Published online Sep 6, 2023. doi: 10.12998/wjcc.v11.i25.5887
Peer-review started: April 11, 2023
First decision: April 26, 2023
Revised: May 31, 2023
Accepted: July 14, 2023
Article in press: July 14, 2023
Published online: September 6, 2023
Processing time: 143 Days and 7.7 Hours
Iterative decomposition of water and fat with echo asymmetry and least squares estimation quantification sequence (IDEAL-IQ) is based on chemical shift-based water and fat separation technique to get proton density fat fraction. Multiple studies have shown that using IDEAL-IQ to test the stability and repeatability of liver fat is acceptable and has high accuracy.
To explore whether Gadoxetate Disodium (Gd-EOB-DTPA) interferes with the measurement of the hepatic fat content quantified with the IDEAL-IQ and to evaluate the robustness of this technique.
IDEAL-IQ was used to quantify the liver fat content at 3.0T in 65 patients injected with Gd-EOB-DTPA contrast. After injection, IDEAL-IQ was estimated four times, and the fat fraction (FF) and R2* were measured at the following time points: Pre-contrast, between the portal phase (70 s) and the late phase (180 s), the delayed phase (5 min) and the hepatobiliary phase (20 min). One-way repeated-measures analysis was conducted to evaluate the difference in the FFs between the four time points. Bland-Altman plots were adopted to assess the FF changes before and after injection of the contrast agent. P < 0.05 was considered statistically significant.
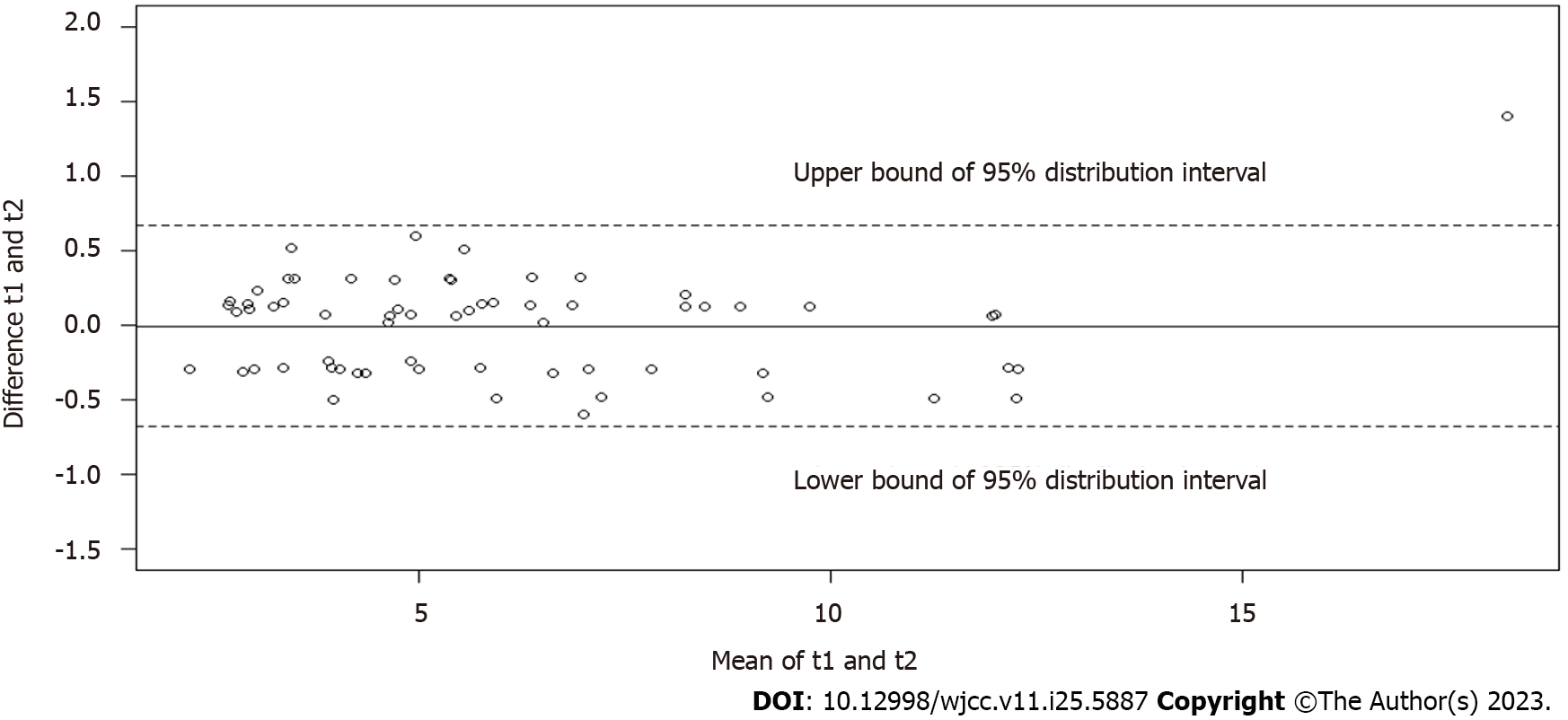
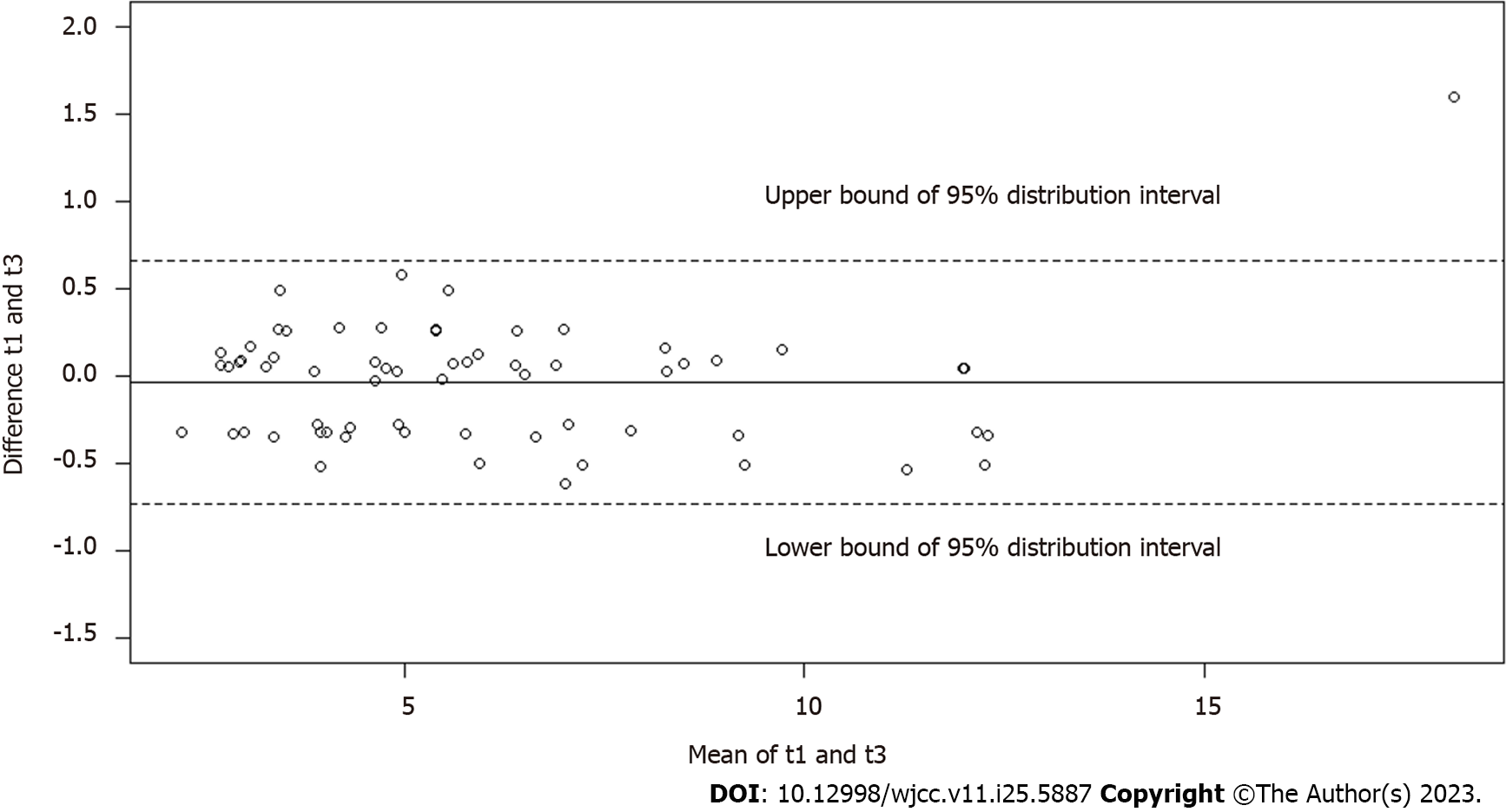
The assessment of the FF at the four time points in the liver, spleen and spine showed no significant differences, and the measurements of hepatic FF yielded good consistency between T1 and T2 [95% confidence interval: -0.6768%, 0.6658%], T1 and T3 (-0.3900%, 0.3178%), and T1 and T4 (-0.3750%, 0.2825%). R2* of the liver, spleen and spine increased significantly after injection (P < 0.0001).
Using the IDEAL-IQ sequence to measure the FF, we can obtain results that will not be affected by Gd-EOB-DTPA. The high reproducibility of the IDEAL-IQ sequence makes it available in the scanning interval to save time during multiphase examinations.
Core Tip: The measurement of fat fraction was stable when using Iterative decomposition of water and fat with echo asymmetry and least squares estimation quantification sequence (IDEAL-IQ) before and after injection of hepatocyte-specific contrast agent. Such strong reproducibility makes IDEAL-IQ available in the interval for saving time during examination and optimizes the scanning protocol.
- Citation: Tian Y, Liu PF, Li JY, Li YN, Sun P. Hepatic MR imaging using IDEAL-IQ sequence: Will Gd-EOB-DTPA interfere with reproductivity of fat fraction quantification? World J Clin Cases 2023; 11(25): 5887-5896
- URL: https://www.wjgnet.com/2307-8960/full/v11/i25/5887.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i25.5887
The defining characteristic of hepatic steatosis (HS) lies in the intracellular accumulation of fat deposits within hepatocytes. Formerly regarded as a comparatively benign entity, it (HS) has the potential to advance towards the development of steatohepatitis, advanced fibrosis, and ultimately cirrhosis[1,2]. Fortunately, if intervention is performed in a timely manner, isolated steatosis is reversible, and a reduction in liver fat may diminish many of its associated risks. The fat fraction, which represents the quantitative ratio of fat mass to liver mass, serves as a pivotal parameter in the diagnosis and ongoing assessment of nonalcoholic fatty liver disease (NAFLD), the prevalence of which is approximately 25%[3]. Rapid radiological detection and diagnosis are essential in the management of patients with NAFLD[2,4,5].
Quantitative diagnosis and efficacy evaluation of HS have been studied clinically. Liver biopsy is not frequently used for HS diagnosis because of its invasive nature, and sampling errors can be caused by heterogeneous fat distribution[6]. Ultrasound detection is economical and convenient but is highly dependent on the procedure and lacks quantitative and objective criteria[7]. Computed tomography (CT) values cannot be used as a noninvasive technique in the evaluation of fat content, but the deposits of iron, copper, glycogen and amiodarone can increase the liver density and severely interfere with the accuracy of CT in the quantification of liver steatosis[8,9].
Currently, magnetic resonance spectroscopy (MRS) is commonly used as a standard fat quantification method[10-12]. However, the accuracy of this technique depends on the homogeneity of the magnetic field, and single spectral data cannot reflect the whole liver condition[11,12]. Recently, multipoint water-fat separation methods based on chemical shifts have been applied to quantitative analyses of liver fat[12-16]. Among these methods, the iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL-IQ) not only generates an accurate evaluation of the FF (0%-100%) but also allows the quantitative analysis of the fat composition. This method utilizes a multiecho acquisition technique to facilitate T2* fitting, enabling the subsequent application of T2* correction. By eliminating the inherent T2* effect, this correction accounts for various factors that can impact the accuracy of FF measurements in chemical shift-based approaches. These factors include field inhomogeneity, T1 relaxation, noise bias, and eddy currents[16]. It facilitates the quantitative measurement of the whole liver and promising clinical application for testing the efficacy of drugs for hepatic disorders. The new quantitative scanning regimen not only facilitates accurate prognosis by monitoring dynamic changes in patients but also provides an objective basis for evaluating the treatment effect.
The primary objective of this study was to assess the potential interference of the liver-specific contrast medium Gd-EOB-DTPA on the measurement of hepatic fat content using the IDEAL-IQ sequence. Additionally, the study aimed to evaluate the reliability and resilience of this technique. By exploring these aspects, the researchers sought to identify and address the challenges associated with the widespread application of Gd-EOB-DTPA in clinical settings.
In this institutional review board-approved study, patients with liver disease who visited the First Affiliated Hospital of Harbin Medical University between October 2015 and June 2016 were enrolled. All patients were diagnosed with hepatopathy using early imaging and subsequently screened with Gd-EOB-DTPA magnetic resonance imaging. The IDEAL-IQ sequence was used before and after injection to detect liver fat levels. In total, 79 patients were enrolled in this study. We excluded 14 cases for the following criteria: (1) Poor image quality caused by factors such as incompatible breath-holding; (2) Focal liver lesions exceeding 50% of the liver volume; and (3) Patients who underwent an abdominal operation. Finally, 65 patients were enrolled (48 males and 17 females with an average age of 54 years old and a range of 36 to 67 years old). Baseline patient characteristics and treatment history are shown in Table 1, and all the patients signed informed consent forms before the study.
| Demographics | Parameter | No. of patients (%) |
| Age | < 60 yr | 51 (65) |
| > 60 yr | 28 (35) | |
| Sex | Male | 48 (60) |
| Female | 17 (40) | |
| Cause | HBV | 20 (30) |
| HCV | 9 (14) | |
| HBV and HCV | 2 (3) | |
| Alcohol | 25 (38) | |
| Cryptogenic | 9 (14) | |
| Cirrhosis | Present | 35 (54) |
| Absent | 30 (46) | |
| Methods of diagnosis | Biopsy | 15 (23) |
| MR imaging | 50 (77) | |
| Tumor characteristics | Unifocal | 43 (66) |
| Multifocal | 22 (34) | |
| Diameter of lesion | < 3 cm | 42 (65) |
| > 3 cm | 23 (35) | |
| No treatment | 45 (69) | |
| Treated | Surgical treatment | 9 (14) |
| Internal medicine | 11 (17) |
A GE Signa HDxt magnetic resonance imaging (MRI) scanner (Milwaukee, United States) was used with 8-channel TORSOPA phased array coils. The patients fasted for 6 h before the test. Fat detection with the IDEAL-IQ sequence was performed four times before and after Gd-EOB-DTPA enhancement. The acquisition parameters were consistent at the four time points, indicating changes in the FF value and R2*. The scanning parameters were as follows: Breath-hold scanning, IDEAL-IQ (a three-dimensional volumetric imaging sequence): repetition time (TR)/first echo time (TE): 3.7 ms/1.7 ms, thickness 5 mm, receiver bandwidth 125 kHz, view: 44 cm× 40 cm, matrix: 256 × 256, flip angle 3°, NEX1. Six echoes in total were obtained for the T2* correction in two consecutive TRs with a 3-echo acquisition maintained for each. Two-dimensional parallel acceleration was used to maintain the scan time, approximately 21 s, within a single breath hold. The breath-hold double arterial phase LAVA enhanced scanning (BH Ax Dual LAVA+C) parameters were as follows: Turn over time 5.0 ms; flip angle 11°; bandwidth 125 kHz; matrix 256×152; view 44 × 40 cm; and the front and back range was 25% larger than the body surface with a thickness of 4-6 mm. The array spatial sensitivity encoding technique (ASSET) was used with a phase accelerating factor of 2.25 Ph. The injection speed of the contrast agent was 2.0-3.0 mL/s. The dosage was 0.025 mmol/kg, which is approved by the FDA, followed by injection of 20 mL saline at the same speed. After the contrast agent was injected, Dynamic-enhanced scanning using LAVA-Flex was employed to capture images during different phases, namely the arterial phase, portal phase, and equilibrium phase. Additionally, IDEAL-IQ was performed four times throughout the study, including a pre-contrast scan, scans between the portal phase (70 s) and late phase (180 s), a delayed phase scan (5 min), and a hepatobiliary phase scan (20 min). These scans were individually labeled as t1, t2, t3, and t4 in the subsequent statistical analyses and tables.
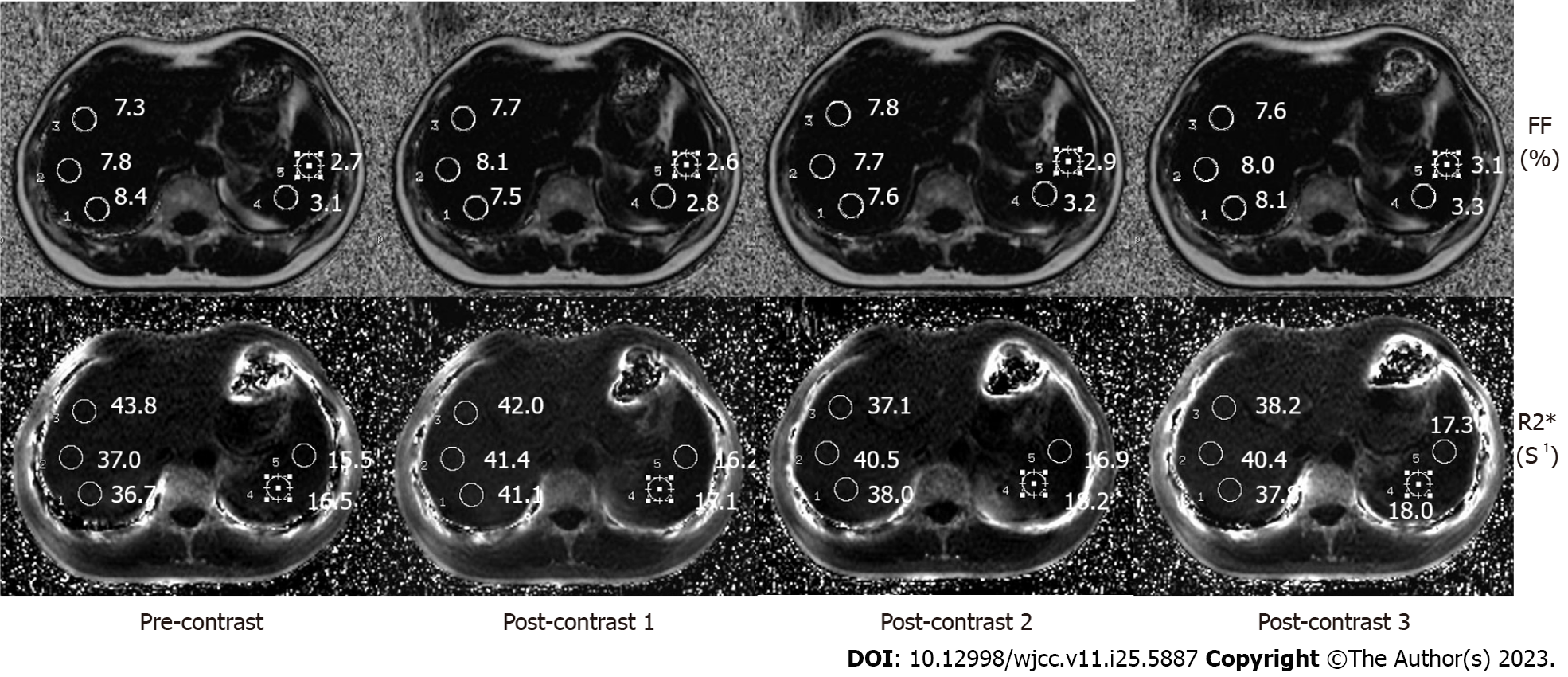
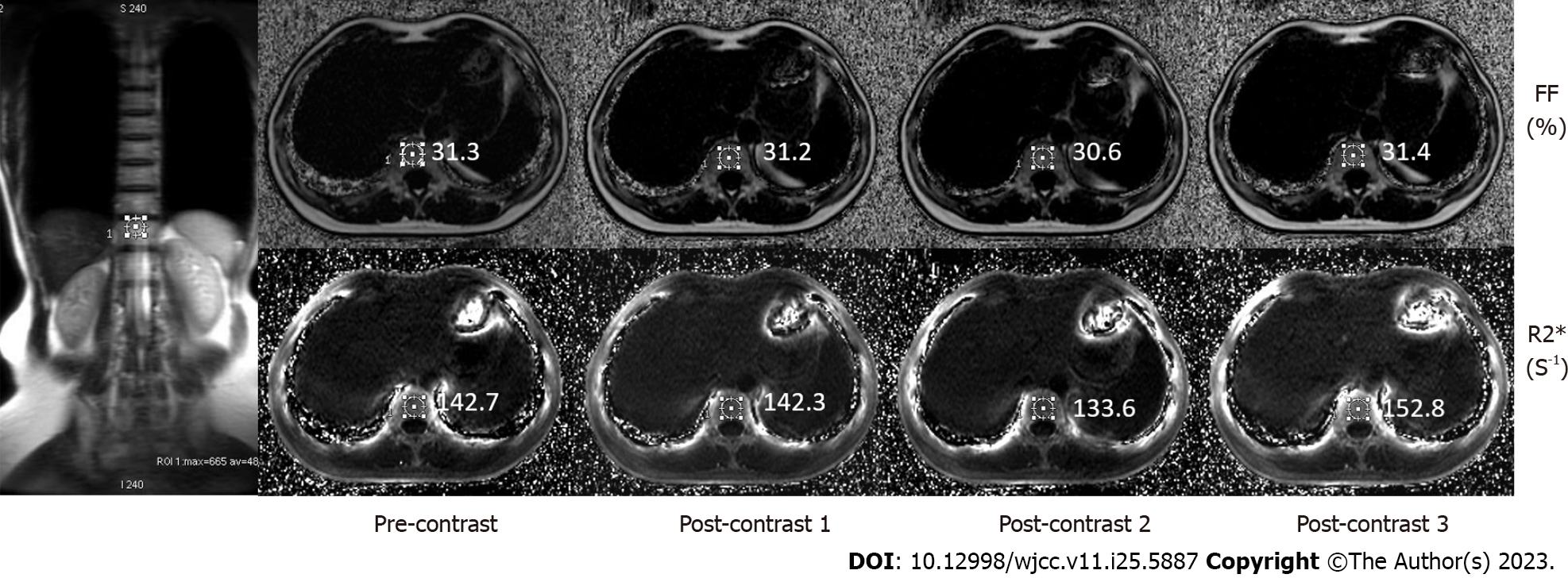
Two radiologists (with 5-7 years of experience in MR imaging of the abdomen) reviewed the image data of the patients in terms of the known pathological and comprehensive diagnosis. FF and R2* maps were autogenerated on the host scanner after each acquisition of the IDEAL-IQ sequence. All the images were independently evaluated by function tool 9.4 on an AW4.5 workstation (Advantage Workstation 4.5; GE Healthcare). Three regions of interest (ROIs) with a diameter of 16 mm in the right liver lobe were used to calculate the mean values (Figure 1). To ensure accurate measurements, regions of interest were carefully selected by excluding intrahepatic bile ducts, blood vessels, and focal liver lesions. The fat fraction detected in the images reflected the amount of fat present in the liver mass, which typically does not exceed 5% in healthy individuals. Similarly, employing the same approach, two ROIs with a diameter of 16 mm were delineated within the spleen for further analysis (Figure 1), one ROI was drawn in the spinal vertebral layer (Figure 2), and the FF and R2* values were recorded. The obtained anatomical images of the spine were considered the region of interest in the vertebral body bone marrow to avoid inclusion of the intervertebral disk. Finally, the FF and R2* values in the liver, spleen and spine determined by the two physicians were averaged to calculate the final result.
The FF and R2* values at the four time points were evaluated, and the data were expressed as the means ± SDss and analyzed by ANOVA for repeated measurements. Furthermore, in each region, the values before and after injecting the contrast agent at each time point were compared, and Bland-Altman plots were applied to analyze the differences. P < 0.05 was considered statistically significant. SAS 9.13 software (SAS Institute Inc, China) was used to perform the statistical analysis.
For the 65 patients, the final FF and R2* values (mean ± SD, n = 65) of the liver, spleen and spine are shown in Table 2. The FF values of the liver, spleen and spine were compared before (time point of T1) and after injection of the contrast agent (T2, T3, and T4). The results showed no significant differences between them (T1-T2, P = 0.85; T1-T3, P = 0.24; T1-T4, P = 0.13), and the FF values were acceptable (Table 3). The R2* value increased from T1 to T2 from 43.14 to 46.21 s-1, respectively (P < 0.0001) in the liver and from 42.97 to 46.08 s-1 (P < 0.0001) in the spleen. Thereafter, the values reached 46.60 s-1 and 47.10 s-1 at t3 and t4 in the liver (P < 0.0001) and 46.38 s-1 and 46.76 s-1 in the spleen (P < 0.0001). R2* values in the spine were 121.75 s-1 at t1 and 124.90 s-1, 125.75 s-1 and 126.45 -1 at t2, t3, and t4, respectively (P < 0.0001). In summary, the R2* of the liver, spleen and spine increased significantly after injection (P < 0.0001), showing a T2* effect of Gd-EOB-DTPA (Table 4).
| Index | T1 | T2 | T3 | T4 |
| Liver FF | 6.0567 ± 3.1295 | 6.0622 ± 3.1026 | 6.0928 ± 3.0863 | 6.103 ± 3.1272 |
| Spleen FF | 2.0183 ± 0.7687 | 2.0363 ± 0.7531 | 2.0478 ± 0.7633 | 2.0652 ± 0.7494 |
| Spine FF | 25.6275 ± 6.6189 | 25.6422 ± 6.5966 | 25.6495 ± 6.5979 | 25.6648 ± 6.5941 |
| Liver R2* | 43.1359 ± 9.95 | 48.211 ± 10.0155 | 49.5981 ± 10.0038 | 52.0894 ± 10.0021 |
| Spleen R2* | 42.9711 ± 13.9805 | 46.0781 ± 13.7729 | 46.3787 ± 13.7615 | 46.7647 ± 13.796 |
| Spine R2* | 121.7495 ± 20.602 | 124.902 ± 20.3045 | 125.7506 ± 20.263 | 126.4444 ± 20.3439 |
| Variation | DF | SS | MS | F | P value |
| T1-T2 (liver) | 1 | 0.001 | 0.001 | 0.0319 | 0.8584 |
| T1-T3 (liver) | 1 | 0.0417 | 0.0417 | 1.3901 | 0.2399 |
| T1-T4 (liver) | 1 | 0.0685 | 0.0685 | 2.2825 | 0.1325 |
| T1-T2 (spleen) | 1 | 0.0103 | 0.0103 | 0.4911 | 0.4843 |
| T1-T3 (spleen) | 1 | 0.0328 | 0.0328 | 1.5601 | 0.2132 |
| T1-T4 (spleen) | 1 | 0.0703 | 0.0703 | 3.3418 | 0.0691 |
| T1-T2 (spleen) | 1 | 0.0069 | 0.0069 | 0.1703 | 0.6803 |
| T1-T3 (spleen) | 1 | 0.0157 | 0.0157 | 0.3859 | 0.5352 |
| T1-T4 (spleen) | 1 | 0.045 | 0.045 | 1.1053 | 0.2944 |
| Variation | DF | SS | MS | F | P value |
| T1-T2 (liver) | 1 | 297.9598 | 297.9598 | 476.8081 | < 0.0001 |
| T1-T3 (liver) | 1 | 377.59 | 377.59 | 604.2357 | < 0.0001 |
| T1-T4 (liver) | 1 | 492.3481 | 492.3481 | 787.8766 | < 0.0001 |
| T1-T2 (spleen) | 1 | 299.2445 | 299.2445 | 1035.896 | < 0.0001 |
| T1-T3 (spleen) | 1 | 359.9598 | 359.9598 | 1246.074 | < 0.0001 |
| T1-T4 (spleen) | 1 | 446.1213 | 446.1213 | 1544.34 | < 0.0001 |
| T1-T2 (spleen) | 1 | 313.126 | 313.126 | 317.1906 | < 0.0001 |
| T1-T3 (spleen) | 1 | 504.28 | 504.28 | 510.826 | < 0.0001 |
| T1-T4 (spleen) | 1 | 694.3318 | 694.3318 | 703.3447 | < 0.0001 |
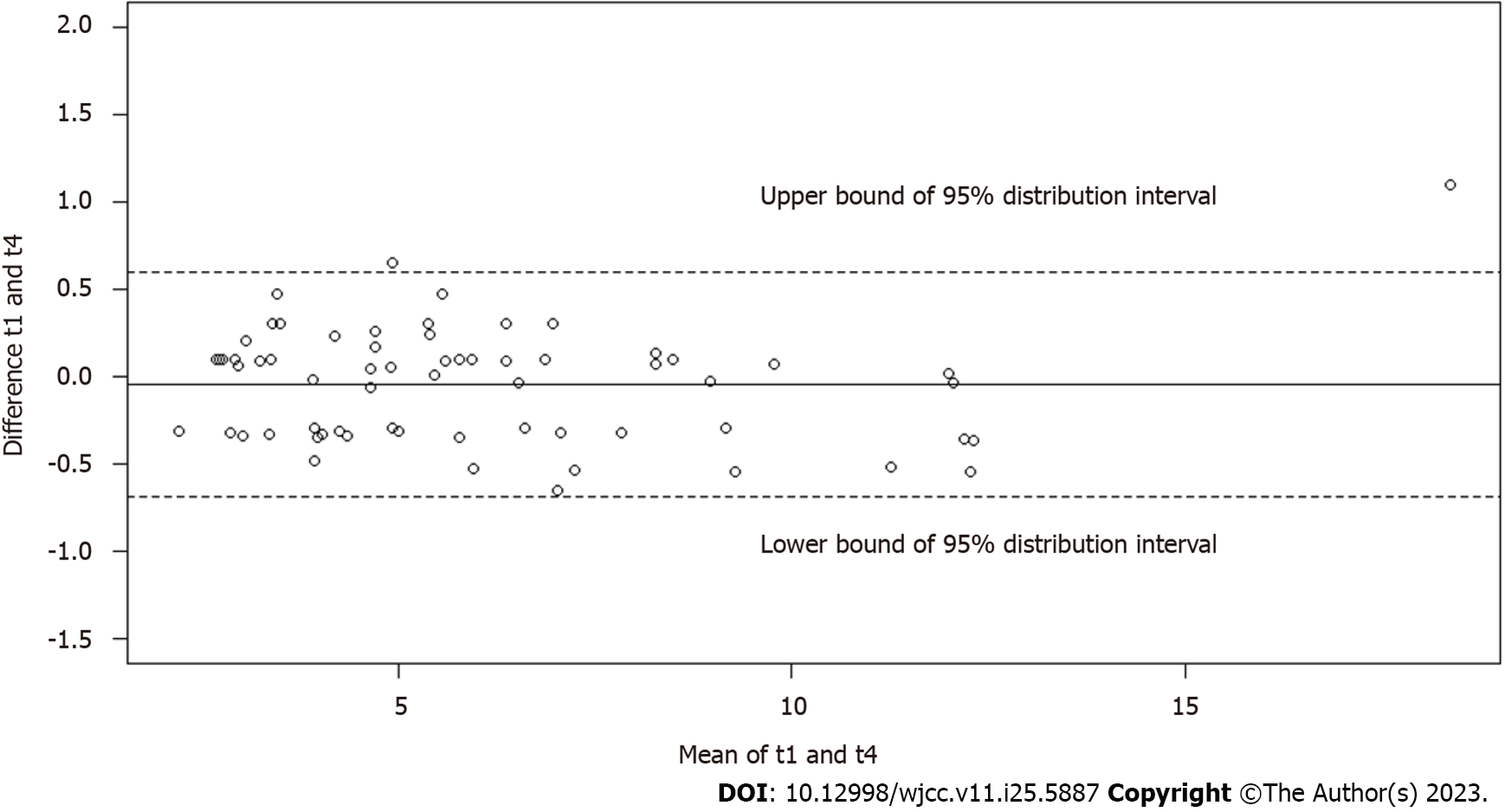
The Bland-Altman plots (Figures 3-5) demonstrated good agreement of hepatic FF measurements between T1 and T2 (95% confidence interval: -0.6768%, 0.6658%), T1 and T3 (-0.3900%, 0.3178%), and T1 and T4 (-0.3750%, 0.2825%). These findings suggested considerable stability and repeatability of the IDEAL-IQ FF measurements under the impact of Gd-EOB-DTPA.
Unlike the R2* values, the FF values of the liver, spleen and spine obtained by the IDEAL-IQ sequence showed no significant differences at the four time points before and after the injection of Gd-EOB-DTPA. Our findings demonstrated that this technique for fat quantification can overcome the strong susceptibility effect induced by contrast media, including hepatocyte-specific contrast agents.
IDEAL-IQ is based on a chemical shift-based water and fat separation technique to obtain the proton density fat fraction (PDFF)[14,17,18]. The accuracy of water-fat separation is affected by many factors, including T2* attenuation and the multiple fat peak model. According to the chemical characteristics of triglycerides, the nine-peak fat model was formed. To remove the influence of T2*, a multiecho technique is used to predict the attenuation rate of R2*, and it is used in the calculation of the water and fat maps[16,19]. As a noninvasive liver fat analysis method, several result maps can be reconstructed in one scan, including the FF, R2*, water and fat phases. Moreover, to decrease the signal deviation between water and fat due to the T1 effect, a small flip angle pulse was used to ensure proton density (PD)[19,20]. The stability and repeatability of testing for liver fat using IDEAL-IQ was acceptable and achieved a high accuracy[12,20].
Approximately 50% of the hepatic cells absorbed Gd-EOB-DTPA, which is derived from a lipophilic EOB compound containing gadolinium. This absorption occurred through the organic anion transporting polypeptide (OATP) mechanism[21]. Although the contrast agent partially entered the hepatic cells via the intercellular space, it did not affect the water or fat composition but altered the magnetic field environment and T2* effect. However, such attenuation was eliminated by IDEAL-IQ. IDEAL-IQ imaging estimated a complex field map via six echo signals using the iterative least-squares method. A complex field map was used to differentiate water and fat and to obtain the dynamic 0 to 100% fat fraction. Amplitude reconstruction was used to quantify the fat fraction, and the phase error was removed. Finally, combined with the results of the two reconstructions, T2* was estimated[20,22]. Thus, elimination of the T2* effect by IDEAL-IQ was relatively stable. A few studies also evaluated the confounding effects of fat detection after injecting a contrast agent, especially SPIO, and found that IDEAL-IQ has stable performance[23]. As a liver-specific contrast agent, SPIO is absorbed by Kupffer cells into hepatic cells. The mechanism was similar to that of Gd-EOB-DTPA, and the difference was mainly in the amounts deposited in hepatic cells. Under T1 deviation, noise difference, T2* decay, a complicated fat resonance spectrum and vortex, the accuracy of the chemical shift in fat quantification was influenced differently[12,20]. However, IDEAL-IQ compensated for and neutralized the effects of interfering factors. Another study illustrated that the FF had no significant difference after injection with Gd-DTPA instead of with Gd-EOB-DTPA in our case[24]. Thus, the presence of contrast agent inside or outside cells does not alter the FF value theoretically.
As a reciprocal of T2*, R2* is related to the water and fat components[17,22]. Due to the application of small 3D flip angle acquisition with gradient echo flyback, the R2* value and FF value can be calculated simultaneously. Therefore, R2* is used to describe the decay of T2* caused by Gd-EOB-DTPA. In our study, the injection of a contrast agent slightly increased R2* due to transverse relaxation of Gd-EOB-DTPA. In the case of contrast agents with similar paramagnetic ions, the R2* influencing factors mainly included micro viscosity, hydrogen proton nuclear dispersion caused by susceptible echo, and the combining capacity with cells[23]. The combining capacity of Gd was lower than that of Gd-EOB-DTPA. However, the influence was limited, which requires further exploration. Being a widely employed liver-specific contrast agent, Gd-EOB-DTPA was similar to Gd in influencing T2* and the T1 relaxation time. At similar dosages, Gd-EOB-DTPA showed a higher relaxation time than Gd, and the paramagnetic effect greatly decreased the T1 relaxation time[25-27]. Nevertheless, IDEAL-IQ effectively addressed the confounding factors that could potentially disrupt accurate fat quantification. It rectified issues such as T2* decay, the distribution of multifat spectrum peaks, and generated separate water and fat images. Additionally, IDEAL-IQ provided valuable information through the fat fraction and R2* maps, further enhancing the reliability of fat quantification[27,28].
However, our study still has a few limitations. First, despite the accuracy and reliability of IDEAL-IQ, Gd-EOB-DTPA intake by hepatic cells was up to 50%, and the role of hepatic function still requires clinical investigation. Second, unstable breath holding resulted in a slight shift in the local scan layer and further deviation in the inconsistent ROI before and after contrast agent administration. However, we used the image calibration software of the AW4.5 workstation and drew ROIs in the same layer as much as possible. Third, in the four periods of IDEAL-IQ after contrast agent injection, the initial time was influenced by several factors. A deviation in the FF value and other parameters was caused by a failure to achieve the target time. Control of the scan time is important for further study.
The measurement of fat fraction was stable when using IDEAL-IQ to detect the FF values in patients scanned by EOB-MRI before and after injecting a hepatocyte-specific contrast agent. The R2* values in the liver, spleen and spine increased after injection of Gd-EOB-DTPA but had no significant effect on fat quantification. Such strong reproducibility makes IDEAL-IQ available in the interval for saving time during examination and optimizing the scanning protocol.
Iterative decomposition of water and fat with echo asymmetry and least squares estimation quantification sequence (IDEAL-IQ) is based on chemical shift-based water and fat separation technique to get proton density fat fraction. Multiple studies have shown that using IDEAL-IQ to test the stability and repeatability of liver fat is acceptable and has high accuracy.
At present, there are few studies on comparing the fat content of different Gadoxetate Disodium (Gd-EOB-DTPA) enhanced organs at different periods. This research can further solve the problem of whether Gd-EOB-DTPA affects fat fraction measurement, and test repeatability of ideal-IQ at Different Periods, which can optimize clinical examination items.
The challenges associated with the widespread applications of liver-specific contrast medium Gd-EOB-DTPA, the purpose of this study is to evaluate whether the Gd-EOB-DTPA would interfere with the measurement of hepatic fat content that was quantified with IDEAL IQ sequence, and the robustness of this technique was also evaluated.
In this study, IDEAL-IQ was employed to quantify liver fat content in a cohort of 65 patients who received injections of Gd-EOB-DTPA contrast material during imaging at 3.0T. Following the injection, IDEAL-IQ was performed four times, and measurements of the fat fraction (FF) and R2* were obtained at specific time points: pre-contrast, between the portal phase (70 s) and late phase (180 s), delayed phase (5 min), and hepatobiliary phase (20 min). These measurements allowed for the assessment of liver fat content at different stages of contrast material uptake and clearance.
The evaluation of the FF at four different time points in the liver, spleen, and spine revealed no significant differences. Furthermore, the measurements of hepatic FF demonstrated good consistency between T1 and T2, T1 and T3, as well as T1 and T4. On the other hand, the R2* values of the liver, spleen, and spine exhibited a significant increase following the injection of the contrast material.
Using IDEAL-IQ sequence in the measurement of FF, we can get results that won’t be affected by Gd-EOB-DTPA. The high reproducibility of IDEAL IQ makes it available in the interval of scanning for saving time during multi-phase examination.
Multi-center prospective studies with larger sample sizes are required to investigate whether the dosage of GD-EOB-DTPA affects IDEAL-IQ FF.
We would like to thank all the study participants and the staff of our hospital. Thanks the National Natural Science Foundation of China.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bangalore S, United States; Pope JE, Canada S-Editor: Ma YJ L-Editor: A P-Editor: Wu RR
| 1. | Clark JM, Cryer DRH, Morton M, Shubrook JH. Nonalcoholic fatty liver disease from a primary care perspective. Diabetes Obes Metab. 2023;25:1421-1433. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 979] [Article Influence: 195.8] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7492] [Article Influence: 832.4] [Reference Citation Analysis (0)] |
| 4. | Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, Loomba R. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease-MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 281] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 5. | Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1565] [Cited by in RCA: 1446] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 6. | Khalifa A, Rockey DC. The utility of liver biopsy in 2020. Curr Opin Gastroenterol. 2020;36:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 7. | Graif M, Yanuka M, Baraz M, Blank A, Moshkovitz M, Kessler A, Gilat T, Weiss J, Walach E, Amazeen P, Irving CS. Quantitative estimation of attenuation in ultrasound video images: correlation with histology in diffuse liver disease. Invest Radiol. 2000;35:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Starekova J, Hernando D, Pickhardt PJ, Reeder SB. Quantification of Liver Fat Content with CT and MRI: State of the Art. Radiology. 2021;301:250-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 9. | Guo Z, Blake GM, Li K, Liang W, Zhang W, Zhang Y, Xu L, Wang L, Brown JK, Cheng X, Pickhardt PJ. Liver Fat Content Measurement with Quantitative CT Validated against MRI Proton Density Fat Fraction: A Prospective Study of 400 Healthy Volunteers. Radiology. 2020;294:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | Wang YS, Ye J, Cao YH, Zhang R, Han XF, Zou LL, Kuang L, Zhang J, Lian H, Xia JX, Zhang Q, Dai W. Association of [(1)H]-MRS quantified liver fat content with glucose metabolism status. Diabetol Metab Syndr. 2020;12:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Idilman IS, Keskin O, Celik A, Savas B, Elhan AH, Idilman R, Karcaaltincaba M. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta Radiol. 2016;57:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 12. | Heba ER, Desai A, Zand KA, Hamilton G, Wolfson T, Schlein AN, Gamst A, Loomba R, Sirlin CB, Middleton MS. Accuracy and the effect of possible subject-based confounders of magnitude-based MRI for estimating hepatic proton density fat fraction in adults, using MR spectroscopy as reference. J Magn Reson Imaging. 2016;43:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Ren W, Feng Y, Li J, Zhang C, Feng L, Cui L, Ran J. Relationship of liver fat content with systemic metabolism and chronic complications in patients with type 2 diabetes mellitus. Lipids Health Dis. 2023;22:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 14. | Eskreis-Winkler S, Corrias G, Monti S, Zheng J, Capanu M, Krebs S, Fung M, Reeder S, Mannelli L. IDEAL-IQ in an oncologic population: meeting the challenge of concomitant liver fat and liver iron. Cancer Imaging. 2018;18:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Hui SCN, So HK, Chan DFY, Wong SKH, Yeung DKW, Ng EKW, Chu WCW. Validation of water-fat MRI and proton MRS in assessment of hepatic fat and the heterogeneous distribution of hepatic fat and iron in subjects with non-alcoholic fatty liver disease. Eur J Radiol. 2018;107:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Wu B, Han W, Li Z, Zhao Y, Ge M, Guo X, Wu X. Reproducibility of Intra- and Inter-scanner Measurements of Liver Fat Using Complex Confounder-corrected Chemical Shift Encoded MRI at 3.0 Tesla. Sci Rep. 2016;6:19339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Reeder SB, Pineda AR, Wen Z, Shimakawa A, Yu H, Brittain JH, Gold GE, Beaulieu CH, Pelc NJ. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med. 2005;54:636-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 556] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 18. | Corrias G, Krebs S, Eskreis-Winkler S, Ryan D, Zheng J, Capanu M, Saba L, Monti S, Fung M, Reeder S, Mannelli L. MRI liver fat quantification in an oncologic population: the added value of complex chemical shift-encoded MRI. Clin Imaging. 2018;52:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Hernando D, Sharma SD, Aliyari Ghasabeh M, Alvis BD, Arora SS, Hamilton G, Pan L, Shaffer JM, Sofue K, Szeverenyi NM, Welch EB, Yuan Q, Bashir MR, Kamel IR, Rice MJ, Sirlin CB, Yokoo T, Reeder SB. Multisite, multivendor validation of the accuracy and reproducibility of proton-density fat-fraction quantification at 1.5T and 3T using a fat-water phantom. Magn Reson Med. 2017;77:1516-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58:354-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 400] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 21. | Caraiani CN, Dan M, Fenesan DI, Badea R. Description of focal liver lesions with Gd-EOB-DTPA enhanced MRI. Clujul Med. 2015;88:438-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Yu H, McKenzie CA, Shimakawa A, Vu AT, Brau AC, Beatty PJ, Pineda AR, Brittain JH, Reeder SB. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging. 2007;26:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 360] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 23. | Liau J, Shiehmorteza M, Girard OM, Sirlin CB, Bydder M. Evaluation of MRI fat fraction in the liver and spine pre and post SPIO infusion. Magn Reson Imaging. 2013;31:1012-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Ge M, Zhang J, Wu B, Liu Z, Song H, Meng X, Wu X. Effect of gadolinium on hepatic fat quantification using multi-echo reconstruction technique with T2* correction and estimation. Eur Radiol. 2016;26:1913-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Shuter B, Tofts PS, Wang SC, Pope JM. The relaxivity of Gd-EOB-DTPA and Gd-DTPA in liver and kidney of the Wistar rat. Magn Reson Imaging. 1996;14:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Bydder M, Yokoo T, Hamilton G, Middleton MS, Chavez AD, Schwimmer JB, Lavine JE, Sirlin CB. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008;26:347-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 340] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 27. | Sofue K, Zhong X, Nickel MD, Dale BM, Bashir MR. Stability of liver proton density fat fraction and changes in R 2* measurements induced by administering gadoxetic acid at 3T MRI. Abdom Radiol (NY). 2016;41:1555-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, Brittain JH, Reeder SB. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 317] [Article Influence: 22.6] [Reference Citation Analysis (0)] |