Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5729
Peer-review started: April 7, 2023
First decision: July 6, 2023
Revised: July 17, 2023
Accepted: August 2, 2023
Article in press: August 2, 2023
Published online: August 26, 2023
Processing time: 133 Days and 9.4 Hours
Uterine intravenous leiomyomatosis is defined as leiomyoma tissue invading the vein outside the leiomyoma. Reports of extension to the right pulmonary artery are relatively rare.
We describe a 31-year-old woman with a benign leiomyoma that extended into the right ventricular lumen, causing mechanical obstruction and corresponding clinical symptoms. Tumors located in the pulmonary artery can cause pulmonary artery obstruction. After diagnosis, surgical treatment should be performed as soon as possible.
In this case, the uterine leiomyoma extended to the right pulmonary system, which is clinically rare.
Core Tip: We describe a 31-year-old woman with a benign leiomyoma that extended into the right ventricular lumen, causing mechanical obstruction and corresponding clinical symptoms. Tumors located in the pulmonary artery can cause pulmonary artery obstruction, and once diagnosed, surgical treatment should be performed as soon as possible.
- Citation: Huang YQ, Wang Q, Xiang DD, Gan Q. Intravenous leiomyoma of the uterus extending to the pulmonary artery: A case report. World J Clin Cases 2023; 11(24): 5729-5735
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5729.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5729
Uterine intravenous leiomyomatosis patients have a clear clinical history of uterine fibroids. The leiomyoma seen by the naked eye extends into the vascular space and can grow in small, medium and large veins; benign leiomyoma tissues have been found to invade the vein outside the leiomyoma but rarely extend to the right ventricular lumen, causing mechanical obstruction and corresponding clinical symptoms[1]. Compared with 2 previous cases of leiomyoma invading the right atrium and right ventricle, the uterine leiomyoma in our case extended to the right pulmonary artery, which is clinically rare.
A 31-year-old female patient was admitted to the hospital due to “paroxysmal syncope for 4 d”. The patient suddenly felt chest tightness and fell due to syncope when she awakened in the morning 4 d previously. After approximately 2 min, she recovered spontaneously but felt exhausted after waking up. During the following 3 d, she had the same symptoms and recovered in the same way. To make a definite diagnosis, she was admitted to our department on November 27, 2022.
The patient felt chest tightness and fell due to syncope when she awakened in the morning 4 d previously.
The patient previously had a hysterectomy that was performed for a “hysteromyoma” in September 2020.
The patient denied any family history of malignant tumors.
The patient’s body temperature was 36.0 ℃, pulse rate was 100 bpm, respiratory rate was 21 breaths/min, and blood pressure was 100/85 mmHg (1 mmHg = 0.133 kPa). A 3 cm traumatic scratch was seen at the corner of the right eye; bilateral lung breath sounds were heavy; no obvious dry and wet rales were heard; and the heart boundary was not large, with low and blunt heart sounds and an even heart rhythm. A tumor flapping sound was heard in the tricuspid valve auscultation area with conduction limitation. The abdomen was flat and soft, the liver, spleen and ribs could not be palpated, and a 10 cm long transverse incision scar was seen below the umbilicus. There was no edema in either lower limb, and arterial pulses in the limbs were good. The vulva was normal, and the vaginal stump had healed well. There was a solid lump that was the size of a small duck egg on the right side of the pelvic cavity, with an unclear boundary on the left side, and was inactive. On the second day after admission, the patient suddenly lost consciousness when using the toilet and did not respond to calls. After approximately 10 s, she recovered spontaneously and had no abnormal vital signs. At 04:35 AM on November 31, 2022, the patient suffered syncope again, which was associated with defecating in bed, accompanied by cyanosis of the lips, without urinary and fecal incontinence. The patient’s syncope lasted for approximately 10 min. After successful bedside cardiopulmonary resuscitation, the patient was transferred to the intensive care unit for further treatment.
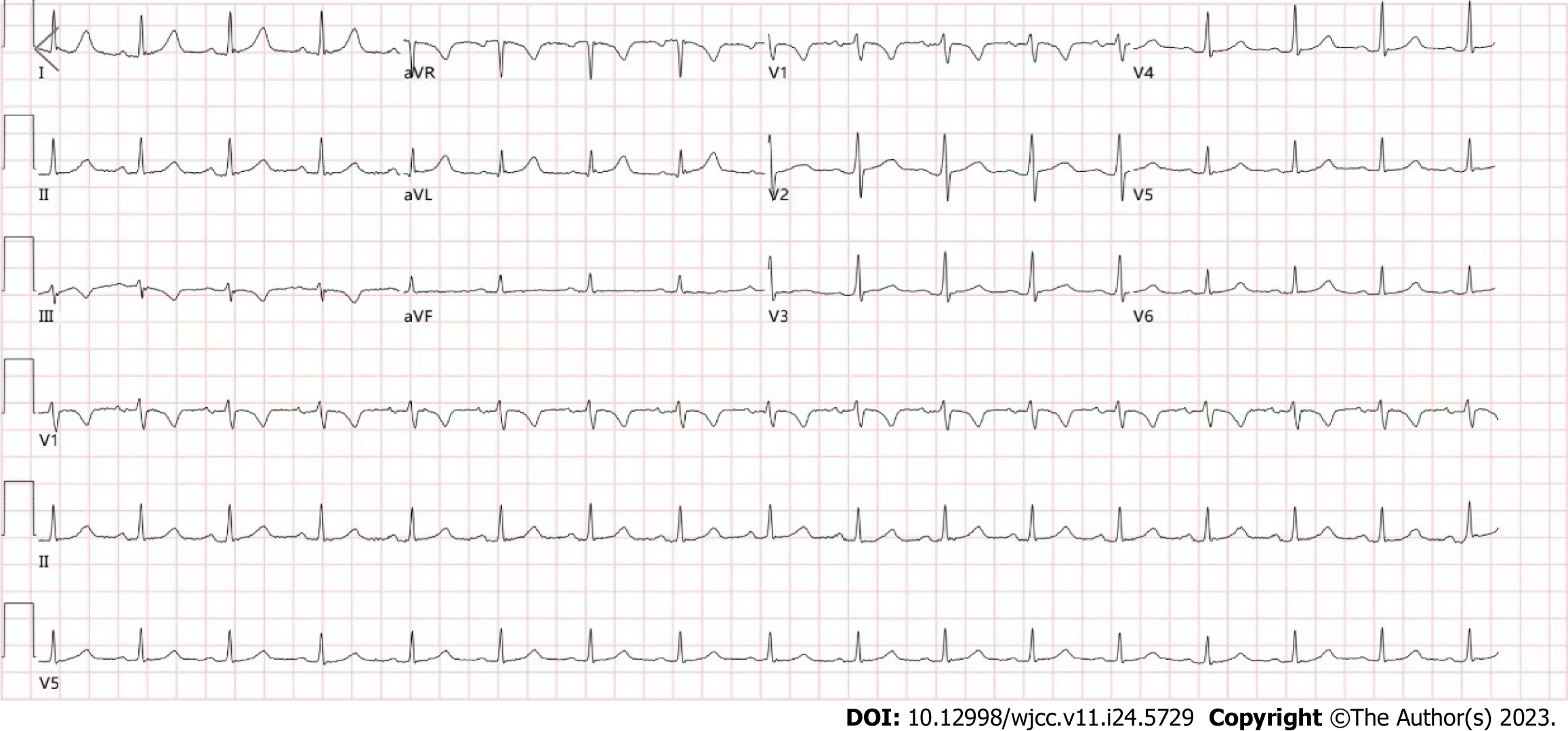
Routine blood examinations showed the following: white blood cell count 11.88 × 109/L; neutrophilic granulocyte percentage 78.11%. Routine urine and stool analyses, liver and kidney function, electrolytes, thyroid function, plasma D-2 polymer and B-type natriuretic peptide were basically normal. Coagulation analysis, carcinoembryonic antigen assay, alpha-fetoprotein assay, infectious disease screening test, high-sensitivity C-reactive protein assay, and erythrocyte sedimentation rate test were normal. Arterial blood gas analysis showed the following: Oxygen partial pressure of 147.7 mmHg and oxyhemoglobin saturation of 99.2%. Electrocardiogram (ECG) showed: (1) Sinus rhythm; and (2) ECG within the normal range (Figure 1).
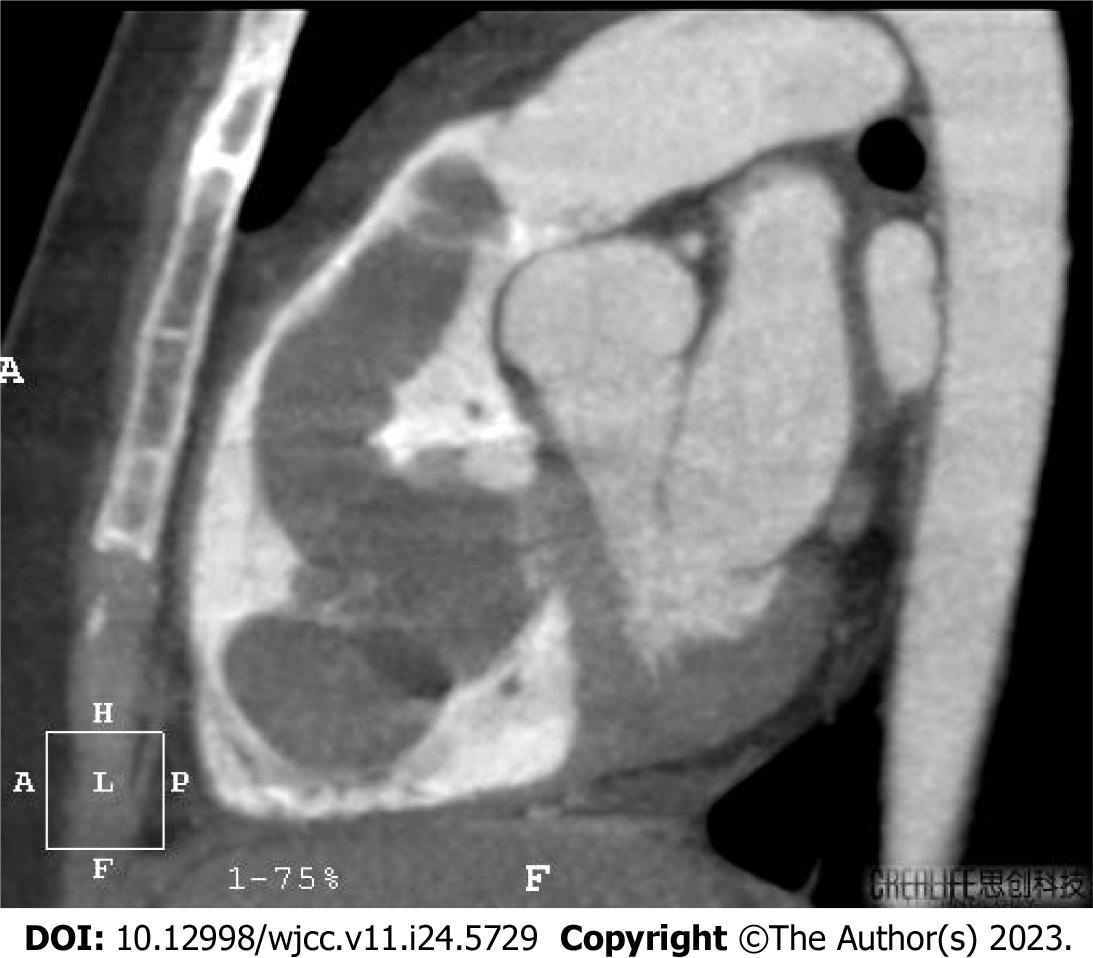
The right ventricle was relatively enlarged, and the left atrium and left ventricle were not enlarged. Multiple moderately heterogeneous echogenic nodules were observed in the right atrium and right ventricle, with loose internal structures and irregular boundaries, one of which was attached to the anterior valve body of the tricuspid valve, approximately 5.7 cm × 3.6 cm in size, and swung between the right atrium and right ventricle with the cardiac cycle. A number of these nodules were attached to the free wall of the right ventricle and prolapsed into the right ventricular outflow tract during the systolic phase, one of which was 3.8 cm × 2.1 cm in size, and no abnormal space was found in the proximal segment of the superior and inferior vena cava. A chest far-reaching radiograph showed the following: the lung texture was heavy, the aortic node was slightly wide, the pulmonary artery segment was flat, the right cardiac margin was round, the right atrium was enlarged, and the heart-chest ratio was 0.51. Abdominal and pelvic ultrasound showed no abnormalities on ultrasonography, no abnormalities in blood flow of the liver, gallbladder, spleen, pancreas and kidney, and after the hysterectomy, no obvious mass images were found in the bilateral adnexal areas. Computed tomography (CT) findings were as follows: In the proximal cardiac segment from the right iliac vein to the inferior vena cava, a cord-like low-density shadow was seen with irregular margins. On delayed scanning, the mass was enhanced. In the systolic phase, the mass protruded through the pulmonary valve into the main pulmonary trunk and right pulmonary artery, and in the diastolic phase, the mass retracted into the right ventricle. The pulmonary artery was not wide; the diameter of the main pulmonary artery was 26 cm; and a filling defect shadow was seen in the right upper pulmonary artery and pulmonary artery of the basal segment (Figure 2). An oval soft tissue shadow was seen in the upper right of the bladder in the pelvic cavity. The CT value was 78 HU, and the size was 58.8 cm × 32.9 cm.
Cardiac tumor and myxoma of the right atrium and right ventricle.
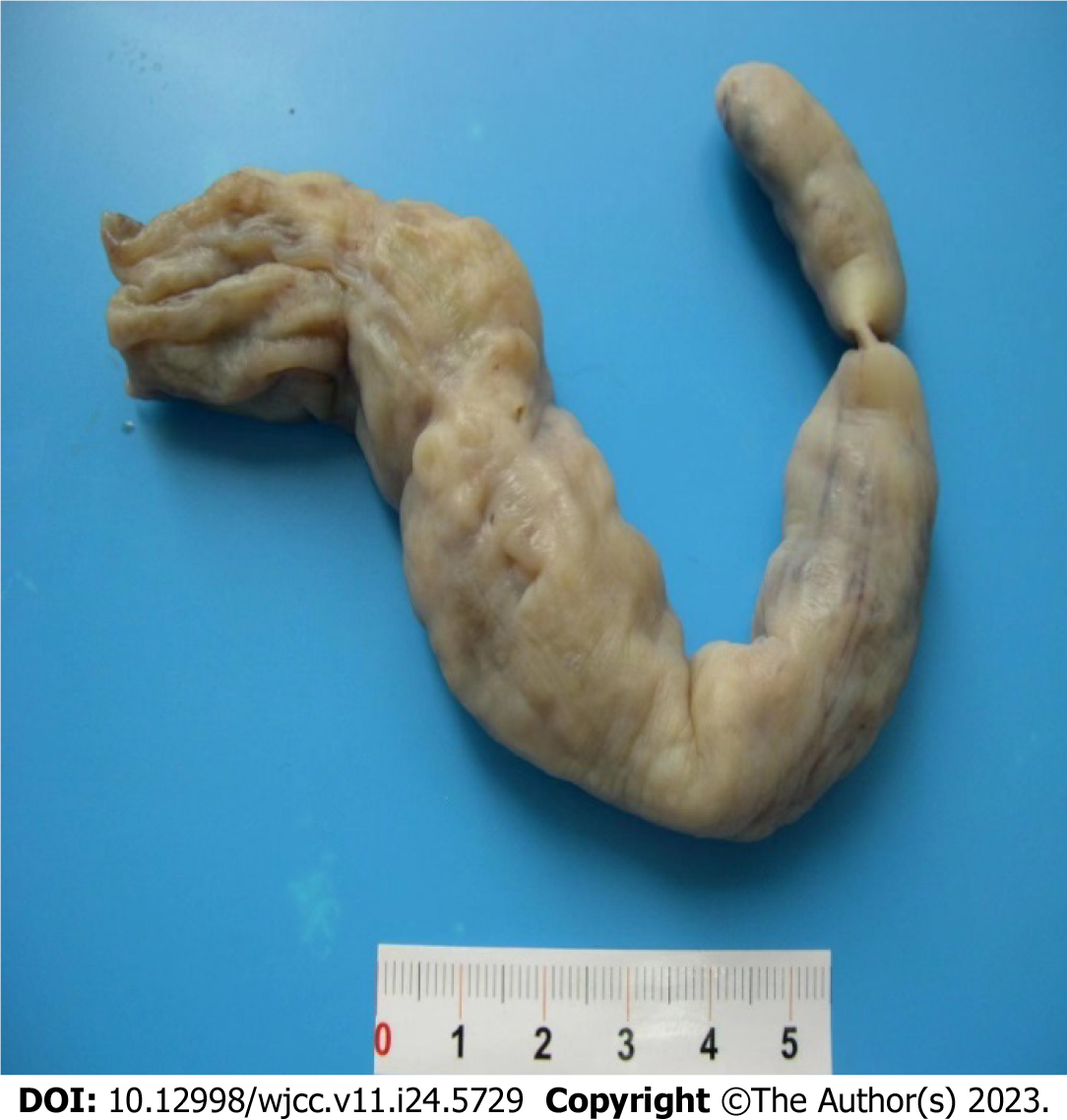
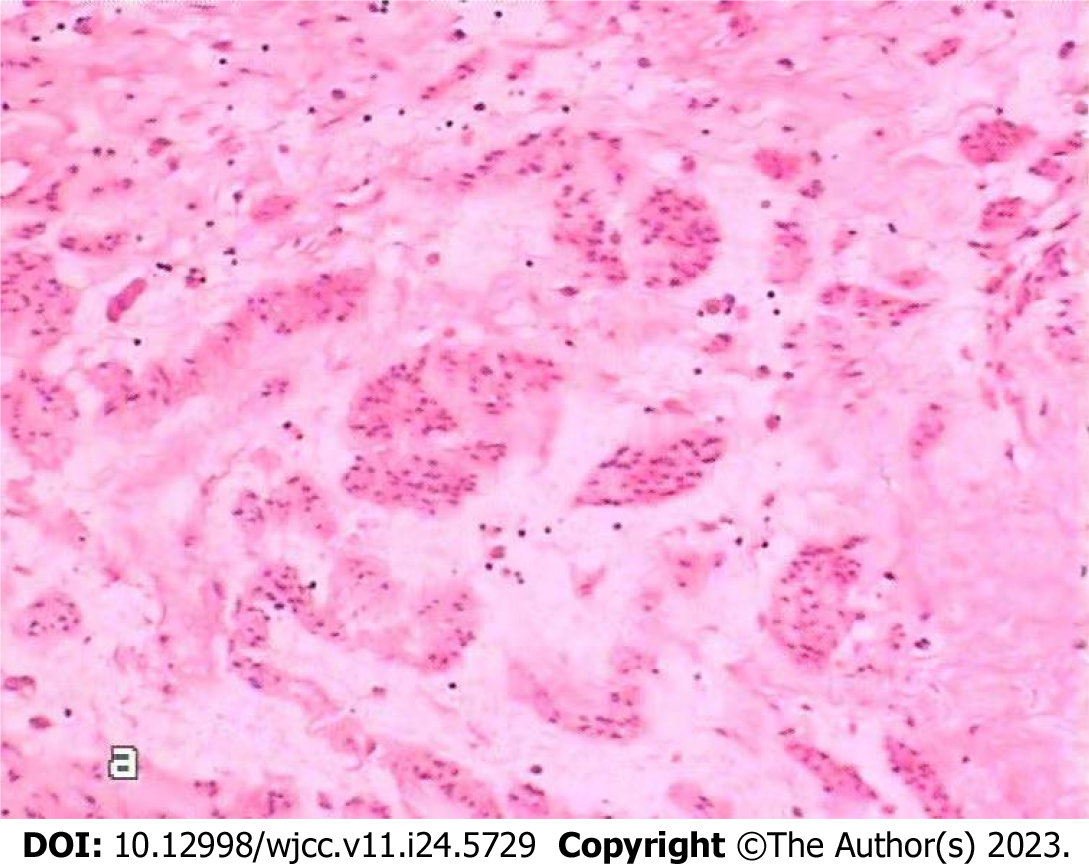
Intracardiac tumor resection was performed under general anesthesia, at room temperature and with extracorporeal circulation on November 31, 2022. Briefly, the skin was disinfected with conventional active iodine, a median thoracotomy was performed, and the pericardium was opened. Extracorporeal circulation was set up with an intubation tube placed in the ascending aorta, superior vena cava and femoral vein through an incision in the lower right atrium. The mass was excised as close as possible to the inferior vena cava and sent for medical examination. Visual observations were as follows: The tumor came from the inferior vena cava in a long strip, with a diameter of approximately 1.5-2.5 cm and a resection length of approximately 20 cm (Figure 3). The surface was smooth, and the capsule was intact. The tumor discontinuously entered the right pulmonary artery. Echocardiography showed that the abnormal echo disappeared in the heart and pulmonary artery during the operation. There were no anesthetic complications during the operation. Postoperative examination (Figure 4) suggested intravenous leiomyomatosis. Cardiac ultrasound was performed postoperatively. After excision of the mass in the inferior vena cava and right ventricle, the right atrium and right ventricle were smaller than they were before the operation, the right ventricle was not enlarged, there was no abnormal echo in the cardiac cavity, and the proximal segment of the inferior vena cava was not abnormal. Postoperative chest X-ray and ECG were normal. The patient was cured and discharged from the hospital, and the second stage of the two-stage operation was performed after rehabilitation. On December 17, 2022, the masses in the inferior vena cava, right iliac vein and abdomen were resected, and postoperative examination results indicated that the disease was of the same origin as the heart mass.
At 3 mo postoperatively, the patient was still alive.
Uterine intravenous leiomyomatosis patients have a clear clinical history of uterine fibroids. The leiomyoma seen by the naked eye extends into the vascular space and can grow in small, medium and large veins. The benign leiomyoma tissues invade the vein outside the leiomyoma (but rarely extend to the right ventricular lumen), causing mechanical obstruction and corresponding clinical symptoms. Unlike 2 previous cases of leiomyoma invading the right atrium and right ventricle, the uterine leiomyoma in our patient extended to the right pulmonary artery, which is clinically rare. Approximately 70%-80% of patients with this disease are women aged 30 to 50 years, and there may be two occurrence mechanisms[2]. Most cases are related to uterine leiomyoma, which is caused by one or more uterine leiomyomas with extensive vascular invasion; a few tumors originate directly from the smooth muscle of the venous wall. Right heart tumors present a variety of clinical manifestations[3] that lack specificity and vary with tumor location, tumor size and tumor nature, and these manifestations generally include systemic manifestations and cardiac manifestations, such as fever, body mass reduction, anemia, shortness of breath, palpitations, syncope and embolism. For example, if the tumor is close to the superior vena cava inlet, it mainly causes superior vena cava obstruction syndrome. If the tumor is located at the inferior vena cava inlet, it may cause inferior vena cava obstruction. If the tumor is located near the tricuspid valve, it often causes tricuspid valve destruction, leading to incomplete closure of the tricuspid valve. In the case of tricuspid valve occlusion, syncope or sudden death may result. Tumors located in the pulmonary artery can cause pulmonary artery obstruction, an increase in right ventricular load, and the occurrence of right heart failure[4] and can be easily misdiagnosed as pulmonary artery embolism. Metastatic cardiac tumors are the first clinical manifestation of cardiovascular system symptoms, and a few of the abnormalities of primary lesions are easily misdiagnosed. The possibility of a metastatic neoplasm should be considered if it is diagnosed as a right ventricular tumor involving the superior or inferior lumen, even if there is no evidence of a primary lesion. According to Giuntoli et al[5], the local recurrence rate of uterine myoma is 53% to 71%, with a tendency for distant spread. Compared with other imaging equipment, methods and techniques, 256-slice spiral CT can clearly show the cardiac structure and tumor shape, size, section and activity and can provide a better basis for diagnosis[6]. For patients with tumor-induced obstruction, valve failure or risk of peripheral embolism, once diagnosed, surgical treatment should be performed as soon as possible. There are two main surgical methods: One-stage radical cure and the staging operation[7]. One-stage radical cure is suitable for patients with a good general condition and mild circulatory disorders. The right heart tumor is resected under extracorporeal circulation, the uterus, appendage and other pathological tissues are removed via the abdomen when the patient is in a stable condition, and pelvic dissection is performed. For the staging operation[8], gynecological surgery or venous surgery involving the abdomen is performed 2 to 4 wk after open heart surgery. The former has a long operation time, major trauma and many postoperative complications, but the staging operation avoids the abovementioned disadvantages. The previous two similar cases in our hospital underwent the staged operation method. Typically, the diagnosis is made after the scheduled myomectomy. In fact, only surgical exploration and pathological analysis of the excised specimen[9] can ultimately determine the diagnosis.
According to some reports, expression of the estrogen receptor was found in the tumor invading the vena cava and right atrium. It was believed that the occurrence and evolution of this disease were related to estrogen, and the effect of estrogen in the body could promote tumor growth and recurrence, thus affecting its prognosis. In 2012, Leitao et al[10] reported 96 patients with extrauterine disease, if tumour residue = 0, these patients had longer progression-free survival, but no overall survival benefit. Hormone-receptor-targeted treatments are currently the main research objective[11], and adjuvant chemotherapy is not recommended. Therefore, the use of anti-estrogen drugs as adjuvant therapy is effective to prevent the recurrence and spread of the disease if necessary after the operation.
How to reduce the morbidity and mortality[12] of this disease remains the subject of debate. According to current clinical data, surgery remains the best treatment compared with other conservative treatments. In conclusion, the best management of this rare uterine tumor is unclear, and further research is needed to clarify the pathogenesis and treatment pathways in patients first diagnosed and patients with relapse of uterine myoma. Molecular biology and genomic profiling may be the most meaningful laboratory tests for patients at high risk of recurrence. More importantly, immune checkpoint inhibitors and targeted therapies need to be studied to determine effective treatments.
In this patient, a uterine leiomyoma extended to the right pulmonary system, which is clinically rare. After such a diagnosis, surgical treatment should be performed as soon as possible.
Special thanks to the Clinical Lab for their relentless support in collecting and storing the pathological specimen. Moreover, many thanks to Xiang DD for her great assistance in collecting and storing the patient’s samples.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Di Donato V, Italy S-Editor: Liu JH L-Editor: Webster JR P-Editor: Zhao S
| 1. | Harris HR, Petrick JL, Rosenberg L. The epidemiology of uterine fibroids: Where do we go from here? Fertil Steril. 2022;117:841-842. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Sohn GS, Cho S, Kim YM, Cho CH, Kim MR, Lee SR; Working Group of Society of Uterine Leiomyoma. Current medical treatment of uterine fibroids. Obstet Gynecol Sci. 2018;61:192-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Huang L, Shi G, Wang Q, Guo Y, Cong M. Pulmonary and mediastinum metastasis of uterine leiomyoma: A case report. Medicine (Baltimore). 2019;98:e18276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34:130-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 356] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 5. | Giuntoli RL 2nd, Garrett-Mayer E, Bristow RE, Gostout BS. Secondary cytoreduction in the management of recurrent uterine leiomyosarcoma. Gynecol Oncol. 2007;106:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Pfenniger A, Silverberg RA, Lomasney JW, Churyla A, Maganti K. Unusual Case of Right Ventricular Intravenous Leiomyoma. Circ Cardiovasc Imaging. 2021;14:e010363. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Kong LY, Chen LL, Xiang W, Liu F. Intravenous Leiomyomatosis With Paradoxical Embolism: Unusual Presentation of Uterine Leiomyoma. Circ Cardiovasc Imaging. 2020;13:e009930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Song BG, Park YH, Kang GH, Chun WJ, Oh JH. Intravenous leiomyomatosis with intracardiac extension. Asian Cardiovasc Thorac Ann. 2011;19:179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Gaillard S, Secord AA. Endometrial stromal sarcoma and related tumors and uterine adenosarcoma. Up To Date. (Last accessed February 19, 2023). Available from: https://www.uptodate.com/contents/endometrial-stromal-sarcoma-and-related-tumors-and-uterine-adenosarcoma. |
| 10. | Leitao MM Jr, Zivanovic O, Chi DS, Hensley ML, O'Cearbhaill R, Soslow RA, Barakat RR. Surgical cytoreduction in patients with metastatic uterine leiomyosarcoma at the time of initial diagnosis. Gynecol Oncol. 2012;125:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Giannini A, Golia D'Augè T, Bogani G, Laganà AS, Chiantera V, Vizza E, Muzii L, Di Donato V. Uterine sarcomas: A critical review of the literature. Eur J Obstet Gynecol Reprod Biol. 2023;287:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 12. | Gadducci A, Zannoni GF. Uterine smooth muscle tumors of unknown malignant potential: A challenging question. Gynecol Oncol. 2019;154:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |