Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5789
Peer-review started: May 24, 2023
First decision: July 3, 2023
Revised: July 7, 2023
Accepted: August 2, 2023
Article in press: August 2, 2023
Published online: August 26, 2023
Processing time: 92 Days and 22.6 Hours
Atrial arrhythmias such as paroxysmal supraventricular tachycardia (PSVT) and atrial flutter (AF) are common in the perioperative setting. They commonly resolve spontaneously. However, occasionally, they may continually progress to fatal arrhythmias or cause complications. Therefore, prompt and appropriate management is important.
A 46-year-old female patient diagnosed with cervical C6-7 radiculopathy characterized by decreased sensation in the right third, fourth and fifth fingers underwent C6-7 anterior cervical disc fusion surgery. Electrocardiography showed PSVT and ventricular tachycardia during C6-7 disc retraction. However, the patient remained stable. Initial treatment with esmolol and lidocaine for ventricular tachycardia was ineffective. Carotid massage and Valsalva maneuver were attempted but PSVT did not resolve. The surgery was paused, and the patient’s fraction of inspired oxygen was set to 100%. Adenosine was admini
Ganglia associated with cardiac arrhythmias in the surgical site should be iden
Core Tip: Paroxysmal supraventricular tachycardia and atrial flutter can occur without structural heart disease and are present at any age. In the current case, the arrhythmia was caused by the surgical stimulation of the stellate ganglion in a patient without a significant medical history. Electrocardiography results were similar, making it difficult to identify the type of arrhythmia. Hence, another arrhythmia was observed even after appropriate treatment. In such a case, if an arrhythmia occurs in the context of stimulation of the right stellate ganglion during cervical spine surgery, identification of triggers to consider, correction of the appropriate triggers, and prevention of migration to fatal arrhythmias should be considered.
- Citation: Seo JH, Cho SY, Park JH, Seo JY, Lee HY, Kim DJ. Intraoperative sudden arrhythmias in cervical spine surgery adjacent to the stellate ganglion: A case report. World J Clin Cases 2023; 11(24): 5789-5796
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5789.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5789
The incidence of intraoperative arrhythmias is high, and some arrhythmias require clinical attention[1]. Paroxysmal supraventricular tachycardia (PSVT) is a common arrhythmia that can occur at any age in patients without structural heart disease. Atrial flutter (AF) is a type of heart rhythm disorder caused by issues in the electrical system of the heart. That is, the upper chambers of the heart (atria) beat extremely rapidly but with a regular rhythm. AF is similar to atrial fibrillation, a common heart disorder[2].
The risk factors of intraoperative arrhythmia include various patient factors, such as age, sex, comorbidities and medications, and surgical factors, such as the type and duration of surgery and the use of anesthetics. In AF and PSVT, measures to lower blood pressure (BP) and the use of medications or other procedures may be required to restore normal heart rhythm[1]. In cervical spine surgery, particularly cervical C6-7 level disc surgery, the proximity of the surgical site to the phrenic nerve and stellate ganglion and the potential for nerve irritation may increase the risk of arrhythmia.
This case shows the importance of assessing potentially arrhythmogenic anatomy at the level of the C6-7 spine while recognizing arrhythmia risk factors in patients undergoing cervical spine surgery, and the need to appropriately differentiate and manage potentially life-threatening arrhythmias such as atrial and ventricular arrhythmias.
A 46-year-old female patient was diagnosed with C6-7 radiculopathy characterized by decreased sensation in the right third, fourth and fifth fingers.
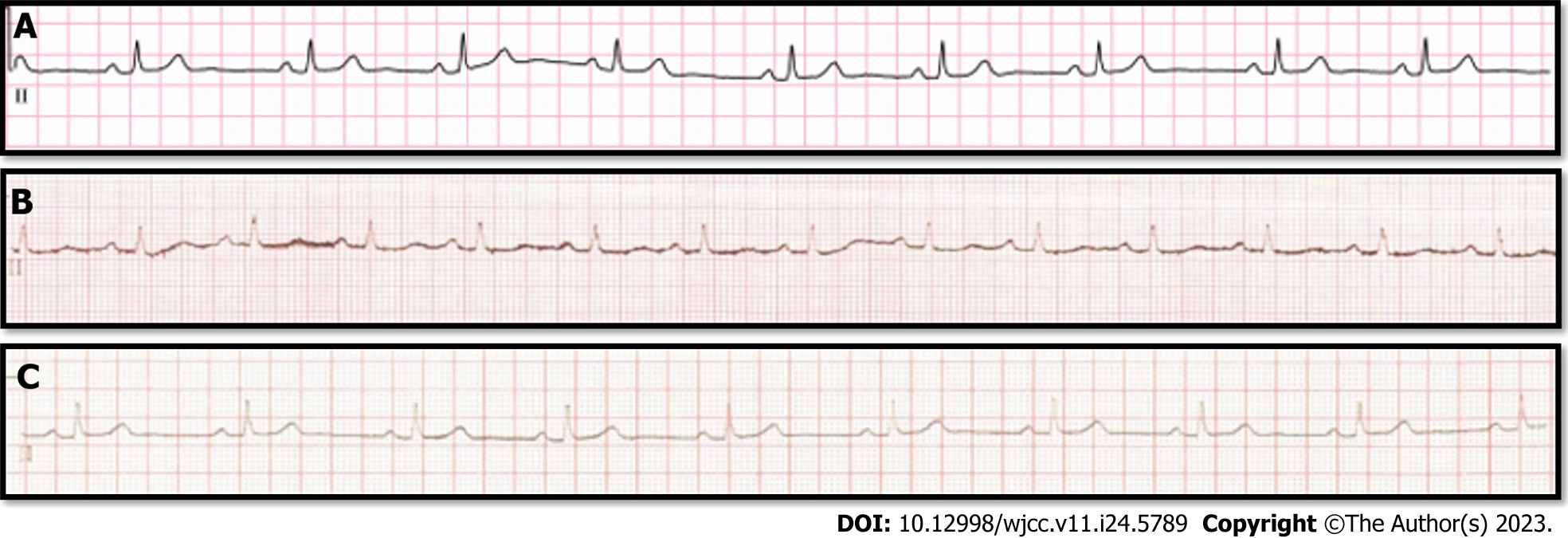
Nonspecific findings were found on preoperative examination and electrocardiography (ECG) (Figure 1A).
The patient’s medical history was unremarkable.
She had no family or genetic history of the disease.
Upon departure from the operating room, the patient’s vital signs were within normal limits: BP, 110/80 mmHg; heart rate (HR), 62 beats/min; SpO2, 99%; and temperature, 36.3°C. ECG revealed a normal sinus rhythm (Figure 2A). After anesthesia induction, the patient’s vital signs were as follows: BP, 128/71 mmHg; HR, 65 beats/min; and SpO2, 99%. Arrhythmias associated with nonspecific findings were observed on neurological examination.
On perioperative laboratory examinations, the cardiac marker, creatine kinase-MB (CK-MB), procalcitonin, and troponin-I levels were within the normal range.
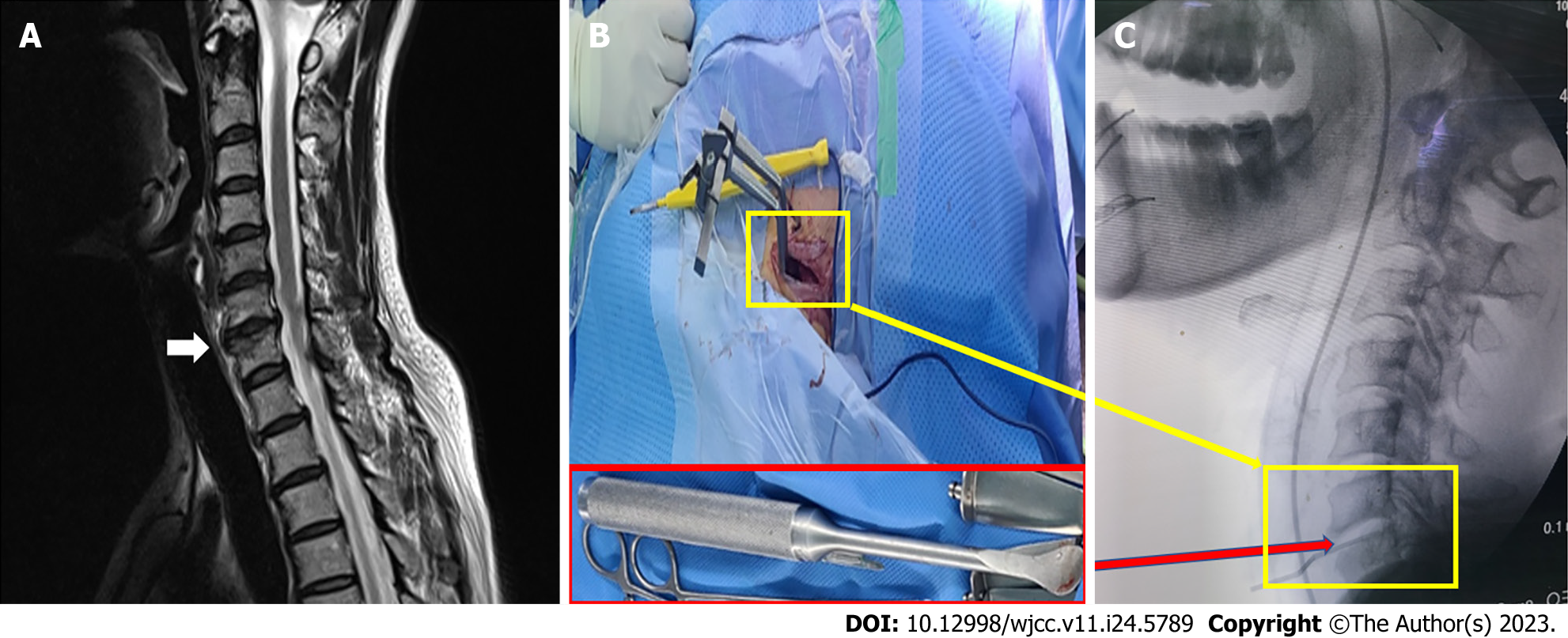
The radiological findings were nonspecific without the C6-7 foraminal stenosis with disc bulging on magnetic resonance imaging (Figure 3A).
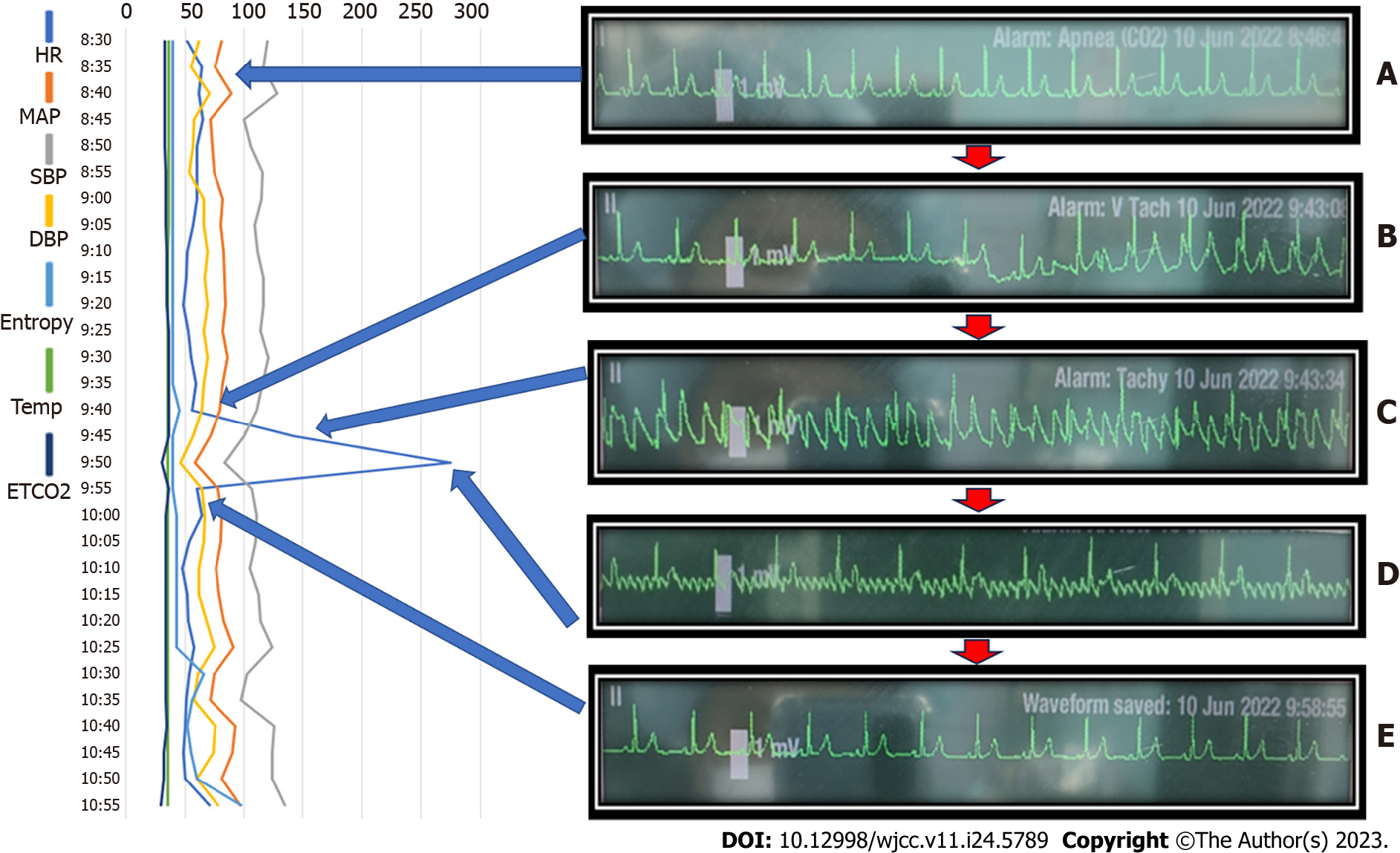
Anesthesia was maintained with desflurane 5%-6% and remifentanil 2 ng/mL via target-controlled infusion, with a sedation scale Entropy® of 30-50. ECG revealed a normal rhythm with the following vital signs: HR, 49-65 beats/min; BP, 100-128/48-65 mmHg; and SpO2, 100%. If there was a sharp increase in HR on ECG at approximately 1 h after the start of surgery (Figure 2B), the Cobb® spinal retractor was used to retract between C6 and C7 to the right of the patient’s neck area (Figure 3B and C). At that time, the patient’s vital signs were as follows: HR, 142 beats/min and BP, 108/62 mmHg. ECG showed looks like PSVT or ventricular tachycardia (VT) (Figure 2C). The AF pattern changed from 1:1 to 4:1-8:1 after drug treatment.
The patient was diagnosed with AF or PSVT r/o VT. However, the final diagnosis was AF.
The electrode pads were immediately applied in preparation for direct current cardioversion. If the patient was hemodynamically stable, esmolol 10 mg and lidocaine 60 mg were administered for VT based on the ECG results. However, they were ineffective. PSVT or AF was suspected. Thus, carotid massage and Valsalva maneuver were attempted to relieve r/o PSVT (Figure 2C). However, arrhythmia persisted. According to the surgeon’s discretion, the surgery was discontinued temporarily. The fraction of inspired oxygen was set at 100%, and adenosine 6 mg was administered for pharmacological management of PSVT, with 200 mL of crystalloid to maintain hemodynamic stability. There was a transient decrease in BP (78/46 to 67/35 mmHg). Nevertheless, it normalized within 2 min. The ECG findings subsequently changed and appeared to show a temporary disappearance of the arrhythmia, and then changed to AF with an atrial rate of 287 beats/min, with only the p wave again being prominent (Figure 2D). Diltiazem 10 mg was added gradually. The flutter persisted. However, cardioversion was not performed as the patient was hemodynamically stable. There was a transient decrease in BP. Nevertheless, the electrocardiogram began to normalize. We requested the surgeon to continue the surgery. During the operation, two episodes of premature venous complex were observed. Nonetheless, the arrhythmia disappeared with treatment with lidocaine 60 mg. To prevent arrhythmia recurrence, lidocaine 60 mg was further administered. BP increased to 109/66 mmHg 3 min after administration of diltiazem. ECG showed a normal rhythm (Figure 2E).
At the end of the surgery, the HR changed from 50 to 58 beats/min and the BP from 105/62 to 126/76 mmHg, and the SpO2 was maintained at 100%. The train-of-four confirmed a neuromuscular blockade level of 0, and sugammadex 200 mg was administered. The patient was fully alert and had spontaneous breathing. Hence, she was extubated. Just before leaving the operating room, the patient’s vital signs were as follows: HR, 71 beats/min; BP, 135/73 mmHg; and the SpO2, 100%. The total anesthesia time was 140 min. Upon arrival in the recovery room, the patient’s vital signs were as follows: HR, 78 beats/min; the BP, 133/82 mmHg; and the SpO2, 100%. The patient did not complain of any symptoms other than pain at the surgical site. No arrhythmias such as AF were detected on ECG performed immediately after arrival in the recovery room (Figure 1B).
After discharge from the recovery room, various tests were conducted for further evaluation. The levels of cardiac markers, including CK-MB (1.72 ng/mL), N-terminal pro B-type natriuretic peptide (38.8 pg/mL), procalcitonin (0.01 ng/mL), and troponin-I (< 0.001ng/mL), were within normal limits. ECG Holter monitoring was normal. Echocardiography showed mild tricuspid regurgitation (peak velocity at 2.29 m/s) without pulmonary arterial hypertension (30 mmHg), which was believed to be within normal limits. Subsequent ECG (Figure 1C) revealed no abnormalities, and both room BP and HR during hospitalization were within normal range. The patient was discharged on postoperative day 6. Currently, she is living a healthy life without arrhythmia recurrence.
Atrial fibrillation is the most common atrial arrhythmia that can occur during surgery, and AF and supraventricular tachycardia are also frequently observed, particularly among arrhythmias with a constant QRS rhythm. PSVT is a typical arrhythmia that can develop at any age in patients without structural heart disease; most commonly between the ages of 12 and 30 years. Its incidence is 2.5 per 1000 adults, and it is more common in women. PSVT can be caused by atrioventricular nodal reentrant tachycardia or atrioventricular reentrant tachycardia. AF is a type of heart rhythm disorder caused by issues in the electrical system of the heart. That is, the upper chambers (atria) beat extremely rapidly but with a regular rhythm. AF is similar to atrial fibrillation, a common disorder[2]. The initial arrhythmia is similar to a wide QRS tachycardia, and several conditions should be distinguished. First, it is important to differentiate true wide QRS tachycardia from tachycardias such as PSVT, which can occasionally mimic a wide QRS. In particular, VT can be life-threatening and requires proper diagnosis and treatment.
The specific arrhythmias experienced by a patient during surgery are based on various factors and should be managed immediately. Antiarrhythmic medication can be used to manage QRS tachycardia only if the tachycardia is regular and the patient is hemodynamically stable, as in low-risk patients with stable ECG and hemodynamic values before and after the arrhythmia, those with normal caffeine intake, and those without a history of suspicious medical conditions, excessive exercise, alcohol abuse, and smoking[3]. However, misdiagnosing VT for supraventricular tachycardia can pose a serious risk. Thus, the condition should be treated as VT when it cannot be appropriately identified. In this case, wide QRS tachycardia was inhibited after the administration of adenosine, and the p wave was tapped more than the QRS on ECG, indicating AF[4]. The intentional atrioventricular block is useful in differentiating narrow QRS tachycardia, and tachycardia termination is achieved with the use of Valsalva or adenosine. In this case, the tachycardia was inhibited. However, only the p-wave morphology changed to that of AF, which was observed evidently.
New-onset AF during general anesthesia induction for noncardiac surgery in a patient without a history of cardiac arrhythmias is rare, and similar cases are challenging to identify[5]. AF is caused by a macro-reentry between the interatrial septum and the free wall of the right atrium, including the isthmus between the tricuspid annulus and the inferior vena cava[6]. The atrial rate is > 240 beats/min. The atrioventricular node has an atrioventricular conduction pattern that varies from 1:1 to 8:1[7]. In this case, the atrioventricular conduction varied from 1:1 to 7:1. The ventricular rhythm was constant. Nevertheless, the atrioventricular rate differed, probably due to the effects of adenosine, which limits atrioventricular conduction. This atrial rhythm was probably attributed to the interaction of the drug with any issues in atrial autonomic control. While the exact cause of atrial tachyarrhythmias is often unknown, several factors have been associated with its development: Age > 60 years, structural heart diseases such as heart valve disorders, history of heart surgery or congenital heart defects, and coronary heart disease. Hypertension can lead to changes in the structure and function of the heart. Thyroid disorders such as hyper- or hypothyroidism can disrupt the normal electrical activity of the heart. Sympathetic stimulation, use of stimulants such as nasal decongestants, hypovolemia or anemia, hypoxemia, hypercarbia, fever, sepsis, and malignant hyperthermia also can lead to it. In addition to these factors, there are possible aggravating factors specific to this tachyarrhythmia, which include direct surgical damage to autonomic nerve fibers innervating the atria and sinoatrial node, high perioperative HR due to sympathetic nervous system excitation, inflammatory changes in the myocardium with age, and premature atrial contraction[8]. In this case, tachycardia was not observed before surgery, and there was no evidence of premature atrial contraction. Preoperative echocardiography was not performed because the patient had no history of cardiac disease. However, a trivial tricuspid regurgitation was observed on postoperative echocardiography. There are some speculations as to the effect of these findings on the development of arrhythmia. The other actions of the autonomic nervous system have also been implicated in AF development, and AF has been reported in a patient without cardiovascular risk factors for arrhythmia and with a normal preoperative electrocardiogram who developed AF due to increased abdominal pressure during laparoscopic gastrectomy[5]. This indicates that even in the absence of an underlying medical condition, AF can be caused by autonomic nervous system activity.
The stellate ganglion is the fusion of the inferior cervical sympathetic ganglion, comprising the sixth and seventh cervical ganglia, and the first thoracic sympathetic ganglion in a space bounded anteriorly by the subclavian and the vertebral artery and posteriorly by the neck of the first rib and the seventh cervical transverse process[9]. The stellate ganglion is the efferent component of the sympathetic nervous system, and the postcardiac ganglionic sympathetic nerves located in the stellate ganglion are distributed throughout the heart, releasing neurotransmitters [e.g., norepinephrine (NE)] bind with b-adrenergic receptors to affect cardiac electrophysiological and contractile functions, including HR, cardiac conduction, and myocardial contraction. It is widely recognized that cardiac postganglionic sympathetic nerve terminals innervate the sinoatrial node, atrioventricular node, His bundle, and contractile myocardium. Although the number of neurotransmitters, including NE, is primarily determined by the intensity of the cardiac postganglionic sympathetic nerve activity, the number of NE molecules that bind to cardiac adrenergic receptors and induce biological effects[10-12]. Increased sympathetic tone and abnormal responsiveness of the parasympathetic nervous system is associated with heart failure and elevated levels of plasma NE correlate with worse outcomes[13]. Based on the above-mentioned mechanisms, clinical studies, and animal experiments have shown that the remodeling of postcardiac sympathetic neurons in the stellate ganglion can cause cardiac sympathetic overactivation and ventricular arrhythmias[14]. Therefore, physical stimulation of the cervical spine adjacent to the stellate ganglion site by the iatrogenic situation made mass effect or such as deformities or damage, may trigger the arrhythmia. Although stellate ganglion block (SGB) is often performed in pain clinics without proper monitoring, the site of the block may directly trigger the ganglion. The location of the surgical approach is almost identical to the approach for SGB, especially right SGB, which may require more careful monitoring for adverse effects. It has also been observed in females that the cricoid cartilage is located cranially, especially when the patient is in a position similar to the present case with neck extension. As the cricoid cartilage is unobstructed and easily exposed to the outside environment, palpation of the transverse process of C6 may increase the likelihood of arrhythmias if force is used[15]. The stellate ganglia are commonly bilateral and there is little evidence of dominant bilateral innervation[16]. The sinoatrial node is primarily innervated by cardiac postganglionic sympathetic nerve terminals from the right stellate ganglion[17]. Therefore, physical cervical sympathetic stimulation, especially right side, such as deformities or damage, may trigger the arrhythmia by sinoatrial node sympathetic tone change. There is only one study showing good results with the left SGB in patients who were not effectively managed with predominantly right SGB[18]. In addition, animal studies have shown that asymmetric electrical stimulation of the supraventricular node induces transient acute AF due to sympathetic overactivation[19]. In a study of patients with a history of atrial fibrillation, the inducibility of atrial fibrillation was significantly reduced after SGB. Even in patients with induced atrial fibrillation, the arrhythmia duration was reduced[20]. In addition, some studies have shown that patients undergoing bilateral stellate ganglionectomy experienced a significant reduction in the incidence of electrically induced atrial fibrillation and the preservation of sinus rhythm[21]. In this case, it might have been necessary to identify which side of the cervical spine was retracted and affected the stellate ganglion during surgery. Although there was a change in the size and rate of the p wave with drug administration, the arrhythmia resolved safely after a few minutes, and the operation could be safely continued.
As ECG remained normal in the postoperative period without any recurrence, the occurrence of AF in this patient was not caused by the patient’s underlying disease but by the presence of some stimulation related to the heart rhythm in the surrounding area caused by the stimulation of the surgical site. In particular, based on the fact that the surgical site was C6-7, the area was stimulated with a retractor to expose C6-7 during surgery at the time of ECG changes. The area was the site of a procedure involving the stellate ganglion. Thus, the arrhythmia was temporarily induced by the overactivation of the autonomic nervous system caused by physical stimulation of the stellate ganglion.
For the acute control of hemodynamically stable AF, beta-blockers such as diltiazem and verapamil are useful. Diltiazem is the preferred calcium channel blocker as it is safe and effective. Verapamil and diltiazem should not be used in patients with advanced heart failure with heart block or dyssynchrony, or in patients with pre-excitation. The heart-rate-lowering effect of beta-blockers is related to the reduced sympathetic nervous system activity. Esmolol is preferred because of its rapid onset of action, which is useful in patients with PSVT and atrial fibrillation. In patients with PSVT, these drugs can be administered intravenously to inhibit tachycardia or when urgent rate control is required in conditions including atrial fibrillation and flutter[4]. Unlike adenosine, these drugs can occasionally cause bradycardia and hypotension. Therefore, they are safe to infuse slowly over 2-3 min. Maintenance doses can be continued for several days if needed[22]. In this case, adenosine was used to differentiate AF from supraventricular tachycardia. Adenosine activates K+ channels by acting on a1 receptors and increasing the permeability of the cell membrane to K+, shortening the action potential period of the freezing node, atrioventricular node, and atrial cells, and activating the membrane potential. It causes atrioventricular conduction disorder. However, its half-life is short, within 10 s. Thus, it is considered an extremely effective and safe drug for terminating supraventricular tachycardia. In addition, adenosine is used for the diagnosis of AF, ventricular fibrillation, and atrial tachycardia based on the fact that it causes temporary atrioventricular block. Moreover, it is used in the differential diagnosis of arrhythmias with a wide QRS[23]. However, there are reports of adenosine causing VT and ventricular fibrillation. Therefore, a more cautious approach should be recommended[24]. During arrhythmia, lidocaine is used. It suppresses electrical activity in depolarized (ischemic or arrhythmogenic) tissues, and it is, therefore, effective in suppressing arrhythmias associated with depolarization (e.g., ischemia or digitalis poisoning). However, it is ineffective against arrhythmias that occur in normally polarized tissues (e.g., AF and fibrillation). In this case, it was effective when used for additional premature ventricular contractions after arrhythmia is controlled, but not when used for atrial arrhythmia.
Atrioventricular block induction is useful in detecting narrow QRS tachycardia. In addition to the Valsalva maneuver, the other methods used to induce atrioventricular block include carotid sinus massage and drugs such as adenosine and verapamil. Atrioventricular node regression tachycardia and atrioventricular regression tachycardia in which the atrioventricular node is essential to the regression circuit are inhibited by this method. AF is easily recognized based on the presence of a distinct flutter wave during the atrioventricular block[25]. In this case, the arrhythmia did not resolve after adenosine tranquilization and the persistence of flutter waves on the electrocardiogram waveform indicated AF rather than PSVT. Electrical cardioversion should be performed when a patient with AF is hemodynamically unstable. However, pharmacological therapy can be attempted if the patient is considered hemodynamically stable. If cardioversion is performed, acute antithrombotic therapy can be beneficial because the short-term stroke rate after cardioversion for AF was 0%-7% based on a meta-analysis. The rate of thrombosis in patients with persistent AF is high, with an annual average of 3%[26]. In this case, the patient was hemodynamically stable; therefore, pharmacological treatment could have been prioritized. The long-term risks of AF also include an increased risk of stroke, heart failure, and mortality[27].
In cases as in our patient, where the anatomy that must be accessed for surgery can cause arrhythmias, appropriate monitoring and the clinician’s empirical ability to act proactively in case of an arrhythmia are required. If an arrhythmia occurs in the context of stimulation of the right stellate ganglion during cervical spine surgery, identification of triggers to consider, correction of the appropriate triggers, and prevention of migration to fatal arrhythmias should be considered. In patients with a high risk of AF, preoperative ECG should be performed to assess the presence of tricuspid regurgitation. Arrhythmias such as AF and PSVT can occur during surgery due to various factors and can have similar electrocardiographic characteristics, thereby making them challenging to differentiate from each other and treat. Hence, clinicians should be knowledgeable about the electrocardiographic features of these arrhythmias.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Amornyotin S, Thailand; Dalfardi B, Iran S-Editor: Lin C L-Editor: Kerr C P-Editor: Yu HG
| 1. | Kwon CH, Kim SH. Intraoperative management of critical arrhythmia. Korean J Anesthesiol. 2017;70:120-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Ferguson JD, DiMarco JP. Contemporary management of paroxysmal supraventricular tachycardia. Circulation. 2003;107:1096-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM, Passman RS, White RD, Hess EP, Tang W, Davis D, Sinz E, Morrison LJ. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S729-S767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 899] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 4. | Jang SW. Differential Diagnosis of Supraventricular Tachycardia. Int J Arrhythm. 2017;18:43-47. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Kwak HJ, Lee KC, Lee D, Kim HS, Kim SH. Atrial flutter associated with high pressure pneumoperitoneum during laparoscopic gastrectomy-A case report. Anesth Pain Med. 2010;5:67-69. |
| 6. | Ortiz J, Niwano S, Abe H, Rudy Y, Johnson NJ, Waldo AL. Mapping the conversion of atrial flutter to atrial fibrillation and atrial fibrillation to atrial flutter. Insights into mechanisms. Circ Res. 1994;74:882-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Song JC, Suk E-H, Cho JH, Ju W, Lee CS, Lim YS. Development of atrial flutter after induction of general anesthesia and conversion to atrial fibrillation −A case report−. Anesth Pain Med. 2017;12:62-67. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Amar D. Perioperative atrial tachyarrhythmias. Anesthesiology. 2002;97:1618-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Brown D. Regional Anesthesia and Local Anesthetic-Induced Seizures. Anesth Analg. 1996;82:699-670. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Verrier RL, Antzelevitch C. Autonomic aspects of arrhythmogenesis: the enduring and the new. Curr Opin Cardiol. 2004;19:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Cuevas J. Molecular mechanisms of dysautonomia during heart failure. Focus on "Heart failure-induced changes of voltage-gated Ca2+ channels and cell excitability in rat cardiac postganglionic neurons". Am J Physiol Cell Physiol. 2014;306:C121-C122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Wallis D, Watson AH, Mo N. Cardiac neurones of autonomic ganglia. Microsc Res Tech. 1996;35:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Zhang DY, Anderson AS. The sympathetic nervous system and heart failure. Cardiol Clin. 2014;32:33-45, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 14. | Li YL. Stellate Ganglia and Cardiac Sympathetic Overactivation in Heart Failure. Int J Mol Sci. 2022;23:13311. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 15. | Park JS, Kim KJ, Lee YW, Yoon DM, Yoon KB, Han MY, Choi JB. Estimation of Stellate Ganglion Block Injection Point Using the Cricoid Cartilage as Landmark Through X-ray Review. Korean J Pain. 2011;24:141-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Lin SY, Hsu WH, Lin CC, Lin CL, Tsai CH, Lin CH, Chen DC, Lin TC, Hsu CY, Kao CH. Association of Arrhythmia in Patients with Cervical Spondylosis: A Nationwide Population-Based Cohort Study. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Meng L, Shivkumar K, Ajijola O. Autonomic Regulation and Ventricular Arrhythmias. Curr Treat Options Cardio Med. 20:38. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Mulvaney SW, Lynch JH, Curtis KE, Ibrahim TS. The Successful Use of Left-sided Stellate Ganglion Block in Patients That Fail to Respond to Right-sided Stellate Ganglion Block for the Treatment of Post-traumatic Stress Disorder Symptoms: A Retrospective Analysis of 205 Patients. Mil Med. 2022;187:e826-e829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Meijborg VMF, Boukens BJD, Janse MJ, Salavatian S, Dacey MJ, Yoshie K, Opthof T, Swid MA, Hoang JD, Hanna P, Ardell J, Shivkumar K, Coronel R. Stellate ganglion stimulation causes spatiotemporal changes in ventricular repolarization in pig. Heart Rhythm. 2020;17:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Leftheriotis D, Flevari P, Kossyvakis C, Katsaras D, Batistaki C, Arvaniti C, Giannopoulos G, Deftereos S, Kostopanagiotou G, Lekakis J. Acute effects of unilateral temporary stellate ganglion block on human atrial electrophysiological properties and atrial fibrillation inducibility. Heart Rhythm. 2016;13:2111-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Ganesh A, Qadri YJ, Boortz-Marx RL, Al-Khatib SM, Harpole DH Jr, Katz JN, Koontz JI, Mathew JP, Ray ND, Sun AY, Tong BC, Ulloa L, Piccini JP, Fudim M. Stellate Ganglion Blockade: an Intervention for the Management of Ventricular Arrhythmias. Curr Hypertens Rep. 2020;22:100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Kwon DS, Badhwar N. Rare Diagnosis of a Common Supraventricular Tachycardia. Card Electrophysiol Clin. 2010;2:221-224. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Shim JK, Lee DI. Atrial Fibrillation with Ventricular Pre-excitation after Intravenous Adenosine as a Treatment of Supraventricular Tachycardia. Korean Circ J. 2003;33:933-935. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 24. | Lee SY, Pothiawala S, Meng Seet C. Adenosine-induced ventricular fibrillation in a patient with supraventricular tachycardia. Qatar Med J. 2021;2021:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Link MS. Clinical practice. Evaluation and initial treatment of supraventricular tachycardia. N Engl J Med. 2012;367:1438-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Shah SR, Luu SW, Calestino M, David J, Christopher B. Management of atrial fibrillation-flutter: uptodate guideline paper on the current evidence. J Community Hosp Intern Med Perspect. 2018;8:269-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Boyer M, Koplan BA. Cardiology patient page. Atrial flutter. Circulation. 2005;112:e334-e336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |