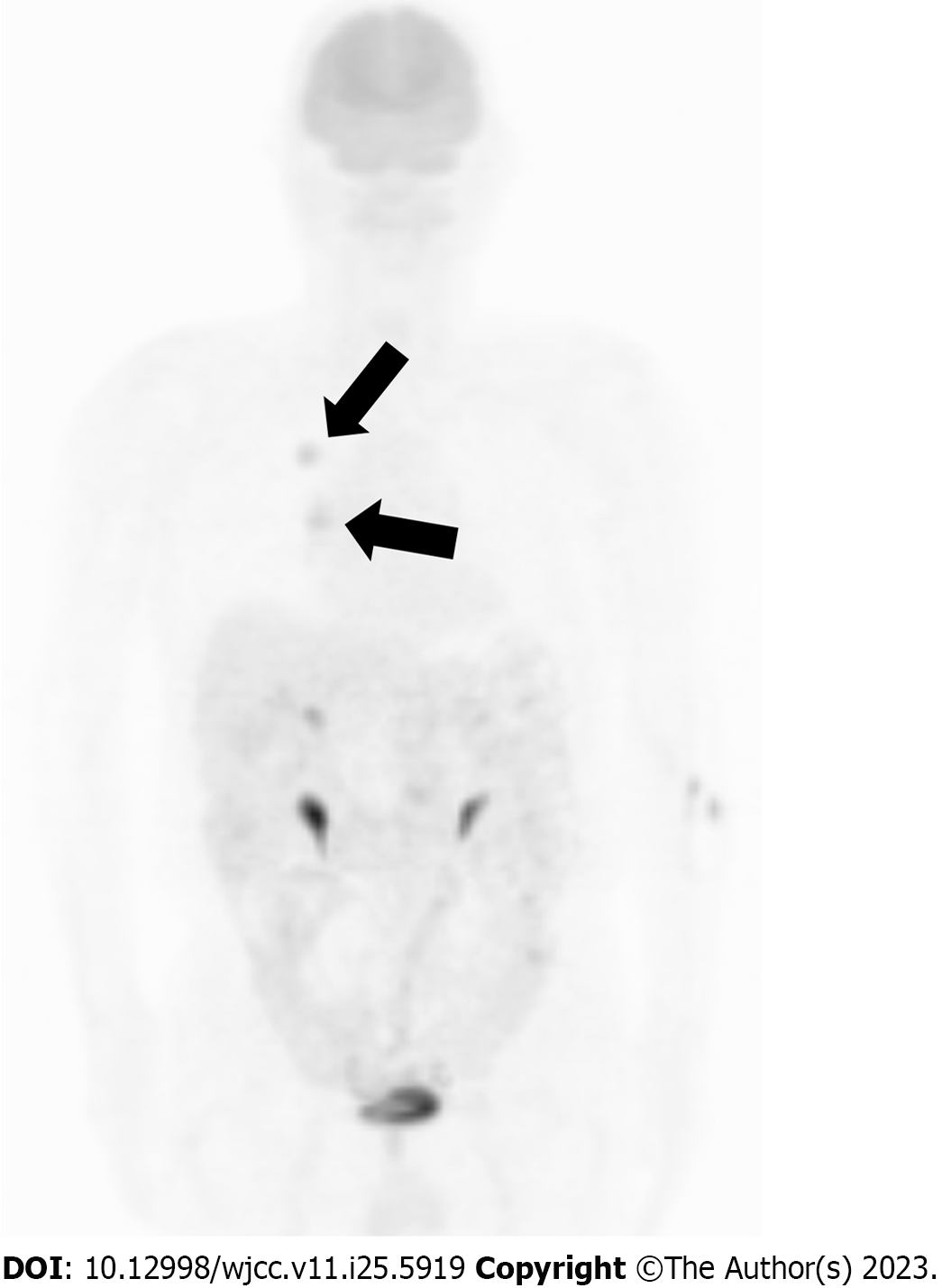
Chest radiography revealed nodular shadows associated with the surrounding tightening of the right upper lung field (Figure 1). Chest CT images (lung window) showed an irregularly shaped mass measuring 2.5 × 2.0 cm in the upper lobe of the right lung and a slightly increased density on the cranial side (Figure 2A and B). CT (mediastinal window) also revealed enlarged lymph nodes in the right hilar region (Figure 2C). Additionally, abdominal CT showed no findings suggestive of metastasis, and magnetic resonance imaging revealed no evidence of brain metastasis. Fluorodeoxyglucose (FDG) positron emission tomography showed FDG uptake, with a maximum standardized uptake value of approximately 5.0, in the right upper lung field and right hilar region (Figure 3), indicating hilar lymph node metastasis of the primary right upper lobe lung cancer.
The specimen collected from the right upper pulmonary lobe during the transbronchial lung biopsy was histologically diagnosed as squamous cell carcinoma.
Intraoperatively, thoracoscopic right upper lobectomy with nodal dissection (ND2a) was performed via a thoracotomy of the fifth intercostal space. The lymph nodes around the truncus artery were enlarged, with strong adhesion. The surgery time was 2 h 4 min, with 10 mL blood loss.
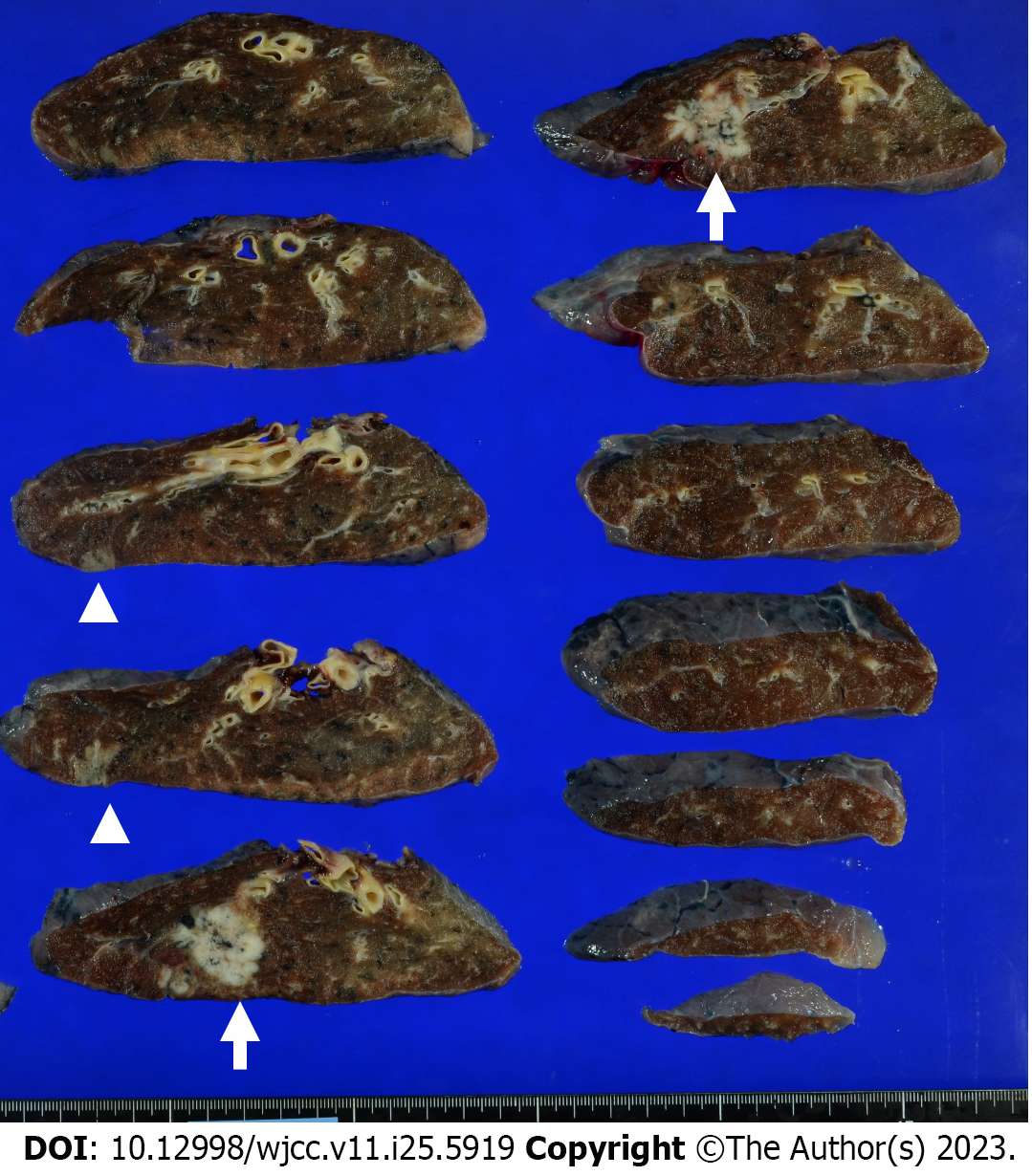
Upon examination of the resected specimen, a tumor measuring 3.0 cm × 2.5 cm × 2.5 cm on cut surfaces was detected in segment 1 of the right upper pulmonary lobe. A lesion with a grayish-white area measuring 1.7 cm × 1.5 cm × 1.0 cm was noted on the cranial side (Figure 4). The lobar bronchial lymph node (#12u) was enlarged to 2.9 cm × 2.8 cm × 2.1 cm.
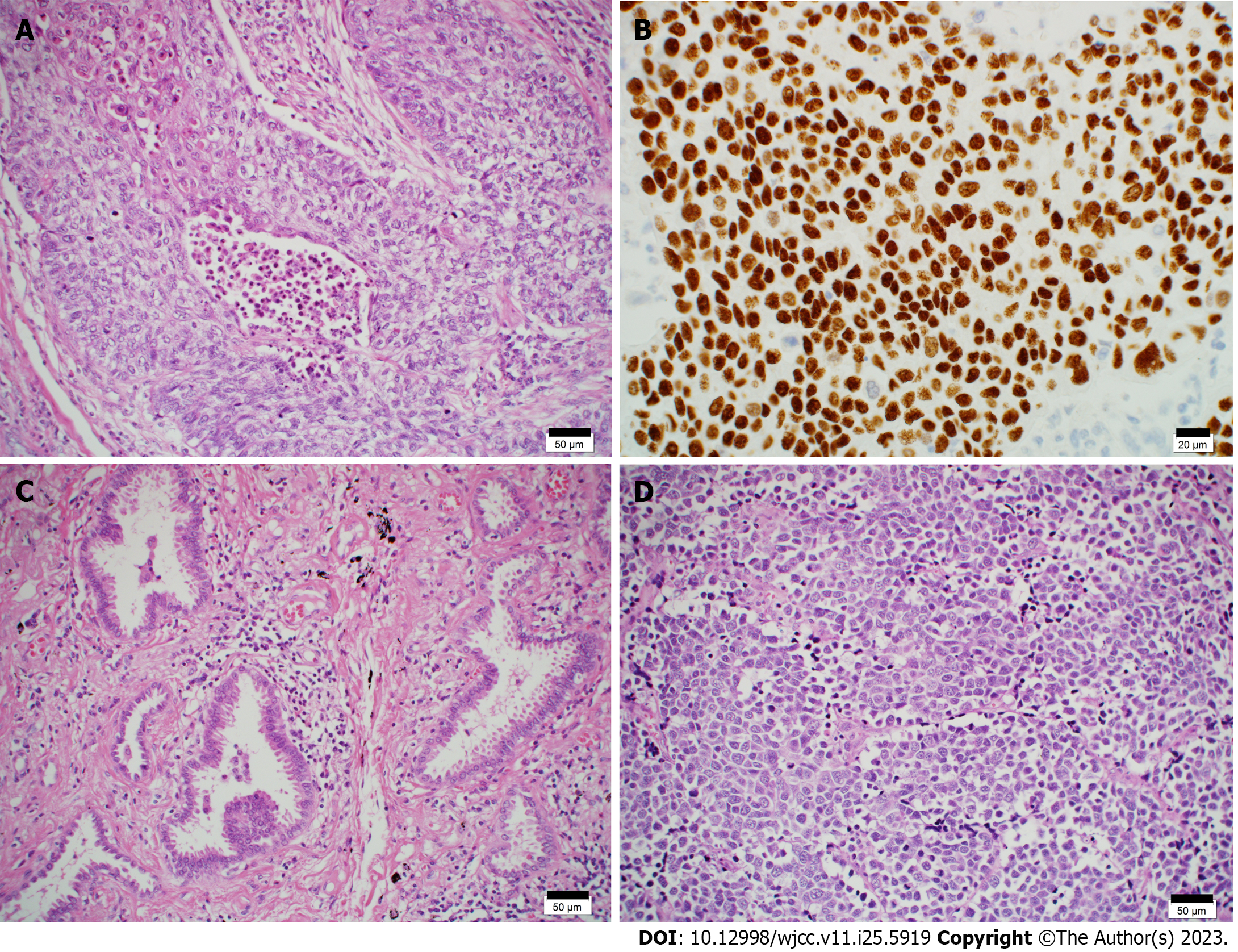
Using hematoxylin-eosin (HE) staining, histopathological examination of the mass in the right upper pulmonary lobe revealed nested and sheet-like growth of atypical cells with hyperchromatic pleomorphic irregularly-shaped nuclei. The nuclei were centrally located, some cells had intercellular bridges, and focal keratinization was observed. These findings were indicative of squamous cell carcinoma (Figure 5A). Immunohistochemical staining was positive for p40 (Figure 5B) and negative for CD56, synaptophysin, and chromogranin A. Vascular and lymphatic invasion was observed. The pathological diagnosis was pT1cN0M0 (pStage IA3).
The cranial side of the tumor in the right upper pulmonary lobe showed lepidic growth of atypical cuboidal cells with enlarged nuclei and eosinophilic cytoplasm (Figure 5C). Focal invasion measuring ≤ 1 mm was noted, indicating microinvasive adenocarcinoma; neither vascular nor lymphatic invasion was observed. The pathological diagnosis was pT1aN0M0 (pStage IA1). HE staining of a lobar bronchial lymph node (#12u) revealed solid and nested growth of atypical cells with finely granular nuclear chromatin and scant cytoplasm. The tumor cells were less cohesive, with no keratinization or intercellular bridges. Apoptotic cells and mitoses were frequently observed. These findings are indicative of small cell carcinoma (Figure 5D). Immunohistochemical staining was positive for cytokeratin CAM5.2 and negative for p40. A few tumor cells were positive for synaptophysin, chromogranin A, insulinoma-associated protein-1 (INSM1), and CD56. Micrometastases of small cell carcinoma were also observed in the lymph nodes of #11s. The pathological diagnosis was pTXN1M0 (Stage IIB).
The final diagnosis was synchronous multiple lung cancers (squamous cell carcinoma and microinvasive adenocarcinoma) with hilar lymph node metastasis of small cell carcinoma of unknown origin.
The patient had no adverse events postoperatively and was discharged on the eighth postoperative day. One month later, the patient received four courses of chemotherapy consisting of carboplatin [area under the curve (AUC), 5] and etoposide (80 mg/m2) combination. No prophylactic cranial irradiation was administered.
There was no evidence of recurrence approximately 2 years after surgery. The patient was followed-up every 6 mo.
Although synchronous multiple lung cancers have been reported previously, they are relatively rare[1]. Martini and Melamed[3] reported the following diagnostic criteria: (1) Each tumor exists independently; (2) Tumors exhibit different histological types, or if they exhibit identical histological types, intrapulmonary metastasis can be ruled out based on the presence or absence of carcinoma in situ, the extent of histodifferentiation, cellular subtypes, the extent of scar formation at the tumor core, and presence or absence of vascular invasion; and (3) Metastasis from other organ cancers can be ruled out. The histological types are often identical. Squamous cell carcinoma is frequently reported in Japan, whereas adenocarcinoma is common in the United States[4]. In the case of simultaneous multiple lung cancers, determining whether these lung cancer foci are separate primary sites is relatively easy when histological examination reveals differences in tumors[5]. However, it is difficult to differentiate between two tumors with similar morphologies[6]. To this end, genetic analysis has recently become a widely used auxiliary diagnostic tool[7,8].
Cancer of unknown origin accounts for approximately 2%–5% of all cancers[9] and can manifest as lymph node metastasis, accounting for approximately 30%–40% of these cases[10]. Cancer detection is even rarer owing to hilar mediastinal lymph node metastasis. In many cases, the primary cancer is assumed to be located in the lungs. Adenocarcinoma is the most common histological type, whereas small cell carcinomas are rare[2]. Cases of synchronous multiple lung cancers with hilar lymph node metastasis of small cell carcinoma of unknown origin are rare, as in the present case. No such cases have been reported in the literature.
Synchronous multiple lung cancers must be differentiated from pulmonary metastases, extrapulmonary metastases, infections, and benign nodules. In the present case, HE staining of the tumor in the right upper pulmonary lobe revealed findings indicative of squamous cell carcinoma. Immunohistochemical staining was positive for p40, consistent with the diagnosis of squamous cell carcinoma[11]. Furthermore, the maximum invasion diameter of the tumor on the cranial side was ≤ 1 mm, and the HE staining results indicated microinvasive adenocarcinoma. HE staining of the lobar bronchial lymph nodes (#12u and #11s) indicated small cell carcinoma. Immunohistochemical staining results were negative for p40; hence, squamous cell carcinoma was excluded. Synaptophysin, chromogranin A, INSM1, and CD56 were focally positive, consistent with small cell carcinoma[12,13]. However, there was no evidence of a primary lesion indicative of small cell carcinoma in the right upper pulmonary lobe. This may be explained as follows: the primary tumor site may be the hilum, a lump of the tumor and lymph node mass may be formed, there may be minute primary lesions in sites that were not collected as specimens, and the primary lesion may be in sites other than the right upper pulmonary lobe. Squamous cell carcinoma is unlikely to transform into small cell carcinoma because squamous cell carcinoma contains no small cell carcinoma components, the lymph nodes contain no squamous cell carcinoma components, and transformation from squamous cell carcinoma to small cell carcinoma is rare.
Our patient received postoperative chemotherapy with four courses of carboplatin (AUC 5) and etoposide (80 mg/m2) combination. When the histological types of tumors vary in multiple synchronous lung cancers, each tumor should be evaluated and treated, regardless of the presence of other tumors. When tumors are in stages II or III, postoperative chemotherapy is recommended[14]. However, no standard effective postoperative treatment regimen has been established for stage I tumors, as in the present case. As our patient had hilar lymph node metastasis of small cell carcinoma of unknown origin, postoperative chemotherapy was administered based on the treatment regimen for small cell lung carcinoma, as stated in the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Approximately 2 years postoperatively, there was no evidence of recurrence.
When multiple synchronous lung cancers are accompanied by hilar lymph node metastasis, diagnosis and therapeutic strategies vary according to the histological type of the tumor. Therefore, immunohistochemical staining is a valuable tool for tumor differentiation. As in our case, the coexistence of synchronous multiple lung cancers with different histological types and histologically different carcinomas of unknown origin is rare. Further investigation of such cases is required to clarify this condition.
We present a case of multiple synchronous lung cancers with hilar lymph node metastasis of small cell carcinoma of unknown origin. Immunohistochemical staining proved to be a valuable diagnostic tool. In addition, synchronous multiple lung cancers and cancers of unknown origin have poor prognoses; hence, an accurate diagnosis is necessary for selecting postoperative adjuvant therapy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Eccher A, Italy; Lin Q, China; Long X, China S-Editor: Lin C L-Editor: Webster JR P-Editor: Zhang XD
| 1. | Trousse D, Barlesi F, Loundou A, Tasei AM, Doddoli C, Giudicelli R, Astoul P, Fuentes P, Thomas P. Synchronous multiple primary lung cancer: an increasing clinical occurrence requiring multidisciplinary management. J Thorac Cardiovasc Surg. 2007;133:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Chang SK, Ano S, Kikuchi N, Masuda M, Osawa H, Kondo Y, Ishii Y. A case of lung and mediastinal and hilar lymph node metastasis in a patient with cancer of unknown primary site. Clin Exp Metastasis. 2022;39:259-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70:606-612. [PubMed] |
| 4. | Wakelee HA, Bernardo P, Johnson DH, Schiller JH. Changes in the natural history of nonsmall cell lung cancer (NSCLC)--comparison of outcomes and characteristics in patients with advanced NSCLC entered in Eastern Cooperative Oncology Group trials before and after 1990. Cancer. 2006;106:2208-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Wu X, Huang W, Geng T, Wei Y. Next-Generation Sequencing of Synchronous Multiple Primary Lung Cancers in a Patient with Squamous Cell Carcinoma and Small Cell Lung Cancer. Onco Targets Ther. 2020;13:11621-11626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Jiang L, Zheng X, Wu S, Zhang J, Ru G, Li Y. A Rare Case Of Synchronous Multiple Primary Lung Cancer: Squamous Cell Cancer And Small Cell Lung Cancer. Onco Targets Ther. 2019;12:8801-8806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Qu R, Tu D, Ping W, Zhang N, Fu X. Synchronous Multiple Lung Cancers with Lymph Node Metastasis and Different EGFR Mutations: Intrapulmonary Metastasis or Multiple Primary Lung Cancers? Onco Targets Ther. 2021;14:1093-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Lim J, Han YB, Park SY, Ahn S, Kim H, Kwon HJ, Lee CT, Cho S, Chung JH. Gene Expression Profiles of Multiple Synchronous Lesions in Lung Adenocarcinoma. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Lee MS, Sanoff HK. Cancer of unknown primary. BMJ. 2020;371:m4050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Greager JA, Wood D, Das Gupta TK. Metastatic cancer from an undetermined primary site. J Surg Oncol. 1983;23:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Takahashi H, Asato Y, Ikeda Y, Kijima S, Tamura Y, Hiraki T, Hatanaka K. Tumor-to-tumor metastasis: lung cancer within a thymoma. Gen Thorac Cardiovasc Surg. 2021;69:147-150. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Wick MR. Immunohistology of neuroendocrine and neuroectodermal tumors. Semin Diagn Pathol. 2000;17:194-203. [PubMed] |
| 13. | Lantuejoul S, Moro D, Michalides RJ, Brambilla C, Brambilla E. Neural cell adhesion molecules (NCAM) and NCAM-PSA expression in neuroendocrine lung tumors. Am J Surg Pathol. 1998;22:1267-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | van Rens MT, de la Rivière AB, Elbers HR, van Den Bosch JM. Prognostic assessment of 2,361 patients who underwent pulmonary resection for non-small cell lung cancer, stage I, II, and IIIA. Chest. 2000;117:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 189] [Article Influence: 7.6] [Reference Citation Analysis (0)] |