Published online Sep 6, 2023. doi: 10.12998/wjcc.v11.i25.5910
Peer-review started: March 27, 2023
First decision: July 3, 2023
Revised: July 20, 2023
Accepted: August 8, 2023
Article in press: August 8, 2023
Published online: September 6, 2023
Processing time: 157 Days and 21.6 Hours
Malignant peripheral nerve sheath tumor (MPNST) is a rare and aggressive soft tissue sarcoma that poses a major diagnostic and therapeutic challenge.
We retrospectively reviewed patients with head and neck MPNSTs treated in our hospital from 2000 to 2021. The clinical features, pathological manifestations, treatments, and prognoses were summarized. We also reviewed the literature, focusing on MPNST in the mandible and maxilla. The study population consisted of five women and five men aged 22–75 years (mean age, 49 years). Of the 10 patients, 7 were initial cases and 3 were recurrent cases. All lesions were sporadic. The most common site was the mandible. The most frequently encountered symptoms were a progressive mass and local swelling. Complete or partial loss of trimethylation at lysine 27 of histone H3 (H3K27me3) was evident on staining in four of nine cases (one case was excluded due to lack of tissue for evaluation of loss of H3K27me3). The 2- and 5-year disease-specific survival rates were 86% and 43%, respectively. The average survival time was 64 mo.
MPNST is a highly malignant tumor with a poor prognosis, prone to a high risk of recurrence and distant metastasis. Complete surgical resection is the main treatment.
Core Tip: We retrospectively reviewed patients with head and neck malignant peripheral nerve sheath tumors treated in our hospital from 2000 to 2021. The study population consisted of five women and five men aged 22–75 years (mean age, 49 years). The 2- and 5-year disease-specific survival rates were 86% and 43%, respectively. The average survival time was 64 mo. Complete or partial loss of trimethylation at lysine 27 of histone H3 (H3K27me3) was evident on staining in four of nine cases (one case was excluded due to lack of tissue for evaluation of loss of H3K27me3).
- Citation: Li L, Ma XK, Gao Y, Wang DC, Dong RF, Yan J, Zhang R. Clinicopathological study of malignant peripheral nerve sheath tumors in the head and neck: Case reports and review of literature. World J Clin Cases 2023; 11(25): 5910-5918
- URL: https://www.wjgnet.com/2307-8960/full/v11/i25/5910.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i25.5910
Malignant peripheral nerve sheath tumor (MPNST) is an uncommon and aggressive tumor that arises from the cells of peripheral nerve sheaths. The most common anatomical sites are the trunk, extremities, and retroperitoneum. The head and neck region accounts for only 2%–9% of cases[1,2]. The clinical presentation of head and neck MPNST includes a rapidly enlarging mass, radicular pain or paresthesia, and neurological defects that depend on the anatomical sites involved[1]. Almost half of all cases are caused by defects in the neurofibromatosis type 1 gene located on chromosome 17; fewer than 10% of cases are radiation-induced (post-radiation sarcomas); the rest are sporadic cases of unknown etiology[3,4]. Histologically, MPNST is a spindle cell sarcoma arising from peripheral nerves, and varies in terms of nerve sheath differentiation; MPNST has been considered as a neurogenic sarcoma, neurofibrosarcoma, malignant schwannoma[1,5]. Most MPNSTs exhibit a tightly packed, stripe-like proliferation of spindle cells, which often complicate the diagnosis for pathologists. It is difficult to differentiate MPNSTs from other spindle cell sarcomas. The level of S-100 expression ranges from 50% to 70%, and is usually focal. In general, more primitive tumors are also more malignant, and are associated with low levels of S-100. Recent studies found that various components of polycomb repressive complex 2 (PRC2) are inactivated in most MPNSTs. Homozygous PRC2 inactivation leads to loss of H3K27me3 (as revealed by immunohistochemical analysis), which may be a new immunohistochemistry marker for MPNST[6-9].
MPNST is a high-grade malignant tumor with a high recurrence rate. The local recurrence rate is almost 50%, and 33% of patients develop bone and lung metastases[1,10]. Similar to most soft tissue sarcomas, wide excision is the main treatment; the utility of adjuvant radiotherapy and chemotherapy remains controversial[1]. In line with the high malignancy, the 5-year overall survival rate is only 20%-51%[3,11,12].
Here, we report on 10 MPNST cases, including 4 in the mandible and one in the maxilla, treated at our institution over a 20-year period. Immunohistochemical evaluation of H3K27me3 was conducted. We also review the literature with a focus on intraosseous MPNST in mandible and maxilla.
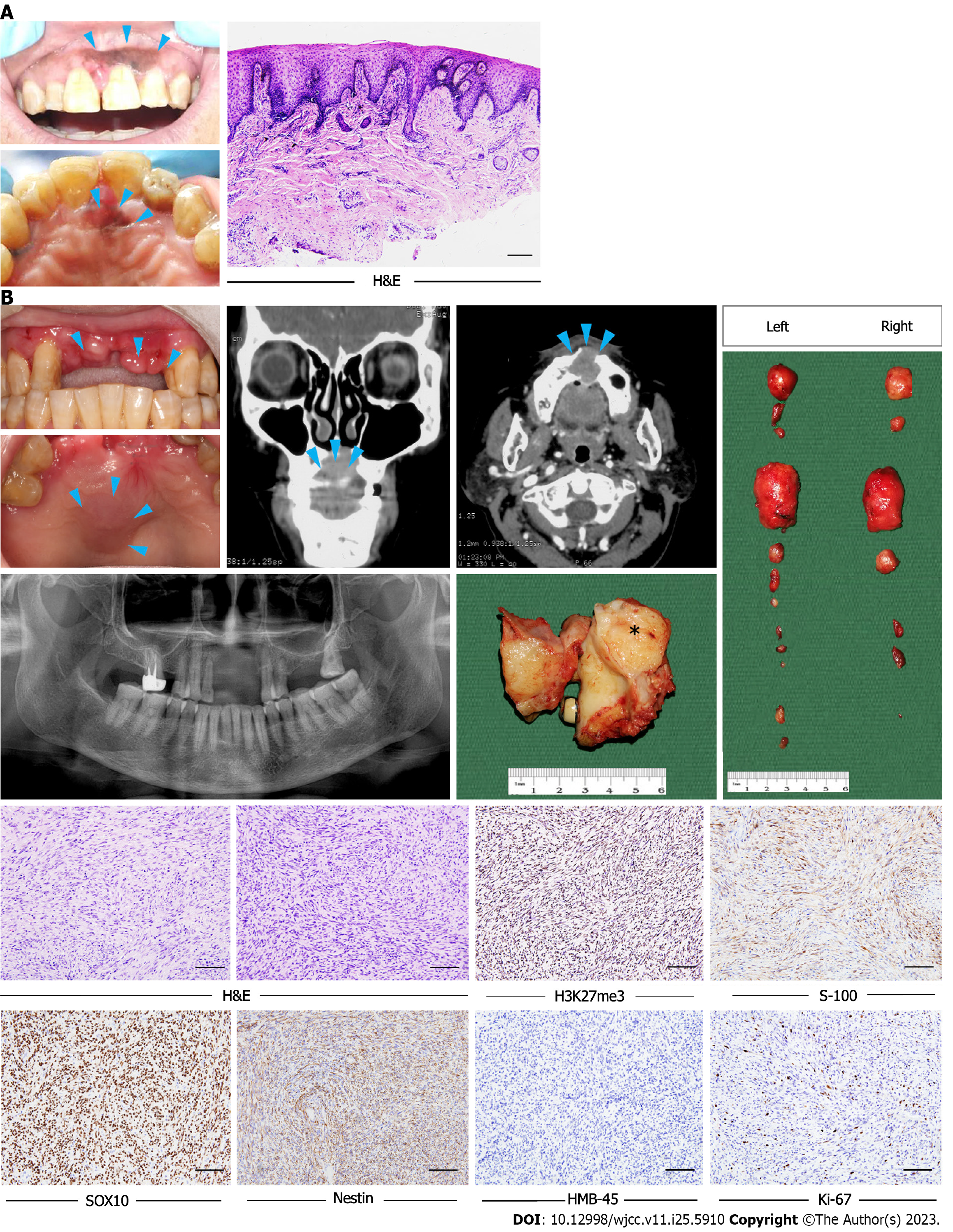
Case 1: A 62-year-old woman was referred to our hospital with a chief complaint of pigmented gingival lesions of the left anterior maxilla.
Case 2: A 28-year-old woman had a 6-mo history of a left mandibular gingival mass.
Case 1: The pathological diagnosis was an oral melanotic macule with increased melanocyte activity (Figure 1A). Two years later, clinical examination revealed a mild asymptomatic swelling on the anterior maxilla and two swollen lymph nodes on each side of the neck.
Case 2: The mandibular mass was located in the region of the 1st premolar to 1st molar teeth. The cortex of the buccal side was not continuous and the soft tissues of the cheek and tongue side were thickened.
Case 1 and Case 2: Personal and family history denies the family history of genetic disease.
Case 1 and Cases 2: Laboratory examinations reveal nothing abnormal.
Case 1: Computed tomography (CT) revealed a soft tissue mass in the anterior maxilla involving the surgical defect in the incisor region (Figure 1B).
Case 2: CT revealed a poorly-defined lytic lesion in the left mandible.
The clinical characteristics of the 10 patients are presented in Table 1; there were 7 de novo cases and 3 recurrences. No patient had a history of NF1 syndrome or radiotherapy before the first consultation. There were five male and five female patients, ranging in age from 22 to 75 years (mean age = 49 years). The mandible was the most common anatomical site. Other locations included the maxilla, gingiva, infratemporal fossa, and palate. The tumor size ranged from 1.5 to 4.5 cm (mean size = 2.9 cm). The most common initial symptoms were a progressive mass and local swelling (10/10 cases), sometimes accompanied by peripheral nerve symptoms including local pain or numbness (4/10 cases).
| Case | Sex/age | Location | Size (cm) | Treatment | Recurrence (site) | Survival | Follow-up (months) | H3K27Me3 |
| 1 | F/62 | Anterior maxilla | 2.5 | S+R+C | DR (lung, bone) | Yes | 28 | Positive |
| 2 | F/28 | Left mandible | 3 | S+R+C | NR | Yes | 24 | Positive |
| 3 | M/22 | Right submandibular region | 2.5 | S | / | / | / | Complete loss |
| 4 | M/74 | Gingival and the left cheek and left temporal | 2.5 | S | / | / | / | Positive |
| 5 | F/48 | Mandible | 3 | S+R+C | LR, DR (lung) | No | 70 | Positive |
| 6 | M/40 | Left facial and upper left gingiva | 3 | S+R | LR, DR (trunk, brain) | No | 216 | Partial loss |
| 7 | F/40 | Right mandible | 3 | S | / | / | / | Partial loss |
| 8 | F/44 | The left inferior temporal fossa | 4.5 | R+C | NR | No | 13 | Partial loss |
| 9 | M/58 | Right palat | 1.5 | S | NR | Yes | 74 | / |
| 10 | M/75 | Mandibular gingival | 3 | S | DR (lung, lymph node) | No | 24 | Positive |
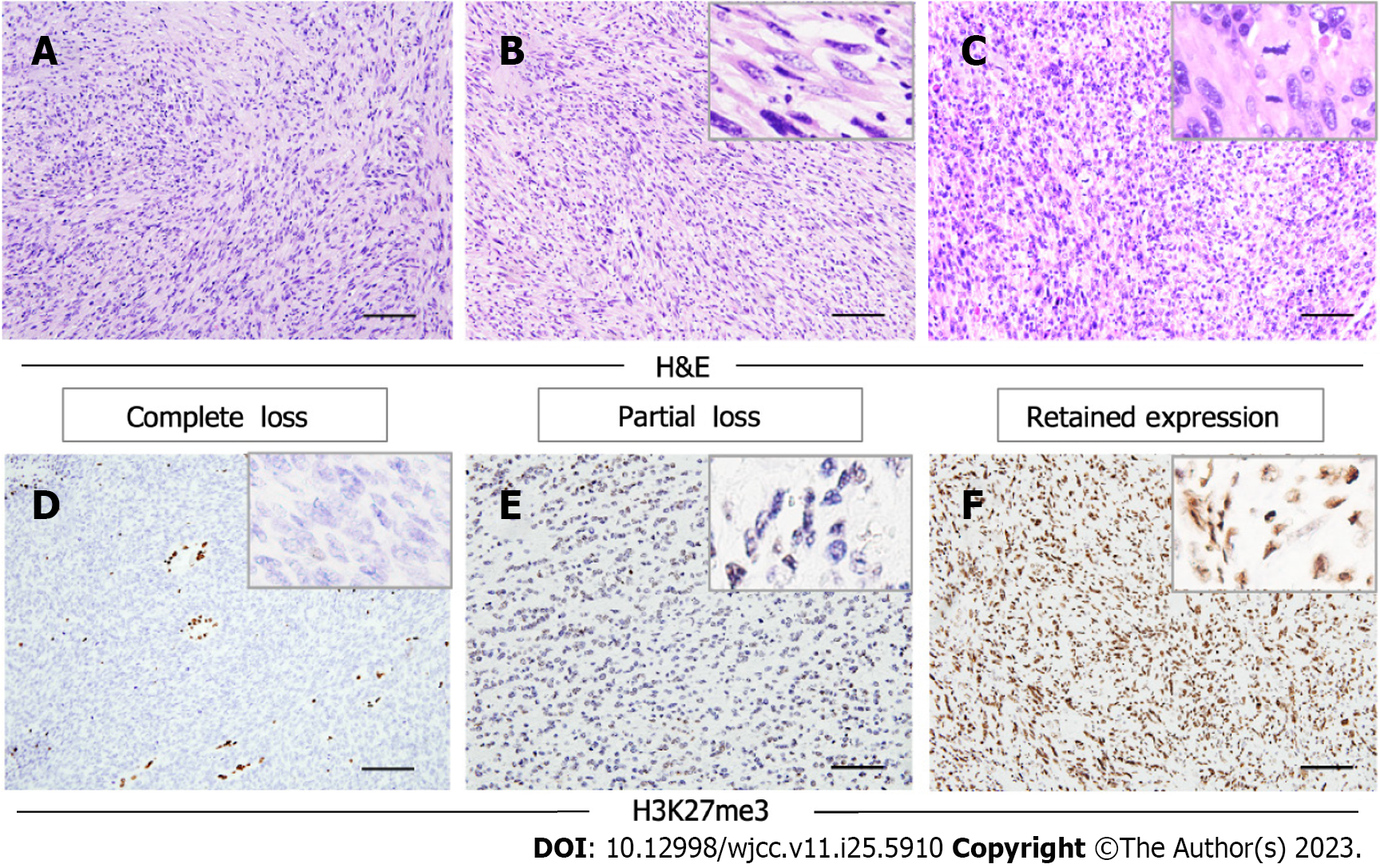
Morphologically, the tumors were composed predominantly of relatively monomorphic spindle cells arranged in intersecting long fascicles. The lesional cells exhibited hyperchromatic ovoid nuclei, with inconspicuous nucleoli and scant cytoplasm. MPNST is generally considered to be a high-grade sarcoma; in line with this, the mitosis score was relatively high in the current study. Large cells exhibiting obvious pleomorphism, or multinucleated giant cells, were found in three cases. Three other cases had areas of epithelial differentiation (Figure 2A-C). All cases evaluated with S100 staining were positive or focal positive, and melanotic markers such as HMB-45 and Melan-A were negative. H3K27me3 staining was evaluated immunohistochemically in the nine surgical cases; one of these (11.1%) exhibited complete H3K27me3 loss, while three (33.3%) showed partial loss (Figure 2D-F).
Nine of the ten patients were initially treated via wide excision; the other patient was prescribed chemotherapy because the tumor was too large to excise. Follow-up information was available for seven patients, who were followed-up for 13–216 mo (mean follow-up = 64 mo). Of these patients, four received both adjuvant radiotherapy and chemotherapy; the remaining patient received radiotherapy only after surgery. Two patients developed local recurrence, and four distant metastasis, within 2 years. Four patients died, while three patients remain under follow-up and are free from disease after 24, 28 and 74 mo, respectively. The 2 and 5-year disease-specific survival (DSS) rates were 86% and 43%, respectively.
Case 1 and Cases 2: Biopsies were used to diagnose MPNST.
Case 1: The patient then underwent partial maxillectomy and radical neck dissection.
Case 2: The patient underwent wide mandibular resection and radical neck dissection, followed by adjuvant radiotherapy and chemotherapy.
Case 1: Two years later, CT performed during regular follow-up revealed multiple pulmonary nodules; further examination confirmed lung and bone metastases.
Cases 2: The patient has remained disease-free for 2 years.
MPNST is an infiltrating and aggressive tumor of neural origin[13]. Although head and neck MPNSTs are histologically similar to MPNSTs in other regions, there are important clinical differences. Patel et al[3] performed a comparative analysis based on the SEER database. The mean age of head and neck MPNST patients was 49.1 years, compared to 46.1 years for patients with MPNSTs in other regions. The former group showed a male predilection (60.2%) while a sex predilection for females (54.2%) in the latter group. Also, the average tumor size of the former group was smaller than in the latter group. The mean age and average tumor size of our patients are consistent with these observations, but we observed no gender differences. This may reflect regional differences among our relatively small sample. MPNSTs develop sporadically, rather than in association with NF-1, in the head and neck region. Ma et al[2] reported that 69.8% (30/43) of MPNSTs were sporadic[14]. None of our cases had a history of NF1 syndrome or malignant transformation after radiotherapy, suggesting that they were all sporadic.
Previous studies found that primary intraosseous MPNST was rare[15]. We report four cases in the mandible and one case in the maxilla in this study; our review of the English language literature revealed 48 cases of primary intraosseous MPNST including 27 such cases in the mandible and 21 such cases in the maxilla. In the mandible, Ma et al[2] reported five patients, of whom three suffered recurrences. The other 22 cases were single cases; the clinical data of these cases, and our four cases, are shown in Supplementary Table 1[15-26]. Among the 26 patients, there were 19 women and 7 men, ranging in age from 4.5 to 76 years [mean age = 33 years (data were unavailable for three patients)]. One patient had a history of NF1 syndrome, and seventeen did not (data were unavailable for five patients). In the maxilla, Ma et al[2] reported 12 patients, of whom seven suffered recurrences. The other 9 cases were single cases; the clinical data of these cases, and our case, are shown in Table 2[19,27-34]. Among the 10 patients, there were 5 women and 5 men, ranging in age from 12 to 65 years (mean age = 44 years). Two patients had a history of NF1 syndrome, and six did not (data were unavailable for two patients). These datas revealed that MPNST in the mandible was more common in women than men, and there were no differences in the maxilla. Most of the patients had no history of NF1 syndrome in both mandible and maxilliary.
| Ref. | Patients (n) | Sex | Age | Treatment | Follow-up (mon) | Recurrence | NF association |
| Kameyama et al[27], 1987 | 1 | F | 61 | / | / | / | No |
| Urade et al[28], 1990 | 1 | F | 47 | S+R+C | 22 | Died of disease | No |
| Che et al[19], 2006 | 1 | F | 13 | R | / | / | No |
| Patil et al[29], 2007 | 1 | M | 45 | / | / | / | No |
| Janardhanan et al[30], 2011 | 1 | M | 40 | S | / | Died of disease | Yes |
| Ali et al[31], 2011 | 1 | M | 50 | R | 8 | No | / |
| Tamgadge et al[32], 2014 | 1 | M | 65 | / | / | / | No |
| Neetha et al[33], 2004 | 1 | F | 12 | R | / | / | / |
| Muraki et al[34], 1999 | 1 | M | 43 | R+C | 20 | Died of disease | Yes |
| Present study | 1 | F | 62 | S+R+C | 28 | Yes | No |
Given the lack of unique histological criteria and a specific immunoprofile, diagnosing MPNST can be very cha
Recent studies have shown that loss of H3K27me3, as revealed by immunohistochemical staining, was described in MPNST.The complete loss rate ranges from 34% to 84%[9,35-42]. Among our patients, 11.1% (n = 1) of the MPNST cases showed complete loss of H3K27me3, while 33.3% (n = 3) showed partial loss. Thus, the H3K27me3 Loss rate was lower than reported previously, perhaps reflecting differences among histological subgroups. Lyskjaer et al[43] found that 76% of MPNSTs with classical histological features had lost H3K27me3, compared to only 23% of MPNSTs with heterologous elements or low-grade components. The complete loss rate of the epithelioid MPNST subtype was 0%.
Patel et al[3] reported that the 5-year head and neck MPNST DSS rate was 65.1%, based on analysis of the SEER database. Similar studies from single institutions reported 5-year DSS rates of 30% and 20%, and a 2-year DSS rate of 21%[14,44]. In our study, the 2- and 5-year DSS rates were 86% and 43%, respectively. Local recurrence and distant metastasis exerted a major influence on DSS. In our study, 43% (3/7) of patients developed local recurrence; two of these patients died and the other one was lost to follow-up. In total, 57% (4/7) of the patients developed distant metastases and 75% died. Both of the patients without local recurrence or distant metastasis are still alive. Other studies revealed that tumor size, stage, and surgical resection significantly influenced DSS[1,2].
Radical surgical resection is the mainstay of MPNST treatment[44,45]. In our study, 9 of 10 patients underwent wide excision; in the remaining case, the tumor was too large to excise. Adjuvant therapies, such as radiotherapy and chemotherapy, are now prescribed for MPNST patients. Some studies have suggested that radiation should be routine to improve survival and decrease the risk of recurrence[44,46]. Chemotherapy has been prescribed for those with large or recurrent tumors, but any role for chemotherapy as an MPNST treatment remains controversial[2]. In our study, both radiation and chemotherapy were prescribed for four patients, and radiation only for one. As our study included a relatively small number of cases, larger series are needed to validate the results.
In summary, MPNST is an uncommon and aggressive soft tissue sarcomas and the head and neck MPNST is extremely rare. Clinical and pathological characteristics of MPNST are not significant. The patients are with poor prognoses and associated with a high risk of recurrence and distant metastasis. Complete surgical resection is the main treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: D'Orazi V, Italy S-Editor: Liu JH L-Editor: A P-Editor: Zhang XD
| 1. | Arshi A, Tajudeen BA, St John M. Malignant peripheral nerve sheath tumors of the head and neck: Demographics, clinicopathologic features, management, and treatment outcomes. Oral Oncol. 2015;51:1088-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Ma C, Ow A, Shan OH, Wu Y, Zhang C, Sun J, Ji T, Pingarron Martin L, Wang L. Malignant peripheral nerve sheath tumours in the head and neck region: retrospective analysis of clinicopathological features and treatment outcomes. Int J Oral Maxillofac Surg. 2014;43:924-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Patel TD, Shaigany K, Fang CH, Park RC, Baredes S, Eloy JA. Comparative Analysis of Head and Neck and Non-Head and Neck Malignant Peripheral Nerve Sheath Tumors. Otolaryngol Head Neck Surg. 2016;154:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | do Amaral TL, Valiati R, de Andrade BA, Rumayor Piña A, Torres SR, Romañach MJ, Agostini M. Malignant peripheral nerve sheath tumor of the lower labial mucosa: case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:e64-e68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Cunha KS, Caruso AC, Faria PA, Silva LE, Pires AR, Geller M, Lopes VS, Moura-Neto RS. Malignant peripheral nerve sheath tumors: clinicopathological aspects, expression of p53 and survival. Clinics (Sao Paulo). 2012;67:963-968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Zhang M, Wang Y, Jones S, Sausen M, McMahon K, Sharma R, Wang Q, Belzberg AJ, Chaichana K, Gallia GL, Gokaslan ZL, Riggins GJ, Wolinksy JP, Wood LD, Montgomery EA, Hruban RH, Kinzler KW, Papadopoulos N, Vogelstein B, Bettegowda C. Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors. Nat Genet. 2014;46:1170-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 230] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 7. | De Raedt T, Beert E, Pasmant E, Luscan A, Brems H, Ortonne N, Helin K, Hornick JL, Mautner V, Kehrer-Sawatzki H, Clapp W, Bradner J, Vidaud M, Upadhyaya M, Legius E, Cichowski K. PRC2 loss amplifies Ras-driven transcription and confers sensitivity to BRD4-based therapies. Nature. 2014;514:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 368] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 8. | Lee W, Teckie S, Wiesner T, Ran L, Prieto Granada CN, Lin M, Zhu S, Cao Z, Liang Y, Sboner A, Tap WD, Fletcher JA, Huberman KH, Qin LX, Viale A, Singer S, Zheng D, Berger MF, Chen Y, Antonescu CR, Chi P. PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat Genet. 2014;46:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 455] [Cited by in RCA: 463] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 9. | Prieto-Granada CN, Wiesner T, Messina JL, Jungbluth AA, Chi P, Antonescu CR. Loss of H3K27me3 Expression Is a Highly Sensitive Marker for Sporadic and Radiation-induced MPNST. Am J Surg Pathol. 2016;40:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 202] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 10. | Farid M, Demicco EG, Garcia R, Ahn L, Merola PR, Cioffi A, Maki RG. Malignant peripheral nerve sheath tumors. Oncologist. 2014;19:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 244] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 11. | Mullins BT, Hackman T. Malignant peripheral nerve sheath tumors of the head and neck: a case series and literature review. Case Rep Otolaryngol. 2014;2014:368920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Wang Y, Wang RR. Malignant Neurilemmoma of the Head and Neck: a clinicopathologic, Immunohistochemical, and Imaging Study of 8 Cases. 2015; 25: 360-364. [DOI] [Full Text] |
| 13. | Probst M, Koerdt S, Ritschl LM, Bissinger O, Liesche F, Gempt J, Meyer B, Burian E, Lummel N, Kolk A. Malignant Peripheral Nerve Sheath Tumor in the Course of the Mandibular Nerve. World Neurosurg. 2018;117:e130-e137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Minovi A, Basten O, Hunter B, Draf W, Bockmühl U. Malignant peripheral nerve sheath tumors of the head and neck: management of 10 cases and literature review. Head Neck. 2007;29:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Lee S, Lee C, Kim JK, Nam W. An unusual presentation of intraosseous malignant peripheral nerve sheath tumour of mandible. Dentomaxillofac Radiol. 2019;48:20180341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Yaga US, Shivakumar R, Kumar MA, Sathyaprakash. Malignant peripheral nerve sheath tumor: A rarity. Indian J Dent. 2015;6:53-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Majumdar S, Kotina S, Mahesh N, Uppala D, Kumar SP. Malignant Peripheral Nerve Sheath Tumor -A Rare Malignancy in Mandible. J Clin Diagn Res. 2016;10:ZD12-ZD13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Zakhary I, Elsalanty M, Ishag I, Taher T, Hassan M, Gehani R, Orafi M, El-Mekkawi H. Malignant peripheral nerve sheath tumor of mandible. J Craniofac Surg. 2011;22:762-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Che Z, Nam W, Park WS, Kim HJ, Cha IH, Kim HS, Yook JI, Kim J, Lee SH. Intraosseous nerve sheath tumors in the jaws. Yonsei Med J. 2006;47:264-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Sham ME, Ghorpade, Shetty A, Hari S, Vinay. Malignant peripheral nerve cell tumour. J Maxillofac Oral Surg. 2010;9:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Prem S, Gangothria S, Reddyb KS. Malignant Peripheral Nerve Sheath Tumor of the Mandible: A Case Report and Review of Literature. Journal of Neurology Research. 2011;. [DOI] [Full Text] |
| 22. | Salla JT, Johann AC, Garcia BG, Aguiar MC, Mesquita RA. Retrospective analysis of oral peripheral nerve sheath tumors in Brazilians. Braz Oral Res. 2009;23:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Patel S, Pathak J, Dekate K, Mohanty N. Malignant peripheral nerve sheath tumour (MPNST) of mandible: solving the perplexity. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Kumar P, Surya V, Urs AB, Augustine J, Mohanty S, Gupta S. Sarcomas of the Oral and Maxillofacial Region: Analysis of 26 Cases with Emphasis on Diagnostic Challenges. Pathol Oncol Res. 2019;25:593-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Naikmasur VG, Guttal KS, Kaveriappa S, Datta KS. Rapidly progressing soft tissue mass of the anterior mandibular region. Malignant peripheral nerve sheath tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:607-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Bullock MJ, Bedard YC, Bell RS, Kandel R. Intraosseous malignant peripheral nerve sheath tumor. Report of a case and review of the literature. Arch Pathol Lab Med. 1995;119:367-370. [PubMed] [DOI] [Full Text] |
| 27. | Kameyama Y, Maeda H, Nakane S, Maeda S, Takai Y, Fukaya M. Malignant schwannoma of the maxilla in a patient without neurofibromatosis. Histopathology. 1987;11:1205-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Urade M, Fujimoto Y, Ogura T, Matsuya T. Malignant schwannoma and melanoma occurring in the maxilla. J Osaka Univ Dent Sch. 1990;30:153-156. [PubMed] |
| 29. | Patil K, Mahima VG, Ambika L. Malignant peripheral nerve sheath tumour: an elusive diagnosis. Indian J Dent Res. 2007;18:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Janardhanan M, Rakesh S, Vinod Kumar R. Intraoral presentation of multiple malignant peripheral nerve sheath tumors associated with neurofibromatosis-1. J Oral Maxillofac Pathol. 2011;15:46-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Ali NS, Junaid M, Aftab K. Malignant peripheral nerve sheath tumour of maxilla. J Coll Physicians Surg Pak. 2011;21:420-422. [PubMed] |
| 32. | Tamgadge S, Modak N, Tamgadge AP, Bhalerao S. Intraosseous malignant peripheral nerve sheath tumor of maxilla: A case report with review of the literature. Dent Res J (Isfahan). 2014;11:405-410. [PubMed] |
| 33. | Neetha MC, Anupama DH, Shashikanth MC. Malignant peripheral nerve sheath tumor of the maxilla. Indian J Dent Res. 2004;15:110-3. [PubMed] |
| 34. | Muraki Y, Tateishi A, Tominaga K, Fukuda J, Haneji T, Iwata Y. Malignant peripheral nerve sheath tumour in the maxilla associated with von Recklinghausen's disease. Oral Dis. 1999;5:250-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Ersen A, Pekmezci M, Folpe AL, Tihan T. Comparision of New Diagnostic Tools for Malignant Peripheral Nerve Sheath Tumors. Pathol Oncol Res. 2017;23:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Otsuka H, Kohashi K, Yoshimoto M, Ishihara S, Toda Y, Yamada Y, Yamamoto H, Nakashima Y, Oda Y. Immunohistochemical evaluation of H3K27 trimethylation in malignant peripheral nerve sheath tumors. Pathol Res Pract. 2018;214:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Cleven AH, Sannaa GA, Briaire-de Bruijn I, Ingram DR, van de Rijn M, Rubin BP, de Vries MW, Watson KL, Torres KE, Wang WL, van Duinen SG, Hogendoorn PC, Lazar AJ, Bovée JV. Loss of H3K27 tri-methylation is a diagnostic marker for malignant peripheral nerve sheath tumors and an indicator for an inferior survival. Mod Pathol. 2016;29:582-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 38. | Schaefer IM, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 206] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 39. | Le Guellec S, Macagno N, Velasco V, Lamant L, Lae M, Filleron T, Malissen N, Cassagnau E, Terrier P, Chevreau C, Ranchere-Vince D, Coindre JM. Loss of H3K27 trimethylation is not suitable for distinguishing malignant peripheral nerve sheath tumor from melanoma: a study of 387 cases including mimicking lesions. Mod Pathol. 2017;30:1677-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Röhrich M, Koelsche C, Schrimpf D, Capper D, Sahm F, Kratz A, Reuss J, Hovestadt V, Jones DT, Bewerunge-Hudler M, Becker A, Weis J, Mawrin C, Mittelbronn M, Perry A, Mautner VF, Mechtersheimer G, Hartmann C, Okuducu AF, Arp M, Seiz-Rosenhagen M, Hänggi D, Heim S, Paulus W, Schittenhelm J, Ahmadi R, Herold-Mende C, Unterberg A, Pfister SM, von Deimling A, Reuss DE. Methylation-based classification of benign and malignant peripheral nerve sheath tumors. Acta Neuropathol. 2016;131:877-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 41. | Pekmezci M, Cuevas-Ocampo AK, Perry A, Horvai AE. Significance of H3K27me3 loss in the diagnosis of malignant peripheral nerve sheath tumors. Mod Pathol. 2017;30:1710-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Makise N, Sekimizu M, Kubo T, Wakai S, Hiraoka N, Komiyama M, Fukayama M, Kawai A, Ichikawa H, Yoshida A. Clarifying the Distinction Between Malignant Peripheral Nerve Sheath Tumor and Dedifferentiated Liposarcoma: A Critical Reappraisal of the Diagnostic Utility of MDM2 and H3K27me3 Status. Am J Surg Pathol. 2018;42:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Lyskjaer I, Lindsay D, Tirabosco R, Steele CD, Lombard P, Strobl AC, Rocha AM, Davies C, Ye H, Bekers E, Ingruber J, Lechner M, Amary F, Pillay N, Flanagan AM. H3K27me3 expression and methylation status in histological variants of malignant peripheral nerve sheath tumours. J Pathol. 2020;252:151-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Kar M, Deo SV, Shukla NK, Malik A, DattaGupta S, Mohanti BK, Thulkar S. Malignant peripheral nerve sheath tumors (MPNST)--clinicopathological study and treatment outcome of twenty-four cases. World J Surg Oncol. 2006;4:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 45. | Zou C, Smith KD, Liu J, Lahat G, Myers S, Wang WL, Zhang W, McCutcheon IE, Slopis JM, Lazar AJ, Pollock RE, Lev D. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249:1014-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 46. | Anghileri M, Miceli R, Fiore M, Mariani L, Ferrari A, Mussi C, Lozza L, Collini P, Olmi P, Casali PG, Pilotti S, Gronchi A. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2006;107:1065-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 291] [Article Influence: 15.3] [Reference Citation Analysis (0)] |