Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5772
Peer-review started: May 12, 2023
First decision: May 31, 2023
Revised: June 18, 2023
Accepted: July 11, 2023
Article in press: July 11, 2023
Published online: August 26, 2023
Processing time: 104 Days and 17.8 Hours
Mantle cell lymphoma (MCL) of the gastrointestinal tract is a rare malignancy, accounting for about 0.2% of malignant colorectal tumors. MCL synchronous with rectal adenocarcinoma is extremely rare. We know of only a few cases reported in the literature. We describe the case of a patient with synchronous rectal adenocarcinoma and intestinal MCL.
A 63-year-old man was admitted to our hospital due to abdominal pain and hematochezia over the past month. The patient was diagnosed with middle rectal cancer cT2N0M0 and underwent surgery. However, we found a large tumor in the small intestine during surgery. The patient underwent total mesorectal excision for rectal cancer and resectioning of the ileal segment containing the large mass. Pathology and immunohistochemistry revealed the presence of both rectal adenocarcinoma and pathognomonic MCL stage IIE presenting as multiple lymphomatous polyposis. The patient subsequently underwent RDHAP/RCHOP chemotherapy and was maintained with rituximab. A Positron Emission Tomography and Computed Tomography (PET/CT) scan showed that the disease responded well to treatment without tumor-increased metabolism in the gastrointestinal tract.
Synchronous rectal adenocarcinoma and intestinal MCL presenting as multiple lymphomatous polyposis are extremely rare. MCL is often discovered fortuitously when rectal cancer is diagnosed. The coexistence of these tumors poses treatment challenges.
Core Tip: Mantle cell lymphoma (MCL) of the gastrointestinal tract is an uncommon malignancy. Rectal adenocarcinoma is more common, but the coexistence of both is extremely rare. Their diagnosis should be based on histopathological and immunohistochemical features. MCL is a typically aggressive disease and has a poorer prognosis than adenocarcinoma. The treatment of two different simultaneously occurring cancers is a challenge, as it depends on the stage, prognosis, and response of each disease to treatment.
- Citation: Vu KV, Trong NV, Khuyen NT, Huyen Nga D, Anh H, Tien Trung N, Trung Thong P, Minh Duc N. Synchronous rectal adenocarcinoma and intestinal mantle cell lymphoma: A case report. World J Clin Cases 2023; 11(24): 5772-5779
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5772.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5772
Mantle cell lymphoma (MCL) of the gastrointestinal tract is a rare malignancy that accounts for about 3%-10% of B-cell non-Hodgkin lymphomas. MCL restricted to the small and large intestine is even more uncommon, accounting for about 0.2% of malignant colorectal tumors[1,2]. Previous reports have shown that MCL is typically present as multiple small- and medium-sized polyps. The disease is characterized by a chromosomal t(11,14) translocation resulting in cyclin D1 overexpression[3]. Furthermore, MCL synchronously accompanied by adenocarcinoma of the colorectum is extremely rare. To our knowledge, only a few cases have previously been reported in the literature[4-8]. This coexistence raises important questions about the mechanism of carcinogenesis, diagnosis, and optimal treatment.
In this report, we present a case of a 63-year-old male patient who was diagnosed with both rectal adenocarcinoma and intestinal MCL, which presented as multiple lymphomatous polyposis. We discuss the challenges in diagnosing and treating these two co-occurring cancers.
A 63-year-old man was admitted to our hospital due to abdominal pain and hematochezia over the past month.
A month prior to presenting at the hospital, the patient began experiencing episodes of cyclical abdominal pain around the navel, with each episode lasting approximately ten minutes before subsiding. In addition to the pain, the patient also reported passing fresh red blood in their stool. These symptoms were not associated with food consumption.
No past illnesses were reported.
No personal history or family history of colorectal polyps or colorectal cancer was recorded.
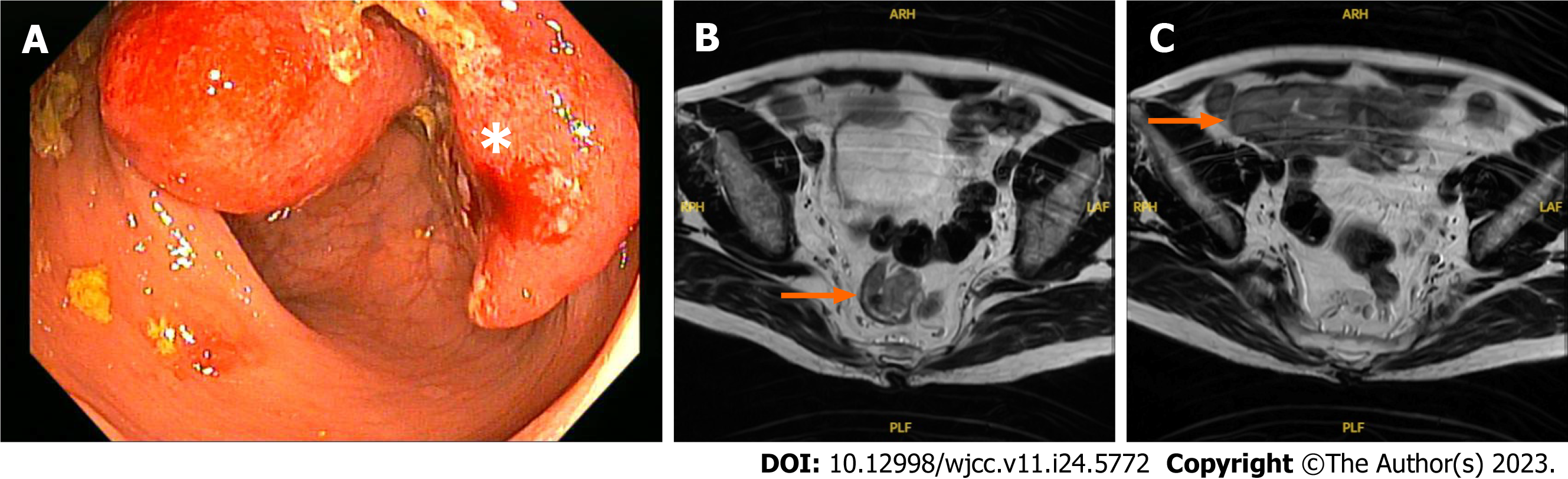
Physical examination showed a soft abdomen and no palpable lymphadenopathy. Digital rectal examination and colonoscopy revealed an ulcerated mass occupying 50% of the circumference of the rectal wall 8 cm from the anal margin. No polyp was detected, and a lesion biopsy demonstrated moderately differentiated adenocarcinoma (Figure 1A). Upper gastrointestinal endoscopy showed no abnormality.
The initial hematologic evaluation obtained the following values: hemoglobin 154 g/L; hematocrit 46.4%; white blood cell count 8000/µL; platelet count 270 × 103/µL; uric acid 286 µmol/L; lactate dehydrogenase (LDH) 116 UI/L; and beta-2-microglobulin 1.44 mg/L. All values were within the normal range.
A pelvic magnetic resonance imaging (MRI) scan showed a middle rectal tumor growing into the muscularis propria, MRF (-), EMVI (-), and an abnormal mass 4 cm × 6 cm in size in the right iliac fossa thought to be an ileal mass (Figure 1B and C). The spleen and liver were unremarkable on the CT scan.
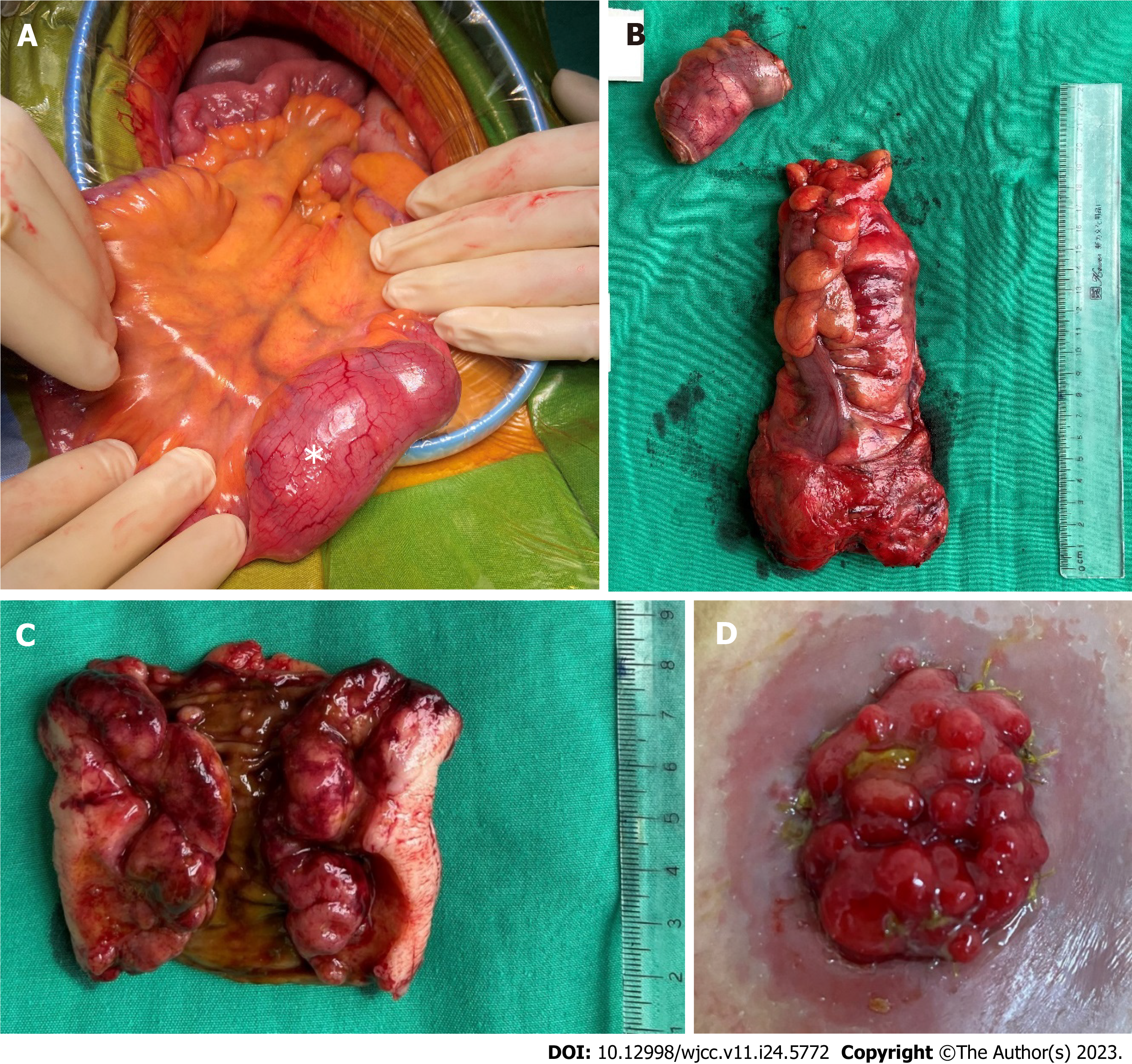
The patient was diagnosed as having a rectal adenocarcinoma and an ileal tumor, which made him suffer abdominal pain cycles. A low anterior resection for rectal cancer was performed to confirm the diagnosis. The primary surgeon was a colorectal cancer expert with 30 years of experience at the national cancer hospital. The intraoperative evaluation showed that in addition to the rectal tumor, both the ileum and the colorectum had multiple polyps ranging in size from 2 to 5 mm. There was also an enormous ileal polyp measuring 4 cm × 6 cm about 15 cm away from the ileocecal valve; therefore, it was further decided to resect the ileal segment containing the large polyp and create a temporary ileostomy (Figure 2).
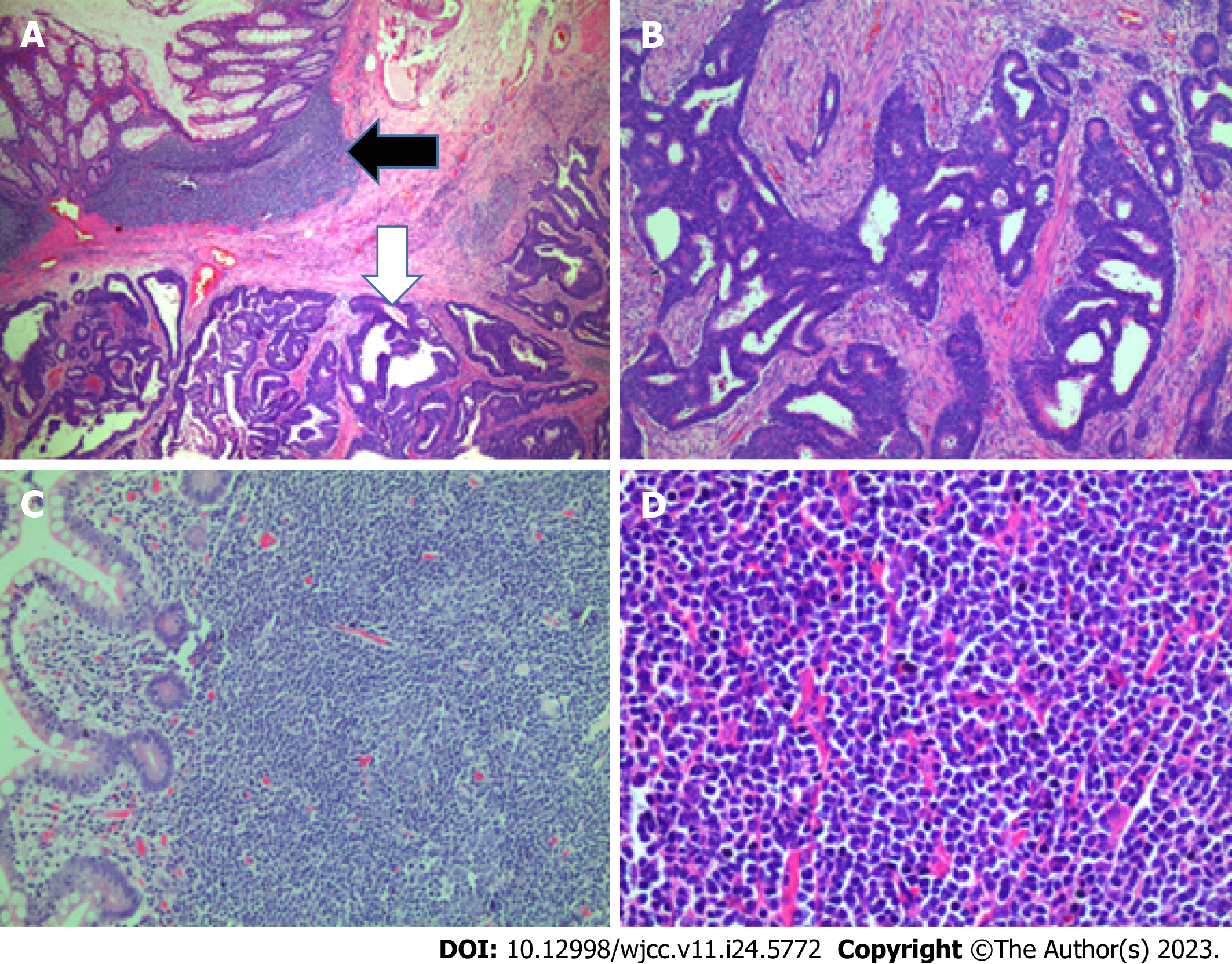
The rectal segment showed a large ulcerative mass, measuring 3.5 cm × 3 cm, a moderately differentiated adenocarcinoma invading into the perirectal fibrous tissue and metastasizing to 2 lymph nodes (pT2N1). The remaining colorectal mucosa had polypoid masses ranging in size from 2 to 5 mm. On microscopic sections, these polyps were shown covered by a cytologically bland columnar epithelium, and the submucosal layer showed diffuse proliferation of lymphocytes with small- or medium-sized nuclei, narrow cytoplasm, and the occurrence of mitosis, suggesting lymphoma.
The resected ileal segment showed a large polypoid mass measuring 6 cm × 5.5 cm with a white-pink cut surface, and the remaining mucosa had small multiple polypoid masses. These polyps showed features similar to those of the polyps in the colon. In addition, a small lymph node was involved with the lymphoma (Figure 3).
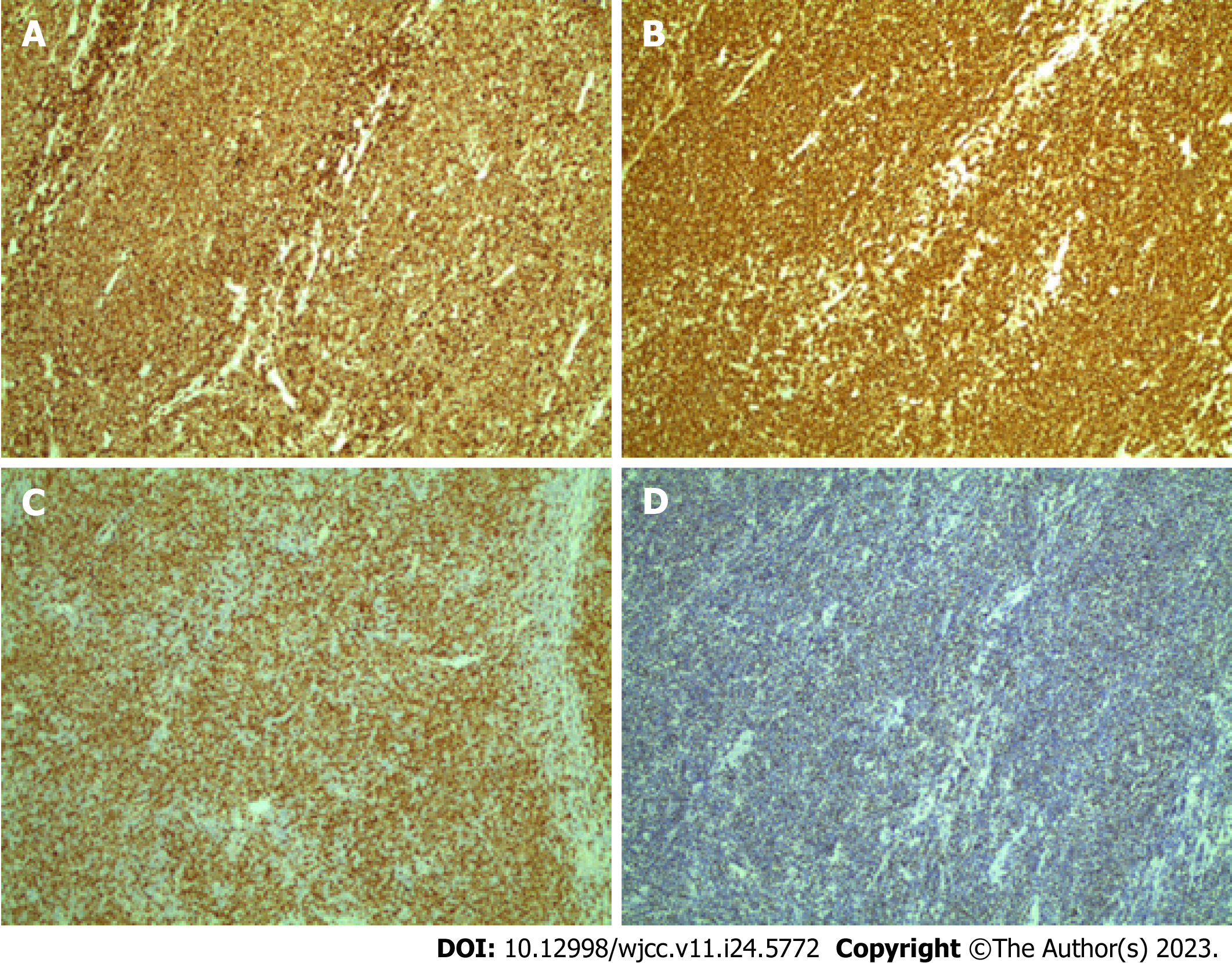
Immunohistochemical analysis of the lymphoid cells showed diffuse positive reactions for CD5, CD20, cyclin D1, and SOX11 (Figure 4) and negative reactions for CD3, CD10, CD23, MUM1, and Bcl6. The tumor cell proliferation index was 70% according to Ki-67 staining. These findings indicated pathognomonic MCL presenting as multiple lymphomatous polyposis (MLP).
A subsequent bone marrow biopsy did not show any abnormality. The postoperative diagnosis was confirmed as synchronous stage IIIa rectal adenocarcinoma and MCL presenting as MLP (Lugano stage IIE). According to the combined Mantle Cell Lymphoma International Prognostic Index (MIPI-c), the patient was classified as high-intermediate risk. Our multi-disciplinary tumor board with surgeons and medical and radiation oncologists decided to treat the MCL with an RDHAP/RCHOP regimen. The patient completed RDHAP/RCHOP chemotherapy and underwent maintenance rituximab.
After the patient completed the induction treatment, a whole-body positron emission tomography (PET)/computed tomography (CT) scan was performed for re-evaluation showed a complete response with no evidence of hypermetabolic lesions present throughout the body. The patient then continued with maintenance treatment and would undergo regular follow-up evaluations every three months to monitor their progress.
MCL is derived from B cell non-Hodgkin lymphoma characterized by chromosomal t(11:14) (q13:32) translocation resulting in overexpression of cyclin D1[3]. Lymphadenopathy occurs in 90% of cases and frequently involves extranodal sites such as bone marrow, the spleen, gastrointestinal tract, Waldeyer’s ring, and lungs[1]. MCL of the gastrointestinal tract is an uncommon malignancy that accounts for 3%-10% of B-cell non-Hodgkin lymphomas and only 0.2% of colorectal malignancies[1,2,9]. In 1984, Isaacson first described and distinguished MCL from other primary gas-trointestinal lymphomas as multiple lymphomatous polyposis because of the predominant polyposis presentation and worse prognosis[10]. In contrast, rectal adenocarcinoma is the third most common cancer after breast and lung cancer. The coexistence of carcinoma at multiple sites in the gastrointestinal tract is uncommon, accounting for about 2%-7% of cases[11].
Synchronous MCL and colorectal adenocarcinoma are extremely rare. Sasaki reviewed 19 cases of coexistence of primary malignant lymphoma and adenocarcinoma in the colorectum based on the literature from 1947 to 2010; of these cases, only three reported MCL[12]. Based on Sasaki’s report and a search on PubMed, we have compiled six previous clinical cases presented in Table 1. Most patients were male, with adenocarcinoma located in the rectum in three cases, the right colon in three cases, and the sigmoid colon in one case. Meanwhile, MCL usually presents as multiple nodules. There was one case of a 54-year-old male patient reported by Kanehira with MCL presenting as a single polyp measuring 4 cm × 3 cm × 1 cm[5]. Most cases of MCL were at a late stage (stage IV accounted for half of the reported cases), while in our case MCL was at stage II. The relationship between rectal adenocarcinoma and gastrointestinal MCL remains unclear. In previous reports, lymphoma appeared one to three years after treatment for adenocarcinoma, but there was no significant association[13,14]. The coexistence of these tumors may be fortuitous rather than showing a specific association[6].
| Ref. | Age/Sex | MCL location | Distribution | MCL stage | Carcinoma location | Stage | Treatment |
| Hopster et al[4], 1995 | 74/F | Rectum, colon and ileal | Rectum | Panproctolectomy | |||
| Kanehira et al[5], 2001 | 54/M | Terminal ileum | Single polyp | I | Ascending colon | IV (hepatic metastasis) | Right hemicolectomy + chemotherapy |
| Kanehira et al[5], 2001 | 74/M | Sigmoid | Multiple small nodules | IV (Bone marrw) | Rectum | III (T3N1) | Low anterior resection + chemoradiation |
| Padmanabhan et al[6], 2003 | 85/M | Colon and ileal | Multiple small nodules | II | Ascending colon | II (T3N0) | Right hemicolectomy |
| Sztarkier et al[7], 2009 | 80/M | Colon | Multiple small nodules | IV (Bone marrow) | Sigmoid colon | III (T3N2) | Sigmoidectomy + R-CHOP |
| Hrudka et al[8], 2016 | 82/M | Colon | Multiple small nodules | IV | Ascending colon | II (T3N0) | Right hemicolectomy + chemotherapy |
| Present case | 63/M | Rectum, colon, and ileal | Multiple nodules | II | Rectum | III | Low anterior resection |
Rectal adenocarcinoma frequently presents with abdominal pain, hematochezia, change in stool shape, and obstructive symptoms such as constipation, abdominal distention, and colic. In contrast, intestinal MCL is more subtle and nonspecific; patients sometimes present with abdominal pain, weight loss, diarrhea, lymphadenopathy, or B symptoms including fever, night sweats, and weight loss[6,15,16]. Our patient was admitted due to abdominal pain and hematochezia, with no peripheral lymphadenopathy or B symptoms on physical examination. Cyclical abdominal pain with increased bowel movements may suggest Koenig’s syndrome, which signifies an incomplete small bowel obstruction. Although MRI revealed no dilated bowel loop or air-fluid level, there was an enormous mass in the ileum. Therefore, the patient underwent surgery to confirm the diagnosis.
On histopathology, the tumor showed a monotonous population of small-to-medium-sized lymphoid cells with irregular nuclear contours, condensed chromatin, and scanty cytoplasm[17,18]. The diagnosis of MCL is often confirmed based on a combination of immunophenotypic characteristics of tumor cells, such as co-expression of CD5, CD20, cyclin D1, and SOX-11 but lack of expression of CD23. In our case, lymphoid cells were positive for CD5, CD20, cyclin D1, and SOX-11. CD20 is a receptor located on B cells and is usually permanently positive even after treatment with anti-CD20 treatment or relapse. Cyclin D1 is a hallmark of MCL (due to > 95% expression) because it is a direct result of t(11:14) translocation, and thus, expression of cyclin D1 on B cells helps to confirm the diagnosis of MCL[18]. In addition, a subgroup of MCLs that lacks cyclin D1 expression because of the absence of t(11:14) translocation is always reactive to SOX-11. SOX-11 is a member of the SOX gene family of transcription factors and is strongly expressed in both positive and negative cyclin D1 groups of MCL, as well as in several other subtypes such as lymphoblastic lymphoma, Burkitt lymphoma, T-cell prolymphocytic leukemia, and anaplastic large-cell lymphoma[19].
MCL is a typically aggressive disease with short-term treatment responses and poor overall survival (OS) compared to other B-cell lymphoma subtypes[20,21]. Survival depends on patient characteristics and histopathological factors. Hoster et al[22] used these features to develop the combined MIPI-c, which is based on age, performance status, LDH level, leukocyte count, and cell proliferation (Ki-67). Our patient had an MIPI-c score of 5.7 and a Ki67 index of 70%, values defining a high-intermediate prognostic group with a five-year OS of 43% despite aggressive treatment[22]. In contrast, rectal adenocarcinoma has a better prognosis than MCL, depending on the tumor stage. The five-year OS of stage IIIa rectal cancer is 73%[23].
The simultaneous occurrence of two different cancers poses treatment challenges. Chemotherapy plays a primary role in the treatment of MCL, which has a poor prognosis but often responds well to chemotherapy. On the other hand, surgery is the principal treatment for rectal adenocarcinoma and chemoradiotherapy has a neoadjuvant/adjuvant role in the advanced stage. Our multi-disciplinary tumor board decided to treat the patient for MCL first for the following reasons. Firstly, although postoperative adjuvant radiochemotherapy is indicated for stage IIIa rectal cancer, our patient underwent total mesorectal excision with a good plane achieved by the lead doctor and a negative rectal and circumferential resection margin. Histopathological findings indicated pT2 tumor stage and metastasis to two mesorectal lymph nodes < 5 mm in size. Recent studies have shown that the local recurrence rate is not associated with metastasis status of the mesorectal lymph node if the total mesorectal excision is of high quality and the preoperative MRI is favorable (tumors T2/T3ab and negative EMVI)[24,25]. Secondly, patients with stage IIE MCL in the high-intermediate risk group receive an RDHAP/R-CHOP regimen lasting about five months. While adjuvant treatment for rectal cancer should be started within eight weeks of surgery, prolongation reduces the effectiveness of treatment. Gao et al[26] showed that the delaying of adjuvant therapy reduced survival outcomes (hazard ratio (HR) = 1.222 for 9-12 wk, HR = 1.252 for 13-16 wk, and HR = 1.969 for > 16 wk vs within eight weeks) and that adjuvant therapy did not improve survival when delayed beyond five months. Our patient completed RDHAP/RCHOP chemotherapy and received maintenance rituximab. A PET/CT scan was performed for re-evaluation showed a complete response with no hypermetabolic lesions present throughout the body.
Although MCL responds well to treatment, it is still prone to recurrence in the future. When this happens, the optimal approach to relapsed or refractory disease remains to be defined. Some of the treatment options available for second-line therapy include monoclonal antibodies, bispecific antibodies, anti-PD-L1 antibodies, lenalidomide, BTK inhibitors, BCL2 inhibitors, epigenetic regulators, PI3K inhibitors, PI3K/mTOR inhibitors, and CAR-T cell therapy[27]. These treatments have recently shown high efficacy in malignant lymphomas, especially nodal lymphomas. However, the choice of the most optimal treatment option is a complex decision that depends on various factors such as the availability of treatments in each locality.
Synchronous rectal adenocarcinoma and intestinal MCL presenting as multiple lymphomatous polyposis are extremely rare. MCL is often discovered incidentally when rectal cancer is diagnosed. Their diagnosis should be based on histopathological and immunohistochemical features. MCL is a typically aggressive disease and has a poorer prognosis than adenocarcinoma. The treatment of two different simultaneously occurring cancers is a challenge.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Viet Nam
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Ani RM, Iraq; Watanabe T, Japan S-Editor: Liu JH L-Editor: A P-Editor: Ji MX
| 1. | Grimm KE, O'Malley DP. Aggressive B cell lymphomas in the 2017 revised WHO classification of tumors of hematopoietic and lymphoid tissues. Ann Diagn Pathol. 2019;38:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Shepherd NA, Hall PA, Coates PJ, Levison DA. Primary malignant lymphoma of the colon and rectum. A histopathological and immunohistochemical analysis of 45 cases with clinicopathological correlations. Histopathology. 1988;12:235-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Li JY, Gaillard F, Moreau A, Harousseau JL, Laboisse C, Milpied N, Bataille R, Avet-Loiseau H. Detection of translocation t(11;14)(q13;q32) in mantle cell lymphoma by fluorescence in situ hybridization. Am J Pathol. 1999;154:1449-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 133] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Hopster D, Smith PA, Nash JR, Elders K, Poston GJ. Synchronous multiple lymphomatous polyposis and adenocarcinomata in the large bowel. Postgrad Med J. 1995;71:443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Kanehira K, Braylan RC, Lauwers GY. Early phase of intestinal mantle cell lymphoma: a report of two cases associated with advanced colonic adenocarcinoma. Mod Pathol. 2001;14:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Padmanabhan V, Trainer TD. Synchronous adenocarcinoma and mantle cell lymphoma of the colon. Arch Pathol Lab Med. 2003;127:E64-E66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Sztarkier I, Levy I, Walfisch S, Delgado J, Benharroch D. Mantle cell lymphoma in a tubular adenoma: unusual presentation with synchronous colonic carcinoma. Ann Diagn Pathol. 2009;13:47-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Hrudka J, Eis V, Lisý P, Gürlich R, Mandys V. [Synchronous colorectal carcinoma and non-Hodgkin lymphoma - two case reports]. Rozhl Chir. 2016;95:369-372. [PubMed] |
| 9. | Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer. 2003;97:2462-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Isaacson PG, MacLennan KA, Subbuswamy SG. Multiple lymphomatous polyposis of the gastrointestinal tract. Histopathology. 1984;8:641-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 93] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Ekelund GR, Pihl B. Mutiple carcinomas of the colon and rectum. Cancer. 1974;33:1630-1634. [PubMed] [DOI] [Full Text] |
| 12. | Sasaki S, Hatanaka K, Sahara N, Uekusa T, Hirayama K, Shirahata A, Ishimaru M. Collision tumor of primary malignant lymphoma and adenocarcinoma in the colon: report of a case. Surg Today. 2010;40:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Yu Q, Liu QY, Wei DM, Luo DZ. Metachronous Sigmoid Carcinoma and Mantle Cell Lymphoma in Intestines. Case Rep Gastroenterol. 2019;13:17-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Liao MT, Cheng MF, Chang WC, Wu YC, Lee HS, Tsai SH. Duodenal mantle cell lymphoma in a patient with advanced sigmoid adenocarcinoma. South Med J. 2009;102:429-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Medeiros LJ, Miranda RN. Diagnostic Pathology: Lymph Nodes and Extranodal Lymphomas E-Book: Elsevier Health Sciences; 2023. Available from: https://www.elsevierhealth.com.au/diagnostic-pathology-lymph-nodes-and-extranodal-lymphomas-9780323847582.html. |
| 16. | Li HP, Zhang WS, He L, Hu H, Ren MQ, Liu XM, Xu LB, Tuo BG. [Clinical and endoscopic characteristics of gastrointestinal mantle cell lymphoma]. Zhonghua Yi Xue Za Zhi. 2022;102:3673-3679. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Braham E, Zarrouk M, Mlika M, Kilani T, El Mezni F. Synchronous mantle cell lymph node lymphoma and pulmonary adenocarcinoma: a case report with literature review. Clin Respir J. 2017;11:430-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Klapper W. Histopathology of mantle cell lymphoma. Semin Hematol. 2011;48:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Soldini D, Valera A, Solé C, Palomero J, Amador V, Martin-Subero JI, Ribera-Cortada I, Royo C, Salaverria I, Beà S, Gonzalvo E, Johannesson H, Herrera M, Colomo L, Martinez A, Campo E. Assessment of SOX11 expression in routine lymphoma tissue sections: characterization of new monoclonal antibodies for diagnosis of mantle cell lymphoma. Am J Surg Pathol. 2014;38:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Ruskoné-Fourmestraux A, Audouin J. Primary gastrointestinal tract mantle cell lymphoma as multiple lymphomatous polyposis. Best Pract Res Clin Gastroenterol. 2010;24:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Maddocks K. Update on mantle cell lymphoma. Blood. 2018;132:1647-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 22. | Hoster E, Rosenwald A, Berger F, Bernd HW, Hartmann S, Loddenkemper C, Barth TF, Brousse N, Pileri S, Rymkiewicz G, Kodet R, Stilgenbauer S, Forstpointner R, Thieblemont C, Hallek M, Coiffier B, Vehling-Kaiser U, Bouabdallah R, Kanz L, Pfreundschuh M, Schmidt C, Ribrag V, Hiddemann W, Unterhalt M, Kluin-Nelemans JC, Hermine O, Dreyling MH, Klapper W. Prognostic Value of Ki-67 Index, Cytology, and Growth Pattern in Mantle-Cell Lymphoma: Results From Randomized Trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34:1386-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 23. | Kozak KR, Moody JS. The impact of T and N stage on long-term survival of rectal cancer patients in the community. J Surg Oncol. 2008;98:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Chand M, Moran BJ, Jones RG, Heald RJ, Brown G. Lymph node status does not predict local recurrence in the total mesorectal excision era. Dis Colon Rectum. 2014;57:127-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, Sebag-Montefiore DJ, Tekkis P, Brown G; MERCURY study group. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 464] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 26. | Gao P, Huang XZ, Song YX, Sun JX, Chen XW, Sun Y, Jiang YM, Wang ZN. Impact of timing of adjuvant chemotherapy on survival in stage III colon cancer: a population-based study. BMC Cancer. 2018;18:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Zelenetz AD, Gordon LI, Chang JE, Christian B, Abramson JS, Advani RH, Bartlett NL, Budde LE, Caimi PF, De Vos S, Dholaria B, Fakhri B, Fayad LE, Glenn MJ, Habermann TM, Hernandez-Ilizaliturri F, Hsi E, Hu B, Kaminski MS, Kelsey CR, Khan N, Krivacic S, LaCasce AS, Lim M, Narkhede M, Rabinovitch R, Ramakrishnan P, Reid E, Roberts KB, Saeed H, Smith SD, Svoboda J, Swinnen LJ, Tuscano J, Vose JM, Dwyer MA, Sundar H. NCCN Guidelines® Insights: B-Cell Lymphomas, Version 5.2021. J Natl Compr Canc Netw. 2021;19:1218-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |