Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5643
Peer-review started: March 17, 2023
First decision: June 19, 2023
Revised: July 2, 2023
Accepted: August 1, 2023
Article in press: August 1, 2023
Published online: August 26, 2023
Processing time: 161 Days and 6 Hours
Multiple myeloma (MM) is a common hematologic malignancy that originates from a malignant clone of plasma cells. Solitary plasmacytoma, history of diabetes, and platelet count are considered as prognostic factors for MM. But some patients are still associated with much worse outcomes without any prognostic predictors. This study aimed to observe the reduction rate of mono
To investigate the reduction rate of M protein after first and fourth cycle che
A total of 316 patients diagnosed with MM for the first time between 2010 and 2019 at the Lishui Municipal Central Hospital were included. All patients were diagnosed according to the National Comprehensive Cancer Network (NCCN) 2020.V1 diagnostic criteria. The risk assessment was performed by the Mayo Stratification for Macroglobulinemia and Risk-Adapted Therapy guidelines. After diagnosis, 164 patients were evaluated and underwent treatment with four to eight courses of continuous induction chemotherapy. The patients with no response after induction treatment were administered additional therapy following the NCCN 2020.V1 criteria. The following baseline data from the patients were collected: Gender, age at diagnosis, Durie-Salmon stage, glutamic-pyruvic transaminase, glutamic-oxaloacetic transaminase, catabolite activator protein, albumin/globulin ratio, lactate dehydrogenase, translocation (t)(6;14), t(11;14), maintenance regimen, total cholesterol (TC), triglyceride, and pho
Multivariate analysis revealed age [hazard ratio (HR): 1.059, 95% confidence interval (95%CI): 1.033-1.085, P ≤ 0.001], International Staging System stage (HR: 2.136, 95%CI: 1.500-3.041, P ≤ 0.001), autotransplantion (HR: 0.201, 95%CI: 0.069-0.583, P = 0.019), TC (HR: 0.689, 95%CI: 0.533-0.891, P = 0.019), C1 reduction rate (HR: 0474, 95%CI: 0.293-0.767, P = 0.019), and C4 reduction rate (HR: 0.254, 95%CI: 0.139-0.463, P = 0.019) as predictors of PFS. The Kaplan-Meier survival analysis and the log-rank tests revealed that a higher reduction rate of M protein after first cycle (≥ 50%) and fourth cycle (≥ 75%) chemotherapy was associated with a longer PFS than the lower one.
Higher reduction rates of M protein after the first and fourth chemotherapy cycles can act as advantageous prognostic factors for PFS in standard-risk group of MM patients during initial diagnosis.
Core Tip: Multiple myeloma (MM) is a common hematologic malignancy that originates from a malignant clone of plasma cells. Solitary plasmacytoma, history of diabetes, and platelet count are considered as prognostic factors for MM. But some patients are still associated with much worse outcomes without any prognostic predictors. This study aimed to observe the reduction rate of monoclonal protein after the first and fourth chemotherapy cycles, which is considered as a new prognostic factor for progression-free survival in standard-risk group of newly diagnosed MM patients.
- Citation: Liu M, Zhang JY. Reduction rate of monoclonal protein as a useful prognostic factor in standard-risk group of newly diagnosed multiple myeloma. World J Clin Cases 2023; 11(24): 5643-5652
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5643.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5643
Multiple myeloma (MM) is the second common hematologic malignancy that originates from B cells, and accounted for approximately 1.8% of all malignancies and led to the death of 30000 patients in 2018[1]. MM can cause kidney injury, anemia, lytic bone disease, hypercalcemia, abnormal functioning of blood coagulation, and damage of other organs[2]. Bone pain is the most common symptom that significantly impairs the quality of life in approximately 60% of patients[3]. Over the past decade, many studies have revealed nonoverlapping and overlapping genetic abnormalities in the myeloma cells and also demonstrated their impact on patient outcomes[4,5]. Del17p, translocation (t)(4;14), t(14;16), and t(14; 20) were considered as predictors of significantly shortened survival in patients with newly diagnosed MM[6-9]. In addition, according to geriatric assessment[10], due to the absence of high-risk cytogenetic abnormalities[11], both the International Staging System (ISS) and the Revised-ISS (R-ISS) were used as prognostic factors for the overall survival (OS) and progression-free survival (PFS) in patients. And ISS 1 and R-ISS 1 patients had a significantly longer PFS and OS[12], while conventional factors such as age below 80 years, beta-2-microglobulin levels, normal hemoglobin, and normal lactate dehydrogenase (LDH) levels were identified as predictors of PFS and OS[13,14]. However, the median survival of patients with MM showed great improvement after undergoing chemotherapy, which consists of proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies[15], while few patients without these predictors still demon
A total of 316 patients diagnosed with MM for the first time between 2010 and 2019 at the Lishui Municipal Central Hospital were included. All patients were diagnosed according to the National Comprehensive Cancer Network (NCCN) 2020.V1 diagnostic criteria. The risk assessment was performed by the Mayo Stratification of Myeloma and Risk-adapted Therapy guidelines. After diagnosis, 164 patients were evaluated and underwent treatment with four to eight cycles of continuous induction chemotherapy. The patients with no response after induction treatment were administered additional therapy following the NCCN 2020.V1 criteria. The following baseline data from the patients were collected: Gender, age at diagnosis, Durie-Salmon (DS) stage, glutamic-pyruvic transaminase (GPT), glutamic-oxaloacetic transaminase (GOT), catabolite activator protein (CRP), albumin/globulin ratio, LDH, t(6;14), t(11;14), maintenance regimen, total cholesterol (TC), triglyceride (TG), and phosphorous (P). All baseline data and the reduction rate of M protein after each chemotherapy cycle from the first to the fourth were assessed by univariate analysis. The factors influencing the OS and PFS were then assessed by multivariate analysis. We found the first cycle (C1) reduction rate and the fourth cycle (C4) reduction rate as predictors of PFS. Then, PFS was compared between patients with a C1 reduction rate of M protein of ≥ 25% vs < 25% and ≥ 50% and < 50%, and betweeb patients with a C4 reduction rate of ≥ 25% vs < 25%, ≥ 50% vs < 50%, and ≥ 75% vs < 75%.
We retrospectively analyzed data from a total of 164 patients in this study, and all patients underwent treatment with four to eight cycles of continuous induction chemotherapy. The median observation time was 48.4 mo (range, 9-114 mo). The baseline characteristics for 164 MM patients diagnosed for the first time based on the reduction rate of M protein after first and fourth chemotherapy cycles are presented in Table 1. There were no significant differences in gender, DS stage, GPT, GOT, CRP, LDH, t(6;14), t(11;14), maintenance regimen, TC, TG, and P concentrations between the groups with different reduction rates of M protein after the first and fourth chemotherapy cycles (Table 1).
| Characteristic | C1 reduction rate | P value | C4 reduction rate | P value | ||
| < 50 | ≥ 50 | < 75 | ≥ 75 | |||
| Age (yr) | ≤ 0.001 | 0.003 | ||||
| < 65 | 25 | 56 | 21 | 60 | ||
| ≥ 65 | 49 | 34 | 40 | 43 | ||
| Gender | 0.912 | 0.903 | ||||
| Male | 36 | 43 | 37 | 42 | ||
| Female | 38 | 47 | 39 | 46 | ||
| ISS stage | ≤ 0.001 | ≤ 0.001 | ||||
| I | 5 | 39 | 2 | 42 | ||
| II | 31 | 34 | 23 | 42 | ||
| III | 38 | 17 | 36 | 19 | ||
| DS stage | 0.087 | 0.783 | ||||
| I | 1 | 1 | 1 | 1 | ||
| II | 7 | 20 | 9 | 19 | ||
| III | 66 | 70 | 51 | 83 | ||
| GPT | 0.657 | 0.985 | ||||
| ≤ 40 | 71 | 85 | 58 | 98 | ||
| > 40 | 3 | 5 | 3 | 5 | ||
| GOT | 0.510 | 0.617 | ||||
| ≤ 40 | 67 | 84 | 57 | 94 | ||
| > 40 | 7 | 6 | 4 | 9 | ||
| CRP | 0.704 | 0.880 | ||||
| ≤ 10 | 53 | 62 | 42 | 83 | ||
| > 10 | 21 | 28 | 19 | 20 | ||
| A/G | 0.916 | 0.041 | ||||
| ≤ 0.5 | 29 | 36 | 18 | 47 | ||
| > 0.5 | 45 | 54 | 43 | 56 | ||
| LDH | 0.215 | 0.530 | ||||
| ≤ 245 | 54 | 73 | 46 | 82 | ||
| > 245 | 20 | 17 | 15 | 21 | ||
| t(6;14) | 3 | 3 | 1.000 | 2 | 4 | 0.405 |
| t(11;14) | 2 | 2 | 1.000 | 1 | 3 | 0.615 |
| Platelet count | ≤ 0.001 | ≤ 0.001 | ||||
| ≥ 100 | 55 | 88 | 45 | 98 | ||
| < 100 | 19 | 2 | 16 | 5 | ||
| Herpes | 13 | 19 | 0.569 | 9 | 23 | |
| Autotransplantation | 5 | 20 | 0.006 | 5 | 20 | 0.020 |
| TC (mmol/L) | 0.903 | 0.767 | ||||
| < 5.2 | 63 | 76 | 52 | 86 | ||
| ≥ 5.2 | 11 | 14 | 9 | 17 | ||
| TG (mmol/L) | 0.546 | 0.778 | ||||
| < 1.71 | 51 | 58 | 41 | 67 | ||
| ≥ 1.71 | 23 | 32 | 20 | 36 | ||
| P (mmol/L) | 0.587 | 0.568 | ||||
| < 1.07 | 17 | 24 | 13 | 26 | ||
| ≥ 1.07 | 57 | 66 | 48 | 77 | ||
Table 2 shows the results of the univariate analysis of the factors influencing the OS and PFS. Multivariate analysis revealed age [hazard ratio (HR): 1.059, 95% confidence interval (95%CI): 1.033-1.085, P ≤ 0.001], ISS stage (HR: 2.136, 95%CI: 1.500-3.041, P ≤ 0.001), autotransplantion (HR: 0.201, 95%CI: 0.069-0.583, P = 0.019), TC (HR: 0.689, 95%CI: 0.533-0.891, P = 0.019), C1 reduction rate (HR: 0474, 95%CI: 0.293-0.767, P = 0.019), and C4 reduction rate (HR: 0.254, 95%CI: 0.139-0.463, P = 0.019) as predictors of PFS (Table 3).
| Prognostic factor | PFS | OS | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (yr) | 1.051 (1.031-1.071) | ≤ 0.001 | 1.034 (1.012-1.055) | 0.002 |
| Gender | 1.265 (0.828-1.931) | 0.277 | 1.412 (0.926-2.152) | 0.109 |
| Classification | 1.037 (0.949-1.132) | 1.037 | 1.093 (0.999-1.196) | 0.053 |
| ISS stage | 1.718 (1.247-2.366) | 0.001 | 2.093 (1.520-2.883) | ≤ 0.001 |
| DS stage | 2.094 (1.082-4.054) | 0.028 | 1.982 (1.015-3.869) | 0.045 |
| GPT | 1.011 (1.002-1.021) | 0.019 | 1.009 (0.999-1.019) | 0.082 |
| GOT | 1.022 (1.011-1.033) | ≤ 0.001 | 1.025 (1.013-1.038) | ≤ 0.001 |
| CRP | 1.002 (0.996-1.007) | 0.593 | 1.002 (0.996-1.008) | 0.491 |
| A/G | 1.041 (0.698-1.553) | 0.844 | 1.149 (0.754-1.751) | 0.518 |
| LDH | 1.003 (1.001-1.004) | ≤ 0.001 | 1.003 (1.002-1.005) | ≤ 0.001 |
| t(6;14) | 1.021 (0.319-3.266) | 0.972 | 1.285 (0.399-4.134) | 0.674 |
| t(11;14) | 1.149 (0.281-4.708) | 0.847 | 1.188 (0.290-4.871) | 0.811 |
| Platelet count | 9.604 (4.965-18.578) | ≤ 0.001 | 8.437 (4.528-15.721) | ≤ 0.001 |
| Herpes | 0.821 (0.451-1.495) | 0.52 | 0.908 (0.498-1.653) | 0.751 |
| Chemotherapy regimen | 1.005 (0.856-1.180) | 0.952 | 0.949 (0.795-1.133) | 0.564 |
| Autotransplantation | 0.339 (0.137-0.842) | 0.020 | 0.347 (0.140-0.860) | 0.022 |
| TC | 0.773 (0.631-0.947) | 0.013 | 0.757 (0.617-0.927) | 0.007 |
| TG | 0.861 (0.666-1.114) | 0.255 | 0.846 (0.642-1.113) | 0.232 |
| P | 1.143 (0.953-1.370) | 0.15 | 1.113 (0.934-1.325) | 0.232 |
| C1 reduction rate | 0.412 (0.325-0.521) | ≤ 0.001 | 0.438 (0.346-0.554) | ≤ 0.001 |
| C2 reduction rate | 0.412 (0.325-0.523) | ≤ 0.001 | 0.441 (0.351-0.553) | ≤ 0.001 |
| C3 reduction rate | 0.390 (0.303-0.501) | ≤ 0.001 | 0.377 (0.290-0.490) | ≤ 0.001 |
| C4 reduction rate | 0.358 (0.283-0.455) | ≤ 0.001 | 0.345 (0.267-0.445) | ≤ 0.001 |
| Prognostic factor | HR (95%CI) | P value |
| Age | 1.059 (1.033-1.085) | ≤ 0.001 |
| ISS stage | 2.136 (1.500-3.041) | ≤ 0.001 |
| DS stage | 1.622 (0.264-1.622) | 0.264 |
| GPT | 1.017 (0.997-1.036) | 0.097 |
| GOT | 1.002 (0.977-1.028) | 0.857 |
| LDH | 1.000 (0.997-1.003) | 0.944 |
| Platelet count | 1.880 (0.732-4.830) | 0.189 |
| Maintenance regimen | 0.410 (0.236-0.710) | 0.001 |
| Autotransplantation | 0.201 (0.069-0.583) | 0.003 |
| TC | 0.689 (0.533-0.891) | 0.005 |
| C1 reduction rate | 0.474 (0.293-0.767) | 0.002 |
| C2 reduction rate | 0.792 (0.440-1.427) | 0.438 |
| C3 reduction rate | 1.974 (0.921-4.230) | 0.08 |
| C4 reduction rate | 0.254 (0.139-0.463) | ≤ 0.001 |
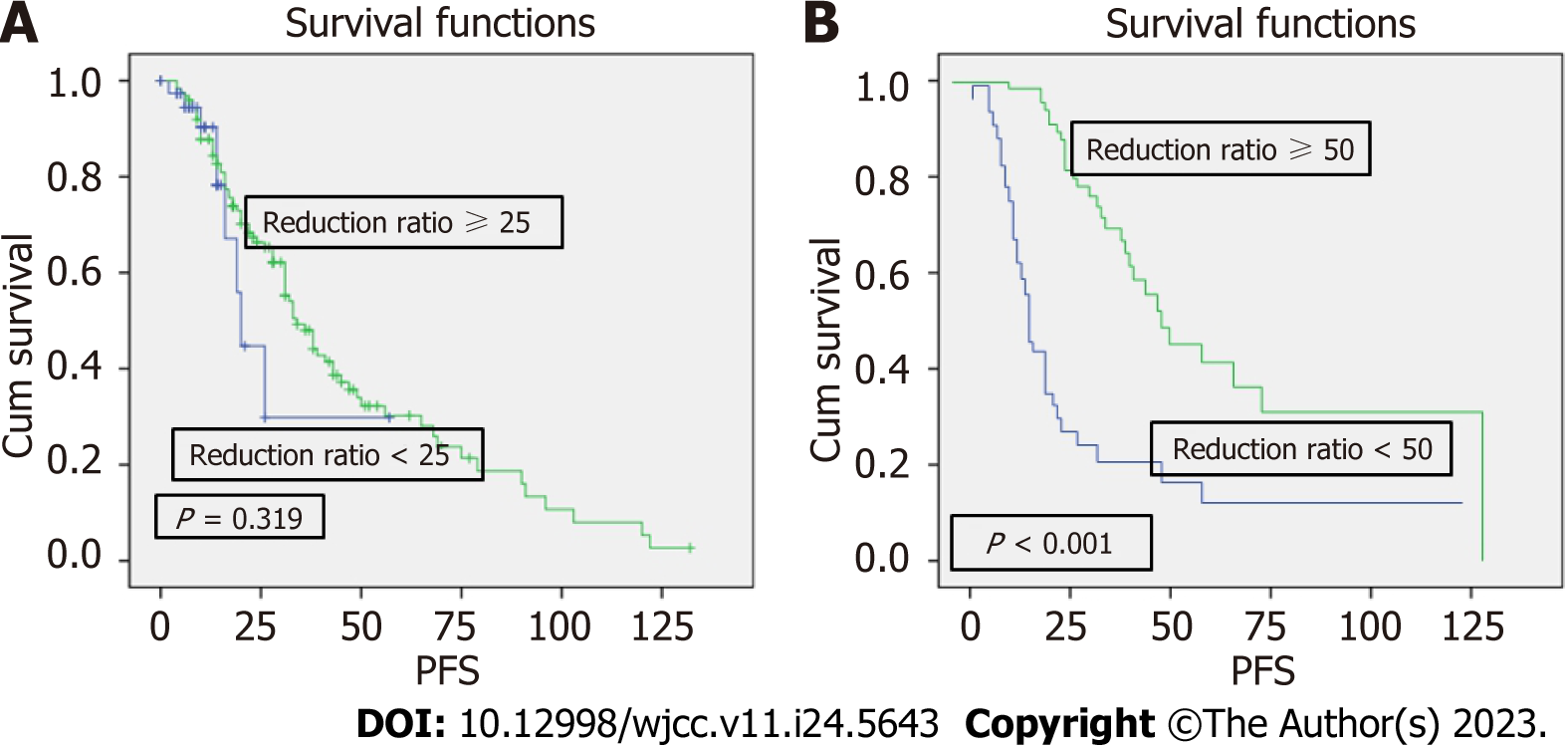
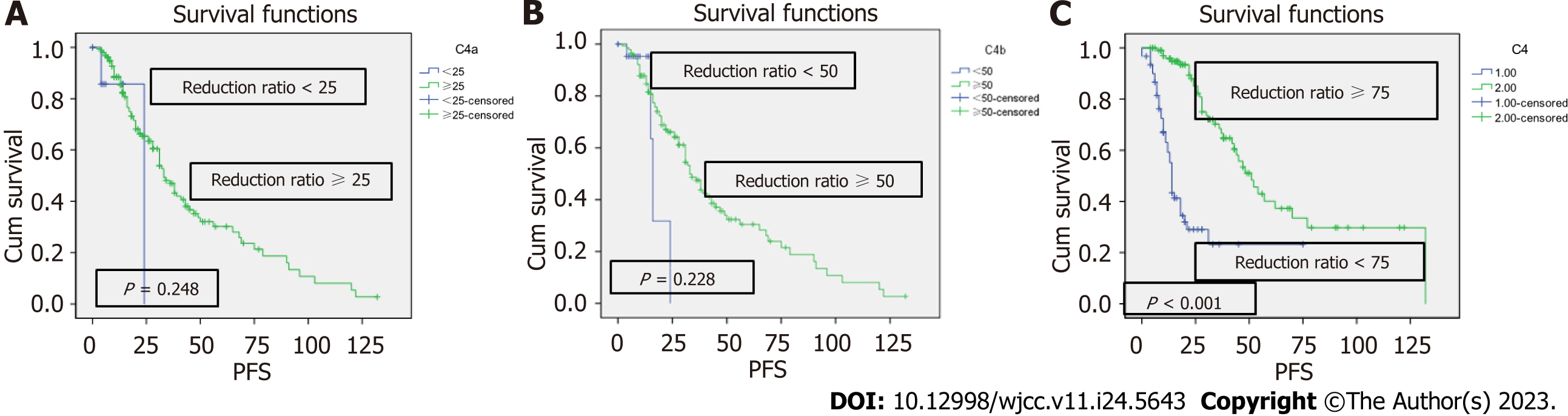
The Kaplan-Meier survive analysis and the log-rank tests revealed that there was no difference in PFS between patients with a C1 reduction rate of M protein of ≥ 25% vs < 25% (P = 0.319), but there was a significant difference between patients with a C1 reduction rate of M protein of ≥ 50% vs < 50% (P ≤ 0.001) (Figure 1). PFS did not differ significantly between patients with a C4 reduction rate of M protein of ≥ 25% vs < 25% (P = 0.248) and ≥ 50% vs < 50% (P = 0.228), but it had a significant difference between patients with a C4 reduction rate of ≥ 75% vs < 75% (P ≤ 0.001) (Figure 2).
Age (HR: 1.054, 95%CI: 1.027-1.081, P = 0.024), ISS stage (HR: 1.879, 95%CI: 1.315-2.686, P = 0.001), platelet count (HR: 2.929, 95%CI: 1.269-6.756, P = 0.012), autotransplantion (HR: 0.211, 95%CI: 0.069-0.647, P = 0.006), and TC (HR: 0.735, 95%CI: 0.573-0.943, P = 0.016) were identified as predictors of OS (Table 4).
| Prognostic factor | HR (95%CI) | P value |
| ISS stage | 1.879 (1.315-2.686) | 0.001 |
| Age | 1.054 (1.027-1.081) | 0.024 |
| DS stage | 1.829 (0.791-4.233) | 0.158 |
| GOT | 1.009 (0.988-1.031) | 0.395 |
| LDH | 0.998 (0.996-1.001) | 0.264 |
| Platelet count | 2.929 (1.269-6.756) | 0.012 |
| Autotransplantation | 0.211 (0.069-0.647) | 0.006 |
| TC | 0.735 (0.573-0.943) | 0.016 |
| C1 reduction rate | 0.868 (0.543-1.387) | 0.553 |
| C2 reduction rate | 0.680 (0.386-1.197) | 0.181 |
| C3 reduction rate | 1.055 (0.592-1.879) | 0.856 |
| C4 reduction rate | 0.608 (0.350-1.058) |
MM is a heterogeneous disease with adverse clinical course, and is characterized by uncontrolled proliferation and accumulation of plasma cells in the bone marrow, which is usually connected with the production of M protein and the differences in the effectiveness of therapeutic strategies and the ability to develop chemoresistance. Risk stratification factors can assist in creating a personalized therapy, thereby improving the treatment outcomes. Prognostic markers such as cytogenetics, molecular biology, and ISS stage showed an association with OS and PFS in MM patients[16]. But there are still many patients with much worse outcomes without any prognostic markers. This study aimed to find more prognostic markers that might help doctors to adjust the therapeutic strategies in time.
M protein refers to monoclonal immunoglobulins or fragments created by abnormal monoclonal B cells or plasma cells to define ISS stage in MM[12]. Its deposition could cause destruction of organs such as the kidneys and skin[17]. The M protein level as a clonal burden is considered to be helpful in predicting the risk of progression of monoclonal gammopathy of undetermined significance (MGUS) to symptomatic diseases[18]. Furthermore, monoclonal gammopathy could affect bone marrow microenvironment, resulting in increased risk of infections, osteoporosis, venous and arterial thrombosis, and bone fractures[18]. In addition, the production of M protein that has autoantibody activity or its deposition in tissues are considered responsible for severe organ damage[18]. González-Calle et al[19] have found Bence-Jones proteinuria as a kind of M protein disorder, and it can act as a tumor burden marker, showing a significant association with the risk of progression to symptomatic progression. Caers et al[20] demonstrated M protein as a significant risk factor in most of the patients with Smoldering MM (SMM) evolving into MM. Another study from Spain revealed that M protein with an increase of ≥ 10% in the first 12 mo of diagnosis was associated with progression to symptomatic MM in 71% of cases at 3 years with a median period of 1.1 year[21]. Gassiot et al[22] found that in patients presenting both a prior MGUS/SMM and partial remission (PR) (PR was defined as a ≥ 90% reduction of urinary M protein in 24 h or < 200 mg per 24 h and a reduction of ≥ 50% of serum M protein) after the first cycle of therapy, the PFS and OS showed significant differences from those of the remaining patients. Another study revealing that a fast response to the first treatment cycle in MM patients is the major predictor of long-term response to lenalidomide and dexamethasone therapy also supported the same concept[22]. Atkin et al[23] believed that M protein production is reduced by treatment with chemotherapy, which improved the outcomes of MGUS.
In this retrospective analysis, we found a significant difference in the outcomes between a standard-risk group of newly diagnosed MM patients with a C1 reduction rate of M protein of ≥ 50% vs < 50%, and between those with a C4 reduction rate of M protein of ≥ 75% vs < 75%; the median PFS was 20 mo vs 33 mo and 18 mo vs 30 mo, respectively, showing a significant difference between groups. In multivariate analysis, a higher reduction rate of M protein after the first and fourth chemotherapy cycles was demonstrated to be advantageous factors for PFS, with the reduction rate of M protein after the fourth chemotherapy cycle of ≥ 75% being stronger. Although the reduction rate of M protein after the first and fourth chemotherapy cycles were not identified as independent prognostic factors for OS in multivariate analysis, there is a trend of a longer OS associated with a higher reduction rate of M protein after the fourth chemotherapy cycle (≥ 75%). It has been more than 30 years since chemotherapy was initially combined with autologous stem cell transplantation (ASCT) for the treatment of MM, which remained to be standard care for few patients with newly diagnosed MM[24-26]. Our study also supported this, and ASCT after chemotherapy was regarded as a protective factor for both PFS and OS. This might be one of the reasons for the association of a higher reduction rate of M protein with a longer PFS. After achieving a high reduction rate, more patients will have a chance to undergo ASCT. Furthermore, our study found TC as a protective factor for both PFS and OS. Jafri et al[27] revealed an inverse correlation between cholesterol level and the risk of hematologic malignancy, but the mechanism remains unclear. A previous study revealed that low platelet count is associated with an unfavorable OS[28]. Similar to previous studies, high ISS stage and age were identified as disadvantageous factors for PFS and OS in this study[29-31].
Our study have identified new independent prognostic factors for patients with newly diagnosed MM, and a higher reduction rate of M protein after the first chemotherapy cycle (≥ 50%) and the fourth chemotherapy cycle (≥ 75%) is associated with a longer PFS. The high reduction rate of M protein after the fourth chemotherapy cycle is associated with OS. To our knowledge, this is the first study to analyze the effects of the reduction rate of M protein after chemotherapy in MM patients. The new prognostic factors could help doctors to administer the treatment in time.
Multiple myeloma (MM) is a common hematologic malignancy that originates from a malignant clone of plasma cells. Solitary plasmacytoma, history of diabetes, and platelet count are considered as prognostic factors for MM. But some patients are still associated with much worse outcomes without any prognostic factors.
To study the potential prognostic factors in MM patients.
This study aimed to observe the reduction rate of monoclonal protein (M protein) after the first and fourth chemotherapy cycles, which is considered as a new prognostic factor for progression-free survival (PFS) in standard-risk group of newly diagnosed MM patients.
We retrospectively analyzed 164 patients diagnosed with standard-risk MM for the first time, and compared the PFS and overall survival (OS) between patients with a reduction rate of M protein after first chemotherapy of ≥ 50% vs < 50% and between patients with a reduction rate of M protein after the fourth chemotherapy cycle of ≥ 75% vs < 75%.
Multivariate analysis revealed age [hazard ratio (HR): 1.059, 95% confidence intervals (95%CI): 1.033-1.085, P ≤ 0.001], International Staging System stage (HR: 2.136, 95%CI: 1.500-3.041, P ≤ 0.001), autotransplantion (HR: 0.201, 95%CI: 0.069-0.583, P = 0.019), total cholesterol (HR: 0.689, 95%CI: 0.533-0.891, P = 0.019), the first cycle reduction rate (HR: 0474, 95%CI: 0.293-0.767, P = 0.019), and the fourth cycle reduction rate (HR: 0.254, 95%CI: 0.139-0.463, P = 0.019) as predictors of PFS. The Kaplan-Meier survival analysis and the log-rank tests revealed that a higher reduction rate of M protein after the first cycle (≥ 50%) and fourth cycle (≥ 75%) chemotherapy was associated with a longer PFS than the lower one.
Our study have identified new prognostic factors for patients with initially diagnosed MM, and a higher reduction rate of M protein after the first chemotherapy cycle (≥ 50%) and the fourth chemotherapy cycle (≥ 75%) is associated with a longer PFS. The high reduction rate of M protein after the fourth chemotherapy cycle could is associated with the OS.
To our knowledge, this is the first study to analyze the effects of the reduction rate of M protein after chemotherapy in MM patients. The new prognostic factors could help doctors to administer the treatment in time.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Roganovic J, Croatia S-Editor: Lin C L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13166] [Article Influence: 1880.9] [Reference Citation Analysis (4)] |
| 2. | Rajkumar SV, Kumar S. Multiple Myeloma: Diagnosis and Treatment. Mayo Clin Proc. 2016;91:101-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 342] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 3. | Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM, Greipp PR. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1522] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 4. | Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, Morgan G, Van Ness B, Chesi M, Minvielle S, Neri A, Barlogie B, Kuehl WM, Liebisch P, Davies F, Chen-Kiang S, Durie BG, Carrasco R, Sezer O, Reiman T, Pilarski L, Avet-Loiseau H; International Myeloma Working Group. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23:2210-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 651] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 5. | Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, Leyvraz S, Michallet M, Yakoub-Agha I, Garderet L, Marit G, Michaux L, Voillat L, Renaud M, Grosbois B, Guillerm G, Benboubker L, Monconduit M, Thieblemont C, Casassus P, Caillot D, Stoppa AM, Sotto JJ, Wetterwald M, Dumontet C, Fuzibet JG, Azais I, Dorvaux V, Zandecki M, Bataille R, Minvielle S, Harousseau JL, Facon T, Mathiot C. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myélome. Blood. 2007;109:3489-3495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 674] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 6. | Dispenzieri A, Rajkumar SV, Gertz MA, Fonseca R, Lacy MQ, Bergsagel PL, Kyle RA, Greipp PR, Witzig TE, Reeder CB, Lust JA, Russell SJ, Hayman SR, Roy V, Kumar S, Zeldenrust SR, Dalton RJ, Stewart AK. Treatment of newly diagnosed multiple myeloma based on Mayo Stratification of Myeloma and Risk-adapted Therapy (mSMART): consensus statement. Mayo Clin Proc. 2007;82:323-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Stewart AK, Bergsagel PL, Greipp PR, Dispenzieri A, Gertz MA, Hayman SR, Kumar S, Lacy MQ, Lust JA, Russell SJ, Witzig TE, Zeldenrust SR, Dingli D, Reeder CB, Roy V, Kyle RA, Rajkumar SV, Fonseca R. A practical guide to defining high-risk myeloma for clinical trials, patient counseling and choice of therapy. Leukemia. 2007;21:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Rajkumar SV. Multiple myeloma: 2012 update on diagnosis, risk-stratification, and management. Am J Hematol. 2012;87:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Avet-Loiseau H, Hulin C, Campion L, Rodon P, Marit G, Attal M, Royer B, Dib M, Voillat L, Bouscary D, Caillot D, Wetterwald M, Pegourie B, Lepeu G, Corront B, Karlin L, Stoppa AM, Fuzibet JG, Delbrel X, Guilhot F, Kolb B, Decaux O, Lamy T, Garderet L, Allangba O, Lifermann F, Anglaret B, Moreau P, Harousseau JL, Facon T. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the intergroupe francophone du myélome experience. J Clin Oncol. 2013;31:2806-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Palumbo A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK, Offidani M, McCarthy P, Evangelista A, Lonial S, Zweegman S, Musto P, Terpos E, Belch A, Hajek R, Ludwig H, Stewart AK, Moreau P, Anderson K, Einsele H, Durie BGM, Dimopoulos MA, Landgren O, Miguel JFS, Richardson P, Sonneveld P, Rajkumar SV. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068-2074. [RCA] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 580] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 11. | Gutiérrez NC, Castellanos MV, Martín ML, Mateos MV, Hernández JM, Fernández M, Carrera D, Rosiñol L, Ribera JM, Ojanguren JM, Palomera L, Gardella S, Escoda L, Hernández-Boluda JC, Bello JL, de la Rubia J, Lahuerta JJ, San Miguel JF; GEM/PETHEMA Spanish Group. Prognostic and biological implications of genetic abnormalities in multiple myeloma undergoing autologous stem cell transplantation: t(4;14) is the most relevant adverse prognostic factor, whereas RB deletion as a unique abnormality is not associated with adverse prognosis. Leukemia. 2007;21:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, Lahuerta JJ, Ludwig H, Morgan G, Powles R, Shimizu K, Shustik C, Sonneveld P, Tosi P, Turesson I, Westin J. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412-3420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 2032] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 13. | Ludwig H, Durie BG, Bolejack V, Turesson I, Kyle RA, Blade J, Fonseca R, Dimopoulos M, Shimizu K, San Miguel J, Westin J, Harousseau JL, Beksac M, Boccadoro M, Palumbo A, Barlogie B, Shustik C, Cavo M, Greipp PR, Joshua D, Attal M, Sonneveld P, Crowley J. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group. Blood. 2008;111:4039-4047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 14. | Stella-Holowiecka B, Czerw T, Holowiecka-Goral A, Giebel S, Wojnar J, Holowiecki J. Beta-2-microglobulin level predicts outcome following autologous hematopoietic stem cell transplantation in patients with multiple myeloma. Transplant Proc. 2007;39:2893-2897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1742] [Cited by in RCA: 1893] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 16. | Kumar SK, Rajkumar SV. The multiple myelomas - current concepts in cytogenetic classification and therapy. Nat Rev Clin Oncol. 2018;15:409-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 229] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 17. | Sethi S, Fervenza FC, Rajkumar SV. Spectrum of manifestations of monoclonal gammopathy-associated renal lesions. Curr Opin Nephrol Hypertens. 2016;25:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | van de Donk NW, Palumbo A, Johnsen HE, Engelhardt M, Gay F, Gregersen H, Hajek R, Kleber M, Ludwig H, Morgan G, Musto P, Plesner T, Sezer O, Terpos E, Waage A, Zweegman S, Einsele H, Sonneveld P, Lokhorst HM; European Myeloma Network. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network. Haematologica. 2014;99:984-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | González-Calle V, Dávila J, Escalante F, de Coca AG, Aguilera C, López R, Bárez A, Alonso JM, Hernández R, Hernández JM, de la Fuente P, Puig N, Ocio EM, Gutiérrez NC, García-Sanz R, Mateos MV. Bence Jones proteinuria in smoldering multiple myeloma as a predictor marker of progression to symptomatic multiple myeloma. Leukemia. 2016;30:2026-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Caers J, Fernández de Larrea C, Leleu X, Heusschen R, Zojer N, Decaux O, Kastritis E, Minnema M, Jurczyszyn A, Beguin Y, Wäsch R, Palumbo A, Dimopoulos M, Mateos MV, Ludwig H, Engelhardt M. The Changing Landscape of Smoldering Multiple Myeloma: A European Perspective. Oncologist. 2016;21:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Fernández de Larrea C, Isola I, Pereira A, Cibeira MT, Magnano L, Tovar N, Rodríguez-Lobato LG, Calvo X, Aróstegui JI, Díaz T, Lozano E, Rozman M, Yagüe J, Bladé J, Rosiñol L. Evolving M-protein pattern in patients with smoldering multiple myeloma: impact on early progression. Leukemia. 2018;32:1427-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Gassiot S, González Y, Morgades M, Motlló C, Clapés V, Maluquer C, Ibarra G, Abril L, Ribera JM, Oriol A. Response to First Cycle Is the Major Predictor of Long-Term Response to Lenalidomide and Dexamethasone Therapy in Relapsed and Refractory Multiple Myeloma: Can We Spare Patients the Toxicity and Costs of Additional Agents? Clin Lymphoma Myeloma Leuk. 2019;19:585-592.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Atkin C, Richter A, Sapey E. What is the significance of monoclonal gammopathy of undetermined significance? Clin Med (Lond). 2018;18:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Choi T. Is autologous stem cell transplantation still relevant for multiple myeloma? Curr Opin Hematol. 2019;26:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Al Hamed R, Bazarbachi AH, Malard F, Harousseau JL, Mohty M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019;9:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 26. | Soekojo CY, Kumar SK. Stem-cell transplantation in multiple myeloma: how far have we come? Ther Adv Hematol. 2019;10:2040620719888111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Jafri H, Alsheikh-Ali AA, Karas RH. Baseline and on-treatment high-density lipoprotein cholesterol and the risk of cancer in randomized controlled trials of lipid-altering therapy. J Am Coll Cardiol. 2010;55:2846-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Kim DS, Yu ES, Kang KW, Lee SR, Park Y, Sung HJ, Choi CW, Kim BS. Myeloma prognostic index at diagnosis might be a prognostic marker in patients newly diagnosed with multiple myeloma. Korean J Intern Med. 2017;32:711-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Sørrig R, Klausen TW, Salomo M, Vangsted AJ, Frølund UC, Andersen KT, Klostergaard A, Helleberg C, Pedersen RS, Pedersen PT, Helm-Petersen S, Teodorescu EM, Preiss B, Abildgaard N, Gimsing P; Danish Myeloma Study Group. Immunoparesis in newly diagnosed Multiple Myeloma patients: Effects on overall survival and progression free survival in the Danish population. PLoS One. 2017;12:e0188988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Andriandi, Kamal AF. Survival rate of multiple myeloma patients in Indonesia: A retrospective study in multiple myeloma at a single institution. Ann Med Surg (Lond). 2019;41:11-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Chretien ML, Hebraud B, Cances-Lauwers V, Hulin C, Marit G, Leleu X, Karlin L, Roussel M, Stoppa AM, Guilhot F, Lamy T, Garderet L, Pegourie B, Dib M, Sebban C, Lenain P, Brechignac S, Royer B, Wetterwald M, Legros L, Orsini-Piocelle F, Voillat L, Delbrel X, Caillot D, Macro M, Facon T, Attal M, Moreau P, Avet-Loiseau H, Corre J. Age is a prognostic factor even among patients with multiple myeloma younger than 66 years treated with high-dose melphalan: the IFM experience on 2316 patients. Haematologica. 2014;99:1236-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |