Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5653
Peer-review started: April 14, 2023
First decision: July 7, 2023
Revised: July 17, 2023
Accepted: August 3, 2023
Article in press: August 3, 2023
Published online: August 26, 2023
Processing time: 132 Days and 21.9 Hours
Microwave endometrial ablation (MEA) is a minimally invasive treatment for menorrhagia. It has been covered by the national insurance in Japan since April 2012, and its demand has been increasing as the importance of women’s health has advanced in society.
To examine the efficacy of MEA as a treatment option for menorrhagia.
In this study, we retrospectively analyzed 76 patients who underwent MEA between January 2016 and March 2020 in our department. MEA was performed in the lithotomy position, under general anesthesia, and with transabdominal ultrasound guidance, including the entire endometrial circumference while confirming endometrial coagulation. The Microtaze AFM-712 and the Sounding Applicator CSA-40CBL-1006200C were used for MEA, and the endometrium was ablated using a Microtaze output of 70 W and coagulation energization time of 50 s per cycle. The visual analog scale (VAS) was used to evaluate menorrhagia, menstrual pain, and treatment satisfaction. Additionally, the hemoglobin (Hb) levels before and after MEA and associated complications were investigated.
The average age of the patients was 44.8 ± 4.0 years. While 14 patients had functional menorrhagia, 62 had organic menorrhagia, of whom 14 had endo
MEA is a safe and effective treatment for menorrhagia.
Core Tip: In the short-term follow-up after microwave endometrial ablation (MEA), we were able to confirm the efficacy and safety of MEA not only in functional hypermenorrhea but also in hypermenorrhea with organic causes. MEA could be a useful option for the treatment of menorrhagia in patients who are at high risk for hysterectomy due to complications or comorbidities, or who desire minimally invasive surgery.
- Citation: Kakinuma T, Kaneko A, Kakinuma K, Matsuda Y, Yanagida K, Takeshima N, Ohwada M. Effectiveness of treating menorrhagia using microwave endometrial ablation at a frequency of 2.45 GHz. World J Clin Cases 2023; 11(24): 5653-5659
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5653.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5653
Menorrhagia is characterized by heavy menstrual bleeding, severe anemia, and decreased quality of life (QoL). As a result, it has become a significant factor limiting social activities in women. Although hemostatic or hormonal agents are often the first treatment for menorrhagia, hysterectomy is chosen as the curative option when conservative treatments are ineffective or when the patient has no desire to bear children. However, some patients require less invasive treatments due to pre-existing conditions, complications, and social backgrounds[1]. Microwave endometrial ablation (MEA) at a frequency of 2.45 GHz is a minimally invasive treatment that can be used instead of conventional hysterectomy. Since April 2012, MEA has been covered by insurance in Japan. It has gradually become a widely used treatment for functional menorrhagia that is caused by a systemic disease or medication and resistant to conservative treatments, as well as organic menorrhagia caused by uterine fibroids and adenomyosis. MEA breaks down the endometrium, including the basal layer, using a protein coagulation device that utilizes dielectric heat generated by microwaves[2]. Thus, MEA reduces menstrual bleeding or induces amenorrhea by impairing the function of the endometrium.
MEA was introduced as a treatment option for menorrhagia at our hospital in January 2016. This retrospective study aimed to clinically examine cases in which MEA was performed for menorrhagia at our hospital.
This study was approved by the Ethics Committee of the International University of Health and Welfare (approval number, 20-B-399; approval date, May 7, 2020). All patients provided written and verbal informed consent for study participation. We retrospectively examined 76 patients with the main complaint of menorrhagia who underwent MEA at our department between January 2016 and March 2020. In all cases, before MEA, cervical and endometrial cytodiagnosis and histodiagnosis were performed as needed to rule out malignant lesions. In addition, magnetic resonance imaging and hysteroscopy were performed to assess the thickness of the myometrium, as well as the shape and length of the uterine cavity to rule out organic diseases. Cervical dilatation was performed using a Lamicel osmotic dilator (Medtronic, Tokyo, Japan) the day before surgery. MEA was performed with the patient in the lithotomy position and under general anesthesia and transabdominal ultrasound guidance and was applied to the entire endometrial circumference while confirming endometrial coagulation. The Microtaze AFM-712 (Alfresa Pharma Co., Ltd., Osaka, Japan) and the Sounding Applicator CSA-40CBL-1006200C (Alfresa Pharma Co., Ltd.) were used for MEA, and the endometrium was ablated with an output of 70 W and coagulation energization time of 50 s per cycle. After MEA, the uterine cavity was observed with a hysteroscope to confirm coagulative necrosis of the endometrium and the lack of ablation in the internal ostium of the uterus or cervical mucosa.
The operative time, number of ablation sessions, length of hospital stay, amount of menstrual bleeding 3 and 6 mo after MEA, menstrual pain, and treatment satisfaction were evaluated using the visual analog scale (VAS). Furthermore, the incidence of amenorrhea, hemoglobin (Hb) levels before and after MEA, and complications from the procedure were examined.
Numerical data are shown as the mean ± standard deviation. All statistical analyses were performed using JMPVR software, version 14.2 (SAS Institute Japan Ltd., Tokyo, Japan). One-way analysis of variance with repeated measures and paired t-tests were used for statistical analyses, and P values < 0.05 were considered statistically significant (online resource).
Table 1 shows the characteristics of the 76 patients included in this study. The average age of the patients was 44.8 ± 4.0 (range, 32-53) years. Fourteen patients had functional menorrhagia, whereas 62 had organic menorrhagia. Of the 62 patients with organic menorrhagia, 14 had endometrial polyps, 40 had uterine fibroids, and 8 had adenomyosis. Comorbidities included autoimmune disease in four patients, deep vein thrombosis in three, thyroid dysfunction in three, cerebral infarction in three, severe diabetes mellitus in two, and multiple sclerosis in one.
| Age (yr) | 44.8 ± 4.0 | |
| Cause of hypermenorrhea | Functional excessive menstruation | 14 |
| (Number of cases) | Endometrial polyps | 14 |
| Uterine fibroids | 40 | |
| Adenomyosis | 8 | |
| Comorbidities | Autoimmune disease | 4 |
| (Number of cases) | Deep vein thrombosis | 3 |
| Thyroid dysfunction | 3 | |
| Cerebral infarction | 3 | |
| Severe diabetes mellitus | 2 | |
| Multiple sclerosis | 1 | |
| (n = 76) |
The mean time taken for the procedure was 37.0 ± 21.6 min, and the mean number of ablation sessions was 7.8 ± 2.9. The patients stayed in the hospital for a mean of 2.6 ± 0.7 d.
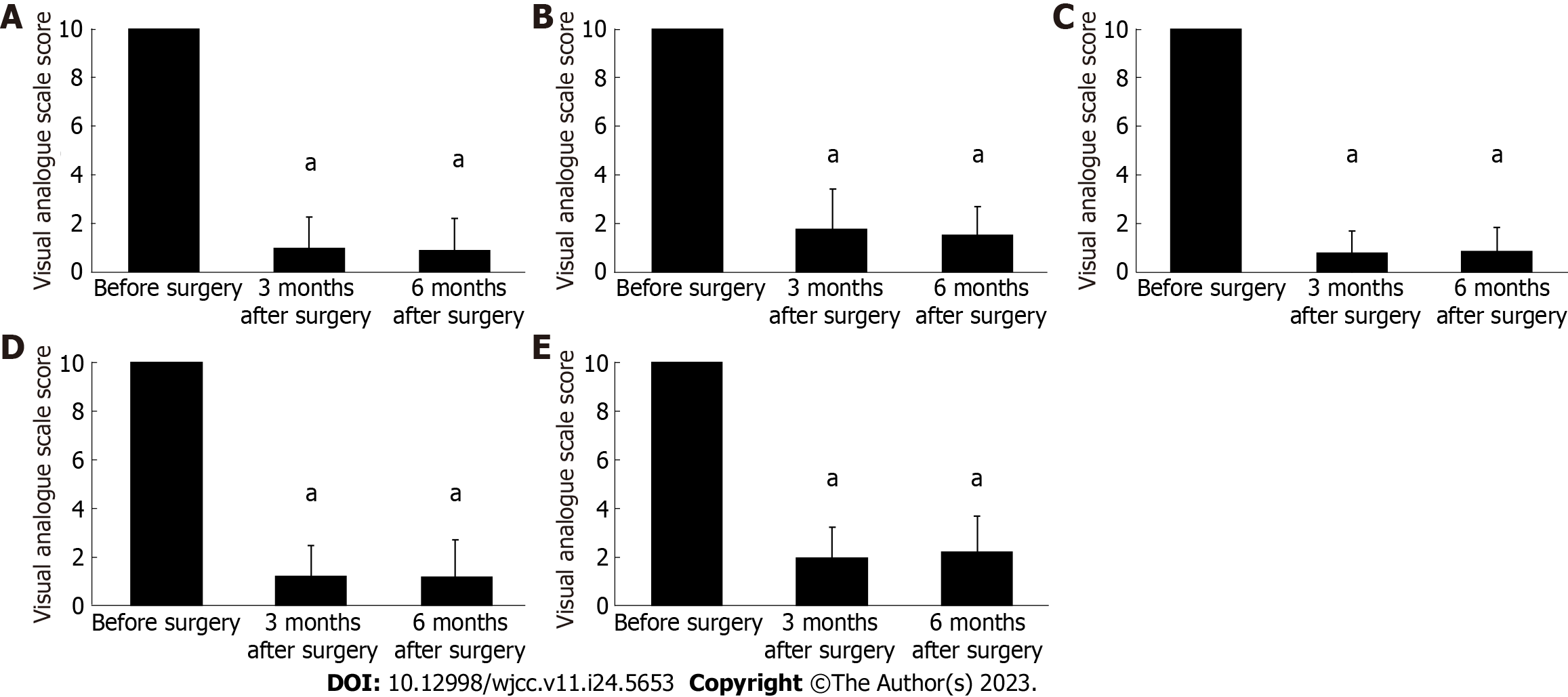
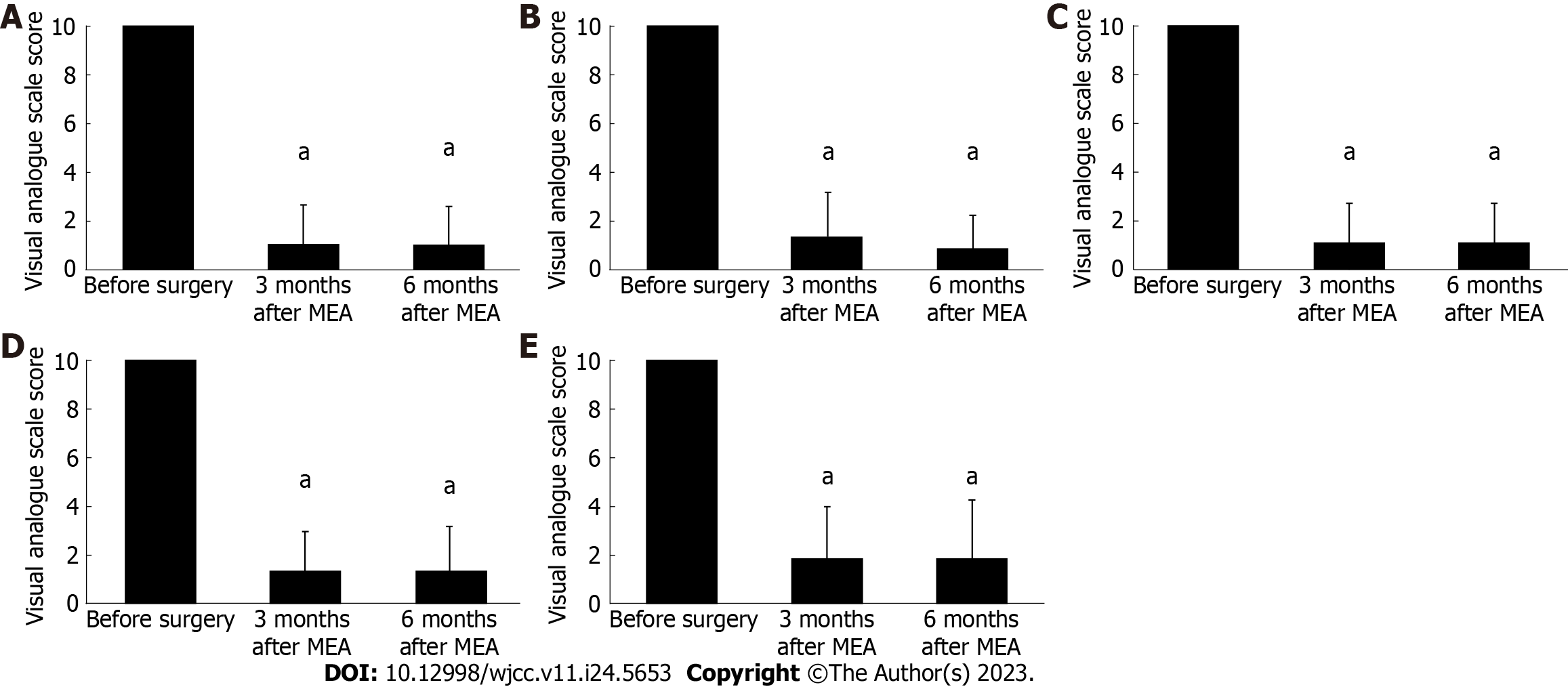
In all MEA cases, the preoperative VAS score for menstrual bleeding significantly improved from 10 to 1.3 ± 1.3 and 1.3 ± 1.3 at 3 and 6 mo after the procedure, respectively (P < 0.001) (Figure 1A). In addition, we evaluated changes in menstrual bleeding following MEA in patients with menorrhagia caused by different conditions. The preoperative VAS score for menstrual bleeding was 10. At 3 and 6 mo after MEA, it decreased to 1.8 ± 1.6 and 1.1 ± 1.2, respectively, in patients with functional menorrhagia; 0.6 ± 0.8 and 0.5 ± 0.9, respectively, in patients with endometrial polyps; 0.9 ± 1.2 and 0.9 ± 1.4, respectively, in patients with uterine fibroids; and 1.9 ± 1.3 and 2.2 ± 1.5, respectively, in patients with adenomyosis. Therefore, regardless of the causative disease, a marked decrease in menstrual bleeding was observed compared with that of the early postoperative period (P < 0.001) (Figure 1B-E).
As observed with menstrual bleeding, the preoperative VAS score for menstrual pain in all MEA cases decreased from 10 to 1.3 ± 1.8 and 1.3 ± 1.8 at 3 and 6 mo after the procedure, respectively, showing a significant improvement from the early postoperative period (P < 0.001) (Figure 2A). We evaluated changes in menstrual pain following MEA based on the causative condition for menorrhagia. The preoperative VAS score for menstrual pain was 10. At 3 and 6 mo after the procedure, it dropped to 0.9 ± 1.7 and 0.5 ± 1.1, respectively, in cases with functional menorrhagia; 0.8 ± 1.5 and 0.9 ± 1.5, respectively, in cases with endometrial polyps; 1.1 ± 1.6 and 1.1 ± 1.7, respectively, in patients with uterine fibroids; and 1.8 ± 2.3 and 1.9 ± 2.5, respectively, in patients with adenomyosis. A significant improvement in menstrual pain was observed from the early postoperative stage, regardless of the causative disease (P < 0.001) (Figure 2B-E).
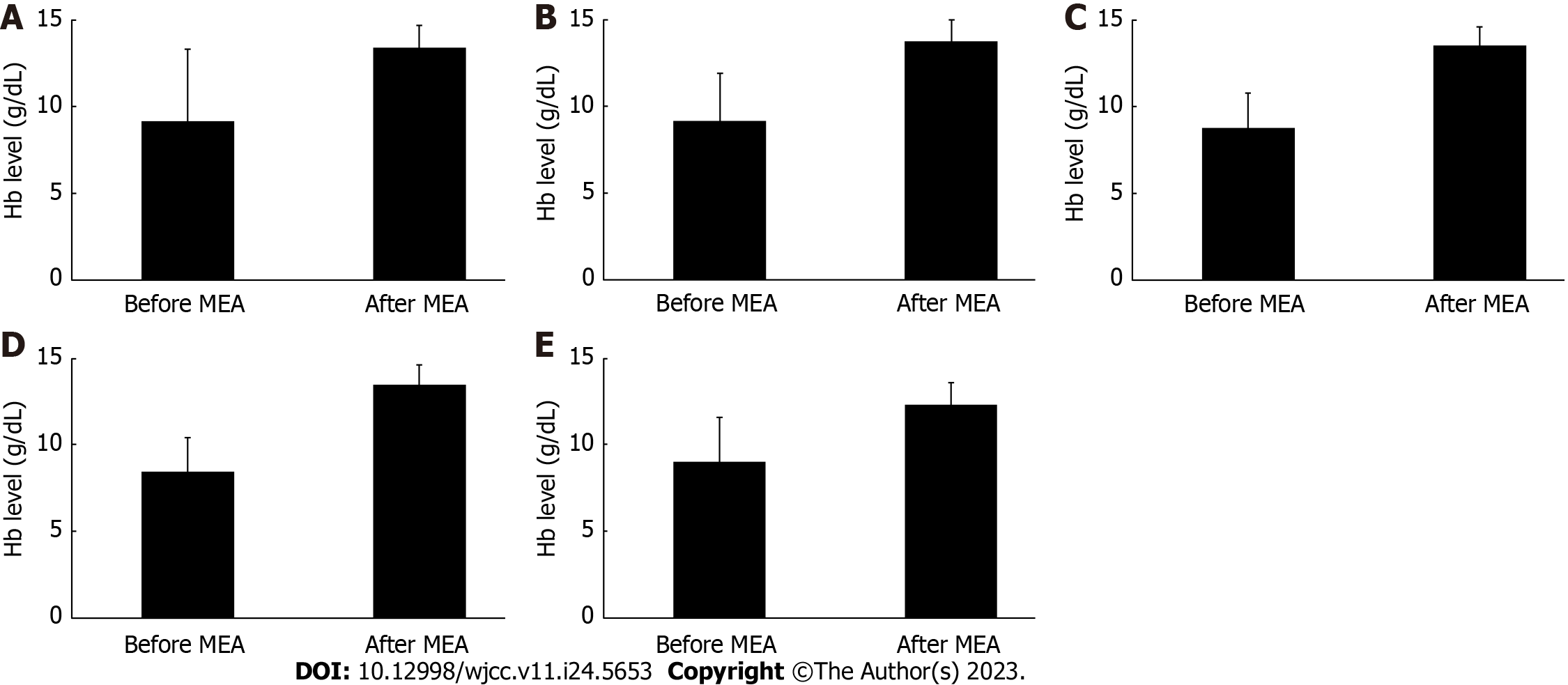
Figure 3 shows the changes in Hb levels following MEA. In all cases, the Hb levels before and after treatment were 9.2 ± 4.2 g/dL and 13.4 ± 1.2 g/dL, respectively, showing a significant improvement (P = 0.003) (Figure 3A). Moreover, we studied the changes in Hb levels following MEA based on the different diseases underlying menorrhagia. The Hb levels before and after MEA treatment were 9.2 ± 2.7 g/dL and 13.8 ± 1.3 g/dL, respectively (P = 0.003), in cases with functional menorrhagia; 8.9 ± 1.9 g/dL and 13.5 ± 1.1 g/dL, respectively (P < 0.001), in cases with endometrial polyps; 8.5 ± 1.9 g/dL and 13.5 ± 1.2 g/dL, respectively (P < 0.001), in cases with uterine fibroids; and 9.1 ± 2.6 g/dL and 12.3 ± 1.3 g/dL (P = 0.034), respectively, in cases with adenomyosis. These findings show a significant increase in Hb levels after MEA, regardless of the causative disease (Figure 3B-E).
Patient satisfaction following MEA was high, with a VAS score of 9.6 ± 0.7. Twenty patients (26.3%) became amenorrheic by 6 mo after MEA. One patient (1.3%) who underwent MEA for menorrhagia caused by adenomyosis developed endometritis as a postoperative complication; however, this was alleviated by administering antibiotics alone. No other serious complications or recurrence of menorrhagia was observed during the postoperative follow-up period.
Studies from outside Japan have reported that the incidence of menorrhagia in women of reproductive age is between 19% and 24%[3,4] and tends to increase with age. Therefore, menorrhagia should be managed to maintain the patient’s lifestyle.
The first choice of management for menorrhagia is drug therapy, such as a combination of estrogen and progestin. However, it is not effective in some cases, and menorrhagia continues to interfere with work and daily life and significantly impairs the QoL of women. Although hysterectomy has been found to improve the symptoms of menorrhagia, it is an invasive procedure that is associated with serious complications, lengthy hospitalization, and leave of absence from work, all of which impose a heavy financial burden on patients. In addition, some patients decline hysterectomy despite not wanting to bear children, and the procedure has shown low postoperative patient satisfaction in many cases. Furthermore, with the recent remarkable advancement of women in society, the number of patients who desire a minimally invasive treatment with a short hospital stay has been increasing.
In Japan, along with the advancement of MEA, the levonorgestrel-releasing intrauterine system (LNG-IUS), developed in the mid-1990s, has been covered by insurance as a treatment for menorrhagia and menstrual pain since 2014, marking a significant change in the management of menorrhagia.
MEA breaks down the endometrium, including the basal layer, using a protein coagulation device that utilizes dielectric heat generated by microwaves[2]. Thus, MEA reduces menstrual bleeding or induces amenorrhea by impairing endometrial function. MEA has been covered by insurance in Japan as a minimally invasive treatment for menorrhagia since April 2012. It was introduced at our hospital in January 2016, and 76 patients have undergone MEA treatment as of March 2020. Consistent with previous studies that have shown the therapeutic effects of MEA[5,6], our study demonstrated an improvement in menorrhagia from the early postoperative stage due to reduced menstrual bleeding, regardless of the cause of menorrhagia. In addition, MEA requires a short hospitalization duration, thereby sufficiently improving patient QoL. Our study included 16 cases of menorrhagia associated with comorbidities, for which the minimally invasive nature of MEA was particularly advantageous. Moreover, our study showed a significant increase in Hb levels after MEA, confirming its therapeutic effects on anemia associated with menorrhagia.
It has been reported that the LNG-IUS treatment, which is a minimally invasive drug therapy for menorrhagia, reduced menstrual bleeding by more than 50% compared with pretreatment levels in 84.8% of the cases and that the incidence of amenorrhea after the treatment was approximately 20%[6,7]. Our findings suggested that MEA is more effective than LNG-IUS, although our study was a subjective evaluation of menstrual bleeding, and these studies cannot be compared. However, from a long-term perspective, 42% of the patients switched from LNG-IUS to other treatments due to relapse of menorrhagia, whereas only 21% switched from MEA[8,9]. Therefore, MEA is believed to have more long-lasting effects on menorrhagia with a lower rate of recurrence.
Furthermore, MEA was initially not expected to improve menstrual pain, as it is not a treatment for dysmenorrhea; however, its effectiveness against menstrual pain has been reported[5,6]. In our study, MEA improved menstrual pain from the first postoperative menses. This is presumed to be a secondary effect due to the relief from uterine contractions accompanied by improved menorrhagia and reduced menstrual bleeding. Although the direct mechanism is unknown, MEA is presumed to be effective in improving menstrual pain associated with menorrhagia.
Complications after MEA include thermal damage to the pelvic viscera, hydrometra due to cervical stenosis and ablation of endometrial tissue, hematometra, endometritis, ascending infection and pelvic inflammation, and pyometra[10]. In this study, only one patient (1.3%) had a postoperative endometrial infection, which was mild, as the condition was relieved by antibiotics alone. The origin of the infection, in this case, was believed to be an effusion from the ablated surface that lasted for several weeks after MEA, as well as necrotic tissue that remained in the postoperative uterus. However, we carefully removed as much accumulated necrotic tissue as possible by examining the endometrial status using hysteroscopy immediately after the MEA. In addition, the incidence of infection after hysteroscopic surgery is reported to be approximately 2%[11]. Thus, a comparable incidence of postoperative infection in our study indicates the safety of MEA.
MEA was associated with high patient satisfaction with a VAS score of 9.6 ± 0.7. Our treatment results showed that 20 patients (26.3%) had become amenorrheic, whereas the remaining continued to menstruate. It is presumed that women with menorrhagia desire to improve their QoL by reducing menstrual bleeding while preserving the uterus, even if they do not become amenorrheic. Further, the established safety of MEA contributed to high patient satisfaction.
This study demonstrated the efficacy of MEA in the management of menorrhagia and its high patient satisfaction rates regardless of the underlying etiology.
The current study results should be interpreted in the light of some limitations. First, our study only followed the patients for up to 6 mo. Second, although not observed in this study, ineffective and recurrent cases of organic menor
This short-term postoperative assessment of MEA confirmed its efficacy and safety not only for functional menorrhagia but also for menorrhagia with organic diseases. Our results suggest that MEA may be a useful therapeutic option for patients with menorrhagia who cannot undergo hysterectomy due to complications or pre-existing conditions or who desire a minimally invasive treatment.
Microwave endometrial ablation (MEA) is a minimally invasive treatment for menorrhagia. It has been covered by the national insurance in Japan, and its demand has been increasing as the importance of women’s health has advanced in society.
MEA was introduced as a treatment option for menorrhagia at our hospital in January 2016.
This retrospective study aimed to clinically examine cases in which MEA was performed for menorrhagia at our hospital.
We retrospectively analyzed 76 patients who underwent MEA between January 2016 and March 2020 in our department.
The visual analog scale scores of menorrhagia and menstrual pain showed improvements after MEA. The hemoglobin levels significantly improved after MEA. Endometritis was observed in only one patient after surgery and was treated with antibiotics.
MEA is a safe and effective treatment for menorrhagia.
MEA could be a useful option for the treatment of menorrhagia in patients who are at high risk for hysterectomy due to complications or comorbidities, or who desire minimally invasive surgery.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Di Donato V, Italy S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Zhao S
| 1. | Bushnell DM, Martin ML, Moore KA, Richter HE, Rubin A, Patrick DL. Menorrhagia Impact Questionnaire: assessing the influence of heavy menstrual bleeding on quality of life. Curr Med Res Opin. 2010;26:2745-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Della Badia C, Nyirjesy P, Atogho A. Endometrial ablation devices: review of a manufacturer and user facility device experience database. J Minim Invasive Gynecol. 2007;14:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Fernandez H. Update on the management of menometrorrhagia: new surgical approaches. Gynecol Endocrinol. 2011;27 Suppl 1:1131-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Amso NN, Stabinsky SA, McFaul P, Blanc B, Pendley L, Neuwirth R. Uterine thermal balloon therapy for the treatment of menorrhagia: the first 300 patients from a multi-centre study. International Collaborative Uterine Thermal Balloon Working Group. Br J Obstet Gynaecol. 1998;105:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Ishikawa M, Katayama K, Yoshida H, Hirahara F. Therapeutic outcomes and postoperative courses in microwave endometrial ablation for menorrhagia. J Microwave Surg. 2012;30:253-257. [DOI] [Full Text] |
| 6. | Nakayama K, Ishibashi T, Ishikawa M, Katagiri A, Katagiri H, Iida K, Nakayama N, Miyazaki K. Microwave endometrial ablation at a frequency of 2.45 GHz for menorrhagia: analysis of treatment results at a single facility. J Obstet Gynaecol Res. 2014;40:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Kaunitz AM, Bissonnette F, Monteiro I, Lukkari-Lax E, Muysers C, Jensen JT. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Hurskainen R, Teperi J, Rissanen P, Aalto AM, Grenman S, Kivelä A, Kujansuu E, Vuorma S, Yliskoski M, Paavonen J. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291:1456-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 220] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Longinotti MK, Jacobson GF, Hung YY, Learman LA. Probability of hysterectomy after endometrial ablation. Obstet Gynecol. 2008;112:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Das S, Kirwan J, Drakeley AJ, Kingsland CR. Pelvic abscess following microwave endometrial ablation. BJOG. 2005;112:118-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Bhattacharya S, Parkin DE, Reid TM, Abramovich DR, Mollison J, Kitchener HC. A prospective randomised study of the effects of prophylactic antibiotics on the incidence of bacteraemia following hysteroscopic surgery. Eur J Obstet Gynecol Reprod Biol. 1995;63:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Nakamura K, Nakayama K, Sanuki K, Minamoto T, Ishibashi T, Sato E, Yamashita H, Ishikawa M, Kyo S. Long-term outcomes of microwave endometrial ablation for treatment of patients with menorrhagia: A retrospective cohort study. Oncol Lett. 2017;14:7783-7790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |