Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5780
Peer-review started: May 19, 2023
First decision: June 13, 2023
Revised: July 3, 2023
Accepted: July 24, 2023
Article in press: July 24, 2023
Published online: August 26, 2023
Processing time: 97 Days and 21.5 Hours
We present a case of focal lymphoblastic transformation to erythroid leukemia following acute myeloblastic transformation in a patient with chronic myeloge
The presence of the Philadelphia (Ph) chromosome was identified through karyotype analysis, while the BCR-ABL fusion gene was detected using quan
Our findings suggest a possible molecular basis for the focal lymphoblastic transformation secondary to myeloblastic transformation in patients with CML.
Core Tip: A case of focal lymphoblastic transformation into erythroid leukemia following acute myeloblastic transformation in a chronic myelogenous leukemia patient is presented, and the mechanism of its occurrence and development is discussed. Multiple new gene mutations and abnormalities in complex karyotypes of chromosomes were found to be the major reason.
- Citation: Wang W, Chen YL, Gou PP, Wu PL, Shan KS, Zhang DL. Focal lymphoblastic transformation of chronic myelogenous leukemia develops into erythroid leukemia: A case report. World J Clin Cases 2023; 11(24): 5780-5788
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5780.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5780
Chronic myelogenous leukemia (CML) is characterized by the presence of the Philadelphia (Ph) chromosome and the BCR-ABL fusion gene. The clinical presentation of CML is often categorized into three phases: Chronic phase, accelerated phase, and blast crisis. Progression to blast crisis substantially worsens the prognosis. In the majority (approximately 70%) of cases of acute transformation of CML, the patients develop acute myelogenous leukemia (AML); only a smaller proportion (about 30%) of patients develop acute lymphocytic leukemia. In rare cases, CML may transform into mixed-phenotype acute leukemia. However, to date, there have been no reported cases of CML with blastic lymphoid transformation, followed by blastic myeloid transformation. In this report, we present one such unique case of CML with blastic transformation in extramedullary lymph nodes, followed by secondary myeloblastic transformation that eventually led to pure erythroid leukemia.
A 44-year-old man presented with fever for 1 wk and a history of CML for over 4 years.
The patient was diagnosed with a mass on the right side of his neck in June 2016. Initially, the mass had a size similar to a quail egg, but it progressively enlarged. The mass had poor mobility and no tenderness. Subsequently, multiple swollen lymph nodes with similar characteristics were found on both sides of the neck. The patient did not experience fever, chills, weight loss, night sweats, skin itching, abdominal pain, or abdominal distension. He sought medical attention at the Department of General Surgery of our hospital, where laboratory tests revealed a white blood cell (WBC) count of 565.21 × 109/L, absolute neutrophil count of 499.09 × 109/L, hemoglobin level of 47 g/L, and total platelet count of 219 × 109/L. The patient was transferred to our department for further investigations, which included bone marrow (WB) aspiration and BCR-ABL fusion gene testing, to confirm the diagnosis of chronic-phase CML. The biopsy of a right neck lymph node, along with the immunohistochemistry results, indicated T-cell lymphoblastic lymphoma (T-LBL)/leukemia. Following the biopsy, fluorescence in situ hybridization (FISH) was performed, which showed the presence of the BCR-ABL fusion gene. Consequently, the patient was diagnosed with CML concurrent with focal blastic transformation of extramedullary lymph nodes.
Oral therapy with imatinib mesylate was initiated, which resulted in the disappearance of the mass, without recurrence. The patient received follow-up treatment for CML in the outpatient department. However, he was not adherent to the treatment regimen and frequently self-discontinued the medication. Additionally, regular monitoring of the fusion gene levels was not done, and molecular remission was not achieved with the treatment.
In September 2019, visible abdominal distension and splenomegaly were noted, accompanied by incomplete intestinal obstruction. The patient had recurrent fever and was diagnosed with a lung infection. Supportive treatment was initiated in the form of fasting, gastrointestinal decompression, enema, and parenteral nutrition, along with anti-infective medications such as sulperazone, imipenem, cilastatin, and voriconazole The patient’s symptoms improved. However, subsequent re-examination revealed accelerated phase CML. Therefore, dasatinib was initiated, which was followed by prolonged pancytopenia lasting for a month. Dasatinib was discontinued, but the pancytopenia persisted; therefore, a blast crisis was considered.
Results of routine blood tests showed a WBC count of 0.43 × 109/L, hemoglobin level of 69 g/L, and total platelet count of 83 × 109/L. Since the onset of the illness, the patient had poor spirits, average physical strength, fair appetite, normal sleep, no significant weight changes, and normal bowel movements.
The patient had no remarkable history of past illness.
The patient had no remarkable family history of similar illnesses.
On physical examination, the patient had a body temperature of 36.4 °C, pulse rate of 103 beats/min, respiratory rate of 20 breaths/min, blood pressure of 105/76 mmHg, with a regular rhythm and no pathological murmurs. The patient was conscious and pale, without superficial lymphadenopathy. Slight tenderness of the sternum was noted. Clear breath sounds were auscultated in both lungs without dry or wet rales. Abdominal bulging and splenomegaly were detected, with measurements along the midclavicular, midabdominal, and third abdominal lines being 28 cm, 35 cm, and +5 cm, respectively. Palpation of the spleen revealed a hard texture with no obvious tenderness, and a palpable spleen notch. There was no swelling in the lower limbs.
BM examination on February 19, 2016 revealed significant proliferation of nucleated BM cells. The ratio of granulocytes to red blood cells (RBC) was 31:1, indicating significant granulocytic hyperplasia. Primitive granulocytes accounted for 3% of the cells. An increase in the proportion of promyelocytes and metamyelocytes, as well as the basophil cell percentage, was noted. Erythroid hyperplasia was suppressed, and the lymphocyte ratio was decreased. The percentage of monocytes was normal. The number of megakaryocytes was greater than 500, with the presence of noticeable megakaryocytes. Peripheral blood smear showed an extremely high total WBC count and a predominance of neutrophils with left shift. Primitive granulocytes accounted for 3% of the cells, with an increased proportion of basophils.
BM examination on August 24, 2020 revealed significant BM hyperplasia, with the percentages of myelocytes and erythrocytes being 22.0%, and 72.0%, respectively. The granulocyte to RBC ratio was 0.31:1, indicating a reduction in the extent of granulocytic hyperplasia. Primordial granulocytes accounted for 7.5% of the cells (34.1% of the nucleated erythroid cells). Cells of various sizes with increased plasma content were detected, with dark blue plasma and possible purple-red granules. The nuclei were round and loosely distributed, with 0-2 nucleoli per nucleus. A predominance of basophils was noted. Marked erythroid hyperplasia was observed, with an increased proportion of young RBCs at different stage, with large cell bodies. The monocyte counts as well as the proportions of binuclear and multinucleated young RBCs and lymphocytes were normal. More than 1000 megakaryocytes were detected in the smear, with the absence of platelets. Examination of the peripheral blood smear showed a normal total WBC count. Primitive cells accounted for 2.0% of the cells, with increased expression of basophils. Four nucleated RBCs per 100 WBCs were noted, with noticeable primordial RBCs, along with various sizes of mature RBCs with deformities such as fragmentation and Cabot rings, among others. Platelet counts were increased. The findings were indicative of the transformation of CML into acute leukemia.
BM examination on October 07, 2020 showed that BM hyperplasia was reduced, with the percentages of myelocytes and erythrocytes being 11.0% and 76.0%, respectively (myelocyte:erythrocyte ratio = 0.14:1). A marked reduction in granulocytic hyperplasia was noted. Erythroid hyperplasia was still noticeable, with a predominance of middle and late stages of young RBCs, which were consistent with the results of the blood smear. Lymphocyte proportions and monocyte counts remained normal. Only seven megakaryocytes were detected in the film, and platelets were rare. No parasites were detected in the blood smear. Peripheral blood smear showed a reduced total WBC count, with a predominance of mature lymphocytes. There was an increased percentage of eosinophils, along with various sizes of RBCs, including fragmented cells with shapes resembling helmets, Cabot rings, etc. Platelets were hardly detected.
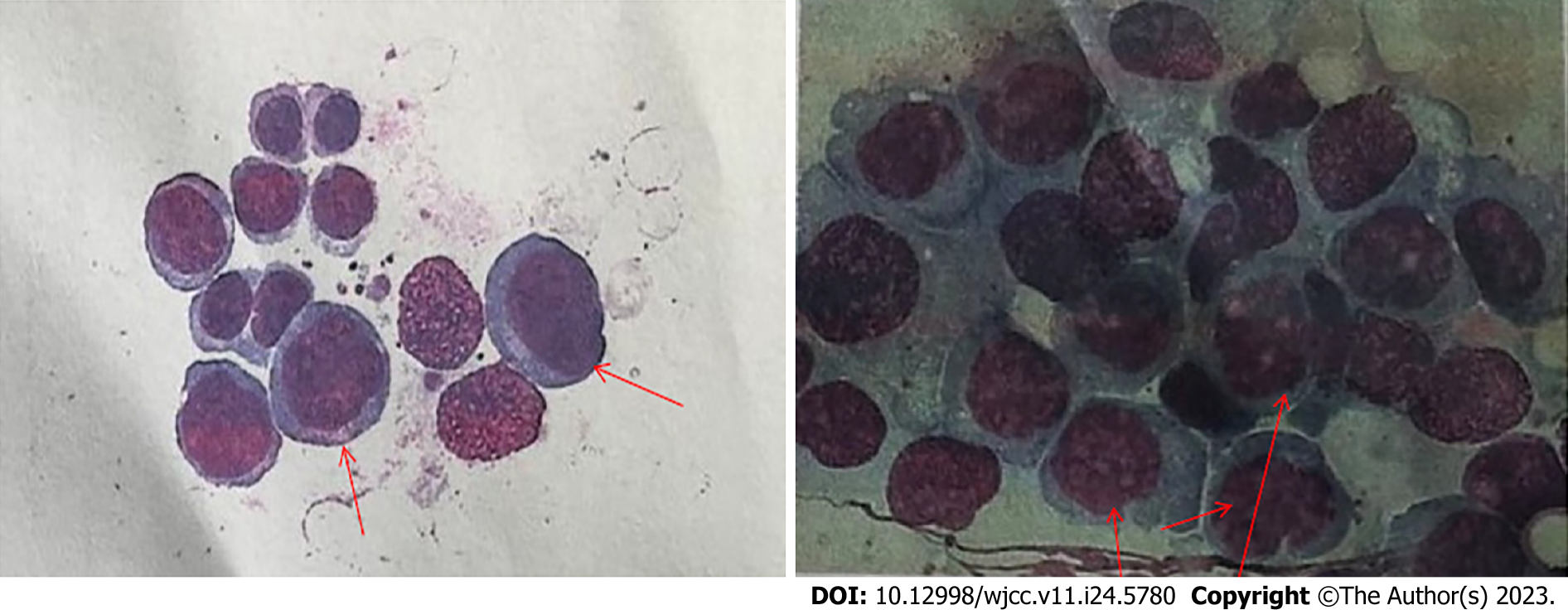
BM examination on October 16, 2020 revealed BM hyperplasia, with the percentages of myelocytes and erythrocytes being 3.5% and 90.0%, respectively. The granulocyte to RBC ratio was 0.04:1, indicating reduced granulocytic hyperplasia. Marked erythroid hyperplasia was observed, with an increased proportion of RBCs in all stages of development. Megaloblasts with malformed nuclei, nuclear budding, and multinucleation were detected. Mature RBCs of various sizes were observed, with nuclear stippling, polychromatic appearance, and helmet-shaped forms. Basophils were detectable. The ratio of lymphocytes to monocytes was normal. Forty-three megakaryocytes were found in the film, with platelets being rare. On the basis of these findings of BM smear examination, transformation of CML into acute erythroid leukemia (AEL) was considered as the diagnosis. However, immunophenotyping and related genetic testing were required to conclusively establish the diagnosis (Figure 1).
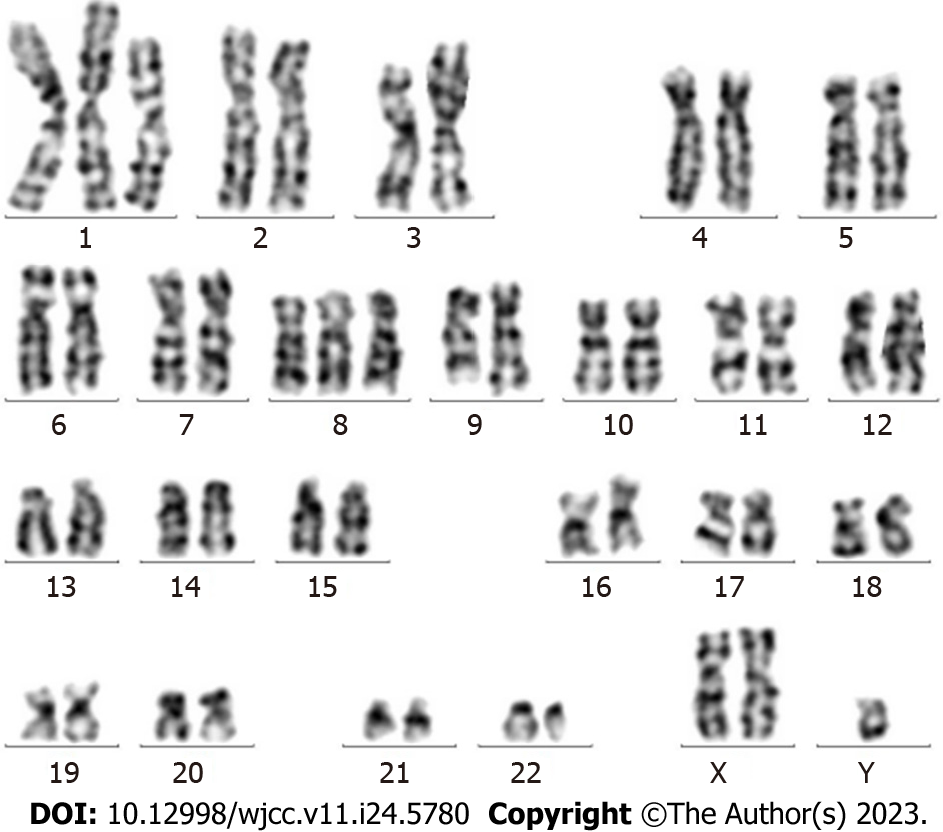
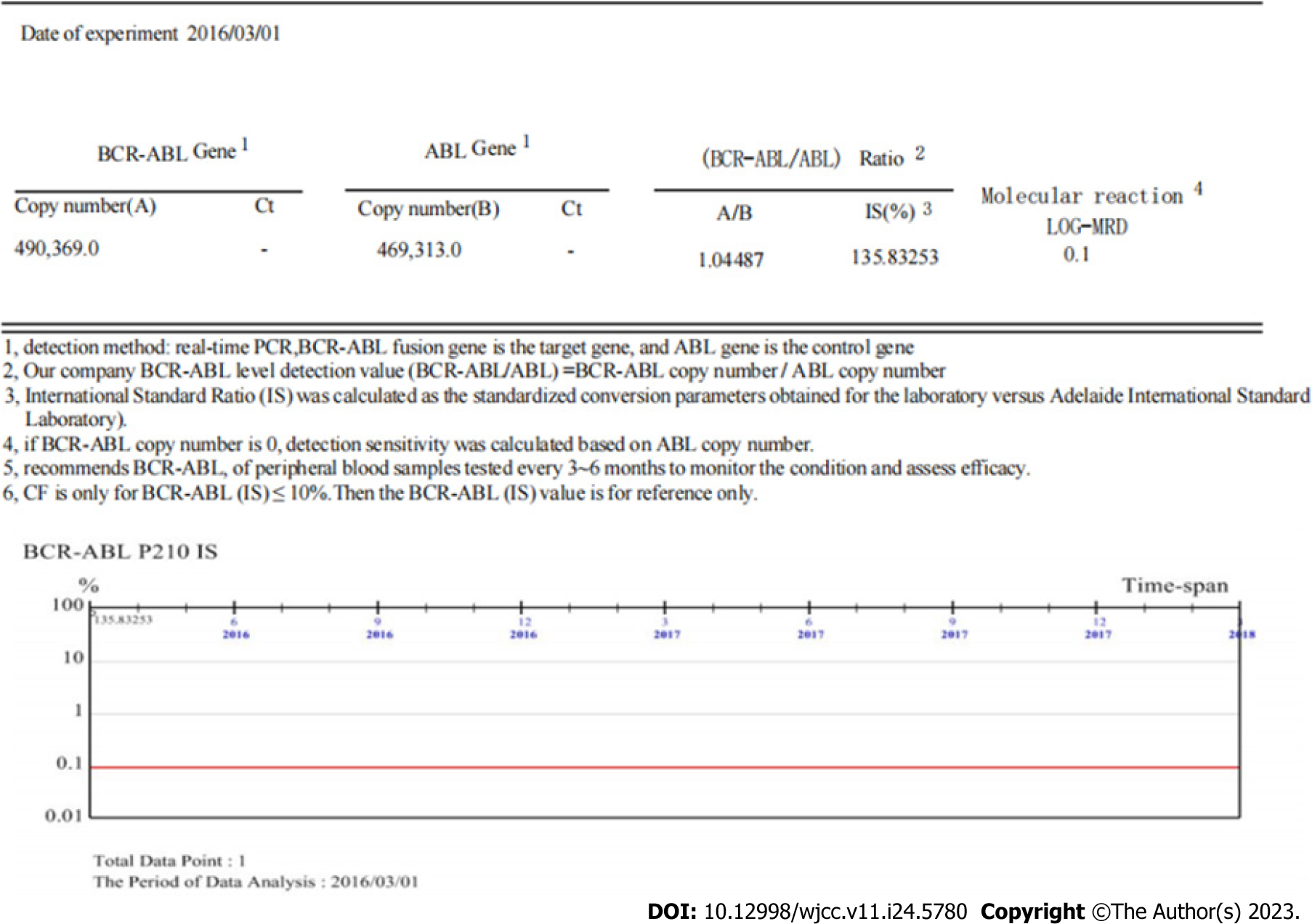
Results for the BCR-ABL1 (p210) fusion gene test on August 20, 2020 were positive. The copy number of BCR-ABL1 and ABL1 was 149082.6 and 127119.0, respectively. The BCR-ABL1/ABL1 ratio was 117.28, and the ratio of IS BCR-ABL1/ABL was 77.40 (Figure 2). Karyotype analysis on August 21, 2020 revealed 47XY,+X,t(9;22)(q34;q11.2)[6]/49,idem,+1,der(1;7) (q10;q10),+7,+8[2]/49,idem,+1,der(1;7),+8,+10[2] (Figure 3).
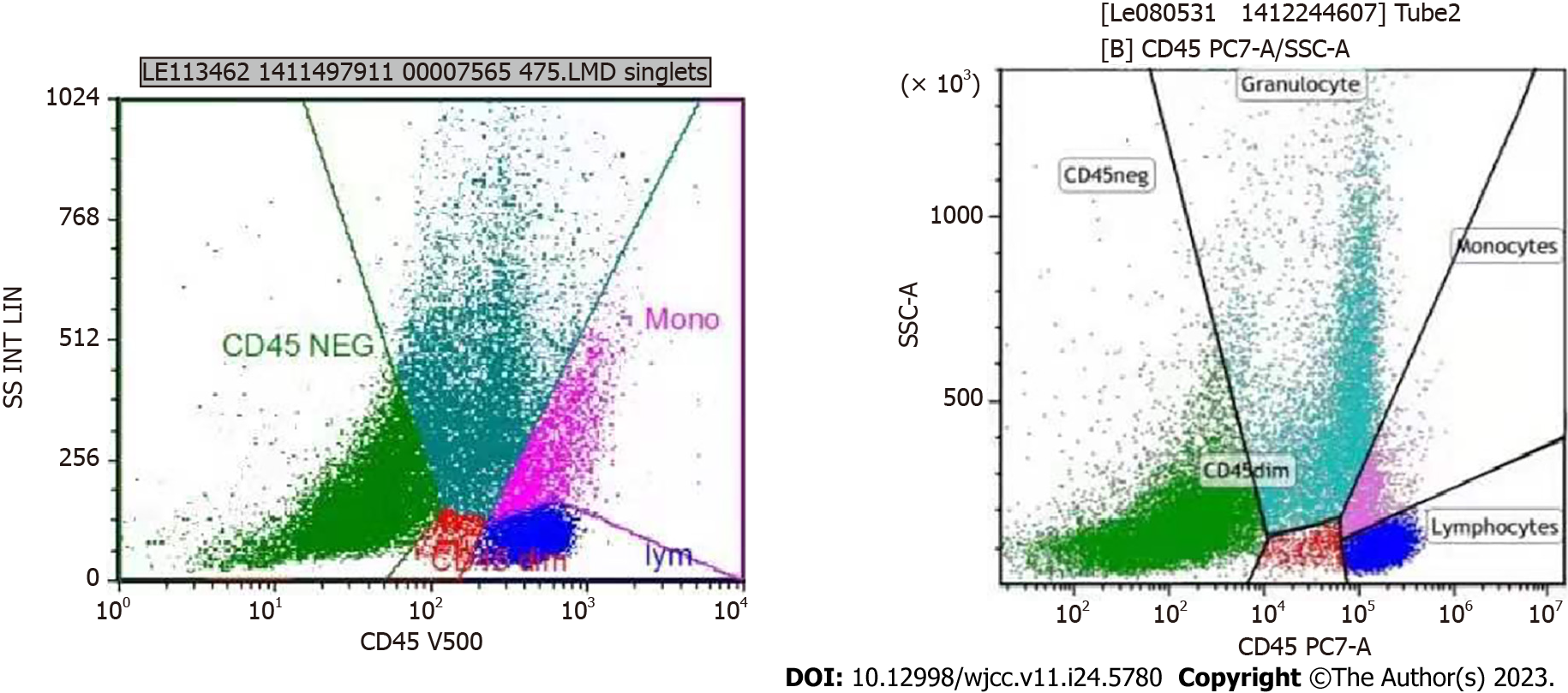
Flow cytometry (FC) immunofluorescence analysis was performed on August 25, 2020. FC of the examined specimens revealed the absence of immunophenotypic abnormalities related to acute leukemia and high-risk myelodysplastic syndrome in the examined specimens. There was no noticeable occurrence of CD34+ cells, and the relative proportion of granulocytes was reduced. No significant changes were observed in the expression levels of CD13, CD15, CD16, and CD11b. T cells accounted for 77.5% of lymphocytes, with a CD4:CD8 ratio of 0.95 and no noticeable abnormalities. Natural killer (NK) cells accounted for 6.9% of lymphocytes, with no noticeable abnormalities. Mature B lymphocytes accounted for 2.5% of lymphocytes, with a dominance of polyclonal B cells.
FC immunofluorescence analysis was also performed on October 8, 2020. The FC results of the examined specimens did not show evidence of immunophenotypic abnormalities associated with acute leukemia, non-Hodgkin lymphoma (NHL), or high-risk myelodysplastic syndrome. There was a significant increase in the relative proportion of nucleated RBCs. The proportion of CD34+ cells in nuclear cells was approximately 0.24%. No noticeable abnormal immunophenotypes were observed. The analysis also showed a reduced relative proportion of granulocytes and significant changes in the expression levels of CD13, CD15, and CD16. The relative proportion of lymphocytes was within the normal range. T cells accounted for 88.68% of lymphocytes, with a CD4:CD8 ratio of 0.69 and no noticeable abnormalities. NK cells accounted for 5.00% of lymphocytes, with no noticeable abnormalities. Mature B lymphocytes accounted for 1.96% of lymphocytes. A significant increase in the relative proportion of nucleated RBCs was noted, accounting for approximately 55.41% of the total RBCs (Figure 4).
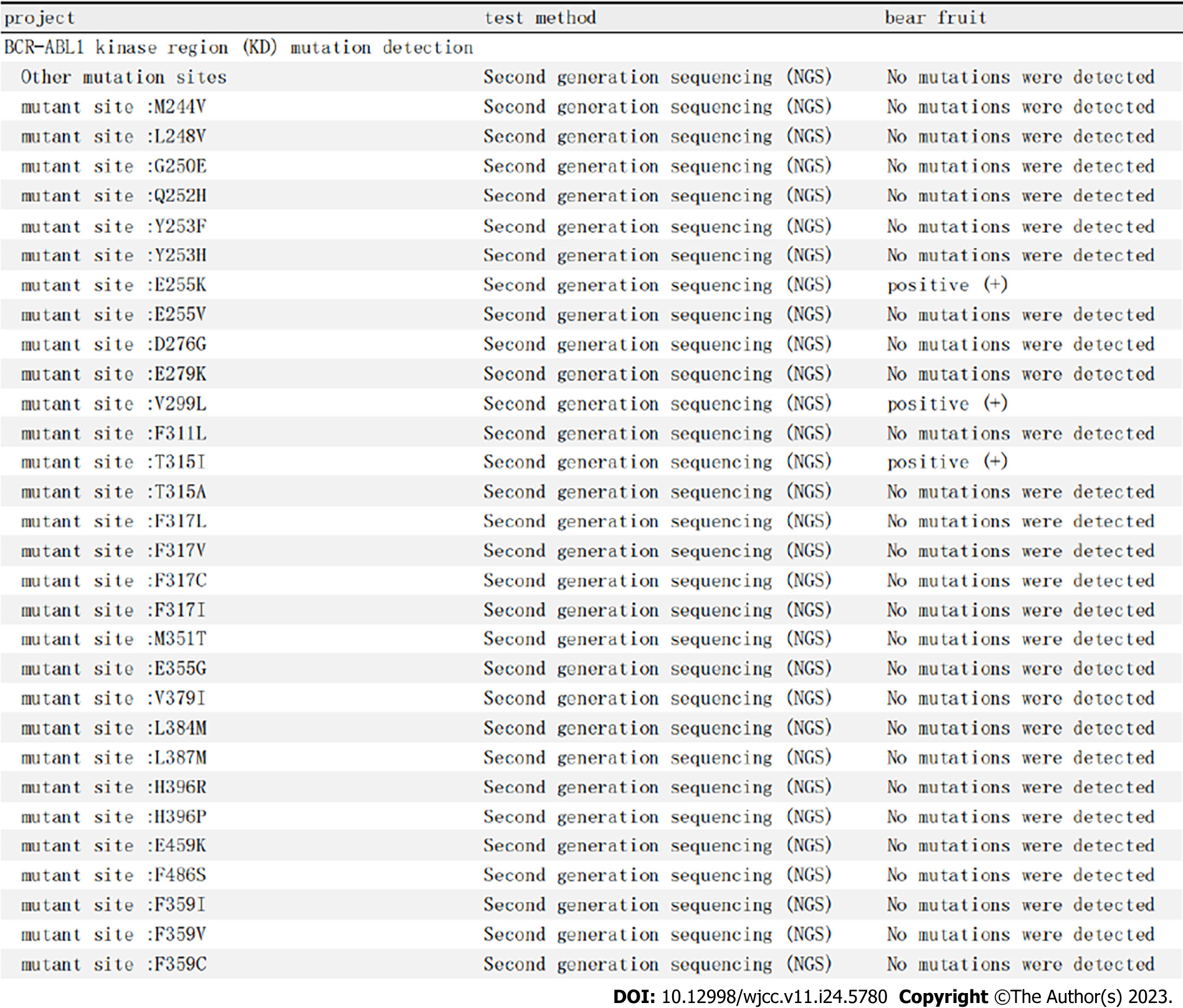
Examination of kinase domain mutations on October 15, 2020 showed that positive results were obtained for E255K, V299L, and T315I mutations, while no M244V, L248V, G259E, Q252H, Y253F, Y253H, E255V, D276G, E279K, F311L, T315A, F317L, F317C, F317I, M351T, E355G, V379I, L384M, L387M, H396R, H396P, E459K, F486S, F359I, F359V, or F359C mutation was detected. On analysis of the gene mutations related to CML progression, no mutations were detected in the genes ASXL1, MYC, CBL, NRAS, CDKN2A, RUNX1, IDH1, TET2, IDH2, TP53, and KRAS (Figure 5).
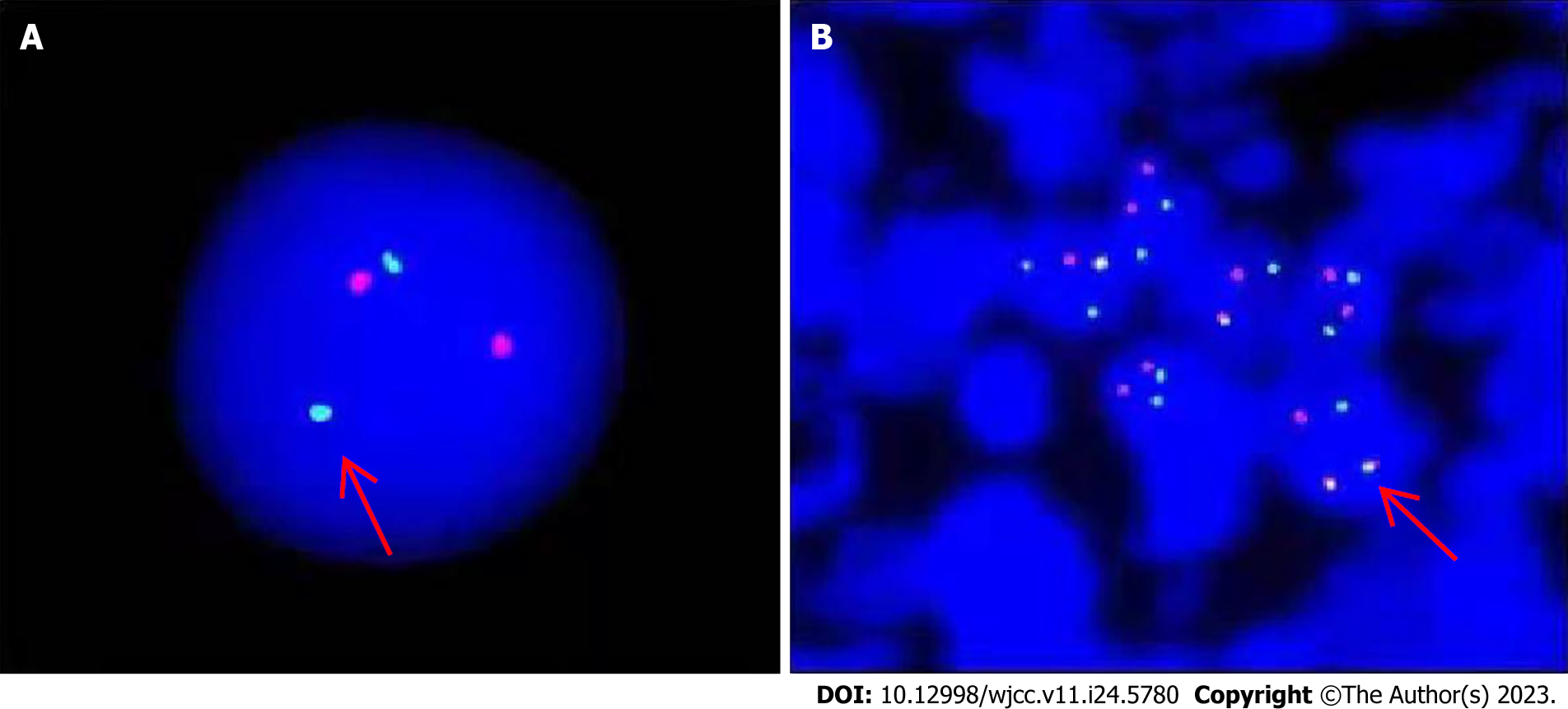
Molecular genetic studies of lymph node biopsy sample and immunophenotyping were performed in March 2016. In the examined lymph nodes, tumor cells were found to be distributed in a flaky shape. The cells were small and round, with uniform size and round or occasionally irregular nuclei. Additionally, nucleoli and residual germinal centers were observed. Proliferation of interstitial fibrous vascular cells was detected. Immunohistochemistry results were as follows: CD3(+), CD79a(+), CD5(+), Bcl-2(+), CD34(++), TdT(+), CK(-), CD43(++), cyclin D1(-), CD20(-), CD10(-), Bcl-6(-), MPO(-), and Ki-67 (approximately 70%). Follicular dendritic cells were CD21(+) and CD23(+). The findings of immunohistochemistry were consistent with T-cell T-LBL/leukemia. The FISH test of the lymph node specimens was positive for the BCR-ABL fusion gene in the tumor cells (Figure 6).
There were no remarkable findings.
Focal lymphoblastic transformation of CML developed into erythroid leukemia.
The patient was admitted to the hospital. Results of BM aspiration tests revealed progression into pure erythroid leukemia following blastic transformation. Due to the ineffectiveness of imatinib and dasatinib, a switch was made to flumatinib after hospitalization. Chemotherapy based on omoharringtonine (HHT) was administered, which resulted in hematological improvement to some extent. The pancytopenia improved, with a significant increase in the total platelet count. However, the CML mutation gene test revealed multiple new gene mutations, including the T315I mutation of BCR-ABL and abnormalities in multiple karyotypes of chromosomes.
Eventually, treatment with flumatinib proved to be unsuccessful. Therefore, we strongly recommended allogeneic hematopoietic stem cell transplantation as soon as possible.
The pathology reports of the patient’s lymph nodes indicated lymphoblastic leukemia, and accordingly, the involved lymph node tissues were removed. Morphological and immunological assessments of tumor cells revealed the presence of the BCR-ABL fusion gene in the FISH test. The results were consistent with CML, and the Ph chromosome was detected. The lymph node mass disappeared after treatment with imatinib, without the need for the chemotherapy that is typically used for acute lymphoblastic leukemia (ALL) or lymphoma. This further confirmed that the patient’s lymph node blastoma was of the focal acute lymphoblastic type induced by the transformation of CML.
With regard to the mechanism of CML involving lymphocytes, the involvement of T cells in CML is widely believed to be due to a common progenitor cell type called CFU-GEMMT, which can differentiate and develop into myeloid cells and T cells in CML patients[1]. Furthermore, CML-Ph-positive T cells have been identified in vitro in the BM cells of chronic-phase CML patients, indicating the involvement of the T-cell lineage in CML[2]. A crucial diagnostic criterion for extramedullary T-LBL/ALL secondary to CML is evidence of blast proliferation in extramedullary tissues. Thus, the morphological, histochemical, and immunophenotypic characteristics of the blasts should align with the features of precursor thymic T cells[3]. Additionally, confirmation of the presence of the Ph or BCR-ABL fusion gene through cellular and molecular genetic assessments is necessary to establish the diagnosis. Genetic diagnosis relies on karyotyping to confirm extramedullary lesions (mostly lymph nodes). However, a single positive detection of the Ph chromosome or BCR-ABL fusion gene using Southern blot or quantitative real-time polymerase chain reaction (RT-PCR) analysis cannot be considered as a piece of conclusive evidence of tumor cells. This is because CML is a clonal disease of pluripotent hematopoietic stem cells. Normal WBCs, including lymphocytes and myeloid cells, in chronic-phase CML patients can also carry the Ph chromosome or BCR-ABL fusion gene, which may be detected when cells of extramedullary lesions are mixed with some CML clonal cells. On the other hand, true tumor cells such as lymphoma cells may test negative. Ichinohasama et al[4] reported two cases of chronic-phase CML patients with secondary Ph(-) NHL. These patients exhibited lymph node enlargement at 33 and 10 mo after the CML diagnosis and pathological examination of the lymph nodes revealed diffuse, large B-cell lymphoma and anaplastic large cell lymphoma, respectively. Southern blot and RT-PCR analysis confirmed the rearrangement of BCR-ABL fusion genes in the BM and lymph node tissues in these two patients. However, FISH testing of the lymph node specimens revealed that the gene fusion signals originated from lymphocytes with normal morphology and karyotype, while the lymphoma cells themselves yielded negative signals. This evidence proved the diagnosis of Ph(-)NHL secondary to CML in these two cases. Therefore, in CML cases presenting with lymph node tumors, it is crucial to differentiate between CML-induced lymphoblastic transformation and secondary NHL.
In our patient, the diagnosis was CML, with extramedullary T-LBL/ALL transformation, as confirmed by FISH. The presence of primitive cells was initially identified through Giemsa (Uiemsa) staining of the lymph node sections, followed by the FISH test to confirm the presence of BCR-ABL fusion signals in the primitive cells. If the FISH test confirms that the tumor cells in the extramedullary lesions carry CML markers, a diagnosis of CML with extramedullary T-LBL transformation can typically be made. In the present case, instead of primary Ph(+)T-ALL, the patient had CML in chronic phase with secondary blastic transformation in the extramedullary lymph nodes, as evident from the clear manifestation of CML in the BM without any signs of T-ALL. Furthermore, FISH analysis of the patient’s BM and lymph node biopsy revealed the presence of M-BCR (p210) type breakpoints, whereas Ph(+)ALL is typically associated with the m-BCR (p190) type breakpoints[5]. This finding further supports the conclusion that the Ph(+)T-ALL in this case was not primary.
Our patient initially presented with chronic phase CML, which then progressed to the accelerated phase and eventually pure erythroid leukemia, which is consistent with the evolution of CML in the BM[6]. CML can persist in the chronic phase for 3 to 5 years before advancing to the accelerated phase and blast crisis[7]. In this case, the patient entered the accelerated phase after 5 years of treatment, with 15.5% of blasts in the BM and a depletion of erythroid cells. Following 3 mo of dasatinib treatment, the patient entered the blast crisis. Morphological changes were observed, showing the transition from the absence of erythroid cells during the accelerated phase to erythroid hyperplasia during the blast crisis. The hyperplasia predominantly affected erythroid precursor cells and giant multinucleated young RBCs, indicating the development of pure erythroid leukemia, which is an uncommon form of acute transformation in CML. This significant shift in morphological characteristics occurred within 3 mo of acceleration, which is clinically rare.
Approximately 2/3 to 3/4 of acute transformations in CML are myeloid, while 1/3 to 1/4 are lymphoid[8]. Cases of transformation into AEL induced by a myeloid transformation in CML have been rarely reported in the literature. AML-M6, also known as acute erythroleukemia, was initially described by Di Guglielmo[9], and later by Dameshek and Baldini[10]. AML-M6 is classified into the phases of simple erythroid hyperplasia, erythroid-granulated mixed hyperplasia, and mononuclear granulated hyperplasia. According to the French-American-British classification system, the diagnostic criteria for acute leukemia require the presence of ≥ 30% blasts. The World Health Organization (WHO) defines acute erythroleukemia as a possible erythroid dysplasia syndrome, excluding cases related to leukemia or erythropoietin treatment. AML-M6 is defined by the WHO as acute leukemia with erythroid hyperplasia. Two main types of acute leukemia are recognized, according to the presence or absence of myeloid (granulated) components - erythroid/granulated type of erythroleukemia and pure erythroid leukemia. The erythroid/granulated type of erythroleukemia is characterized by BM erythroid precursor cells that account for ≥ 50% of nuclear cells and primitive granulocytes that account for ≥ 20% of non-erythroid cells. In contrast, pure erythroid leukemia is defined by immature erythroid hyperplasia accounting for > 80% of BM cells[11]. Our patient’s BM shows a proportion of primitive RBC exceeding 90%, confirming the diagnosis of pure erythroid leukemia.
The presence of additional chromosomes, aside from the Ph chromosome, in the accelerated phase or blast crisis is indicative of a poor prognosis. The appearance of extra chromosomes often precedes the clinical blast crisis by 2-4 mo. Common chromosomal aberrations include +8, +19, and i(17q)[12], which primarily occur during myeloblastic transformation. However, other aberrations in acute lymphoblastic transformation have rarely been reported. In the present case, multiple complex extra karyotype changes were identified during the transition to erythroleukemia. The occurrence of these complex karyotype changes may be an important reason for the rapid development of secondary erythroleukemia and may also serve as a high-risk factor for the poor prognosis of AEL[13].
The patient initially presented with a combination of CML and T-LBL/ALL. Treatment with imatinib led to the disappearance of the extramedullary T-LBL/ALL without any relapse, which indicates the reliable therapeutic effect of imatinib alone in treating CML cases with secondary T-LBL/ALL transformation. This also confirms that T-LBL/ALL can occur secondarily to CML. Additionally, in our patient, CML progressed gradually to the accelerated phase and eventually underwent an acute transformation to erythroleukemia. The treatment response to dasatinib, a second-generation tyrosine kinase inhibitor (TKI), was poor, while flumatinib had an even worse effect. Therefore, TKIs can be considered as the main treatment for both the acute lymphoid and acute myeloid transformations of CML, despite their low curative effect. However, secondary TKI resistance remains a challenge for patients with blasts, particularly after the T315I mutation. Bonatinib has been shown to have efficacy in CML with T351i mutation, but its effectiveness for CML blast crisis with multiple gene mutations and complex karyotypes, still requires more evidence-based medical support. Moreover, bonatinib is expensive and requires long-term administration, which may make it difficult for patients to accept. HHT can temporarily control disease progression in the short term, but will eventually fail, with an effectiveness rate of only around 30%. Allogeneic hematopoietic stem cell transplantation is considered as the only therapeutic modality useful for the control or even cure of such cases with dual blasts. We intend to continue observing the patient for subsequent treatment response.
This rare case of focal lymphoblastic transformation secondary to acute myeloblastic transformation of CML to erythroid leukemia gives us insight into the clinical presentation, investigative findings, and treatment in such cases.
We would like to thank KINGMED for Clinical Laboratory for medical testing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kupeli S, Turkey; Sultana N, Bangladesh S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Yu HG
| 1. | Michor F. Chronic myeloid leukemia blast crisis arises from progenitors. Stem Cells. 2007;25:1114-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Soliman DS, Amer AM, Mudawi D, Al-Sabbagh Z, Alkuwari E, Al-Sabbagh A, Ibrahim F, Yassin MA. Chronic Myeloid Leukemia with cryptic Philadelphia translocation and extramedullary B-lymphoid blast phase as an initial presentation. Acta Biomed. 2018;89:38-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Jain N, Lamb AV, O'Brien S, Ravandi F, Konopleva M, Jabbour E, Zuo Z, Jorgensen J, Lin P, Pierce S, Thomas D, Rytting M, Borthakur G, Kadia T, Cortes J, Kantarjian HM, Khoury JD. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood. 2016;127:1863-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 246] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 4. | Ichinohasama R, Miura I, Takahashi N, Sugawara T, Tamate E, Endoh K, Endoh F, Naganuma H, DeCoteau JF, Griffin JD, Kadin ME, Ooya K. Ph-negative non-Hodgkin's lymphoma occurring in chronic phase of Ph-positive chronic myelogenous leukemia is defined as a genetically different neoplasm from extramedullary localized blast crisis: report of two cases and review of the literature. Leukemia. 2000;14:169-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Khan M, Siddiqi R, Tran TH. Philadelphia chromosome-like acute lymphoblastic leukemia: A review of the genetic basis, clinical features, and therapeutic options. Semin Hematol. 2018;55:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Russo D, Garcia-Gutierrez JV, Soverini S, Baccarani M. Chronic Myeloid Leukemia Prognosis and Therapy: Criticisms and Perspectives. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Boddu P, Benton CB, Wang W, Borthakur G, Khoury JD, Pemmaraju N. Erythroleukemia-historical perspectives and recent advances in diagnosis and management. Blood Rev. 2018;32:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Dong Y, Shi O, Zeng Q, Lu X, Wang W, Li Y, Wang Q. Leukemia incidence trends at the global, regional, and national level between 1990 and 2017. Exp Hematol Oncol. 2020;9:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 9. | Di Guglielmo G. [Erythroleukemia]. Prog Med (Napoli). 1953;9:97-101. [PubMed] |
| 11. | Park S, Picard F, Azgui Z, Viguie F, Merlat A, Guesnu M, Leblond V, Dreyfus F. Erythroleukemia: a comparison between the previous FAB approach and the WHO classification. Leuk Res. 2002;26:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Ryu S, Park HS, Kim SM, Im K, Kim JA, Hwang SM, Yoon SS, Lee DS. Shifting of erythroleukemia to myelodysplastic syndrome according to the revised WHO classification: Biologic and cytogenetic features of shifted erythroleukemia. Leuk Res. 2018;70:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Jondreville L, Krzisch D, Chapiro E, Nguyen-Khac F. The complex karyotype and chronic lymphocytic leukemia: prognostic value and diagnostic recommendations. Am J Hematol. 2020;95:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |