Published online Aug 16, 2023. doi: 10.12998/wjcc.v11.i23.5589
Peer-review started: May 16, 2023
First decision: June 13, 2023
Revised: June 27, 2023
Accepted: July 25, 2023
Article in press: July 25, 2023
Published online: August 16, 2023
Processing time: 92 Days and 2.7 Hours
Polyethylene glycol (PEG) is widely used as an additive because of its hydrophilic and chemically inert properties. However, there are been increasing reports of PEG allergies, including anaphylaxis, although they are still rare. This case report aims to raise awareness, that the commonly used bowel cleansing agent contain
Prior to surgery for sigmoid colon cancer, a 63-year-old man was prescribed a bowel cleansing agent containing PEG. Within 30 min of ingestion, he developed symptoms of anaphylactic shock and did not respond to initial intramuscular epinephrine injection. Under diagnosis of anaphylaxis to PEG, he was stabilized with fluid hydration and continuous norepinephrine infusion.
While allergic reactions to PEG are rare, they can be life-threatening. Therefore, it is crucial for clinicians to be aware of this possibility and to diagnose and resu
Core Tip: Polyethylene glycol (PEG) is a widely used additive and generally considered a non-allergenic substance due to its chemical inertness and poor absorption in the gastrointestinal tract. Due to its hydrophilic nature, it is also used as bowel cleansing agents before colonoscopy and colorectal surgery. Although allergic reactions to PEG are rare, reports of such allergies are increasing and can be life-threatening anaphylactic shock. By presenting a 63-year-old man who experienced anaphylactic shock during bowel preparation using PEG, we announced the allergic potential of PEG for timely diagnosis and proper management.
- Citation: Park GW, Park N, Kuk JC, Shin EJ, Lim DR. Anaphylactic shock induced by polyethylene glycol after bowel preparation for the colorectal cancer surgery: A case report. World J Clin Cases 2023; 11(23): 5589-5594
- URL: https://www.wjgnet.com/2307-8960/full/v11/i23/5589.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i23.5589
Polyethylene glycol (PEG) is a widely used additive in medical, pharmaceutical, cosmetic and food industries due to its hydrophilic nature. It acts as an osmotic laxative and a bulking and stabilizing agent[1]. Depending on its molecular weight (MW), various types of PEG are available, with larger MW being less absorbed in the gastrointestinal tract[2,3]. Although allergic reactions to PEG are rare, reports of such allergies are increasing and can range from mild hypersensitivity reactions like urticaria and pruritus to life-threatening anaphylactic shock[4].
Therefore, it is important to be aware of the allergic possibility of PEG and to provide proper management. In this case report, we present a 63-year-old man who experienced anaphylactic shock triggered by PEG 3350 for better under
A 63-year-old male developed systemic urticaria and pruritus after taking the PEG-containing agent for 30 min.
The patient was scheduled for the laparoscopic anterior resection under the diagnosis of sigmoid colon cancer. The day before the surgery he took a bowel cleansing agent containing PEG for bowel preparation, and after 30 min of ingestion he developed symptoms.
The patient has taken aspirin and clopidogrel due to history of acute myocardial infarction, and is also a carrier of hepatitis B virus controlled with tenofovir, with no history of allergies. He received three doses of the Moderna vaccine without any other complications.
The patient has no family history of allergy and cancer.
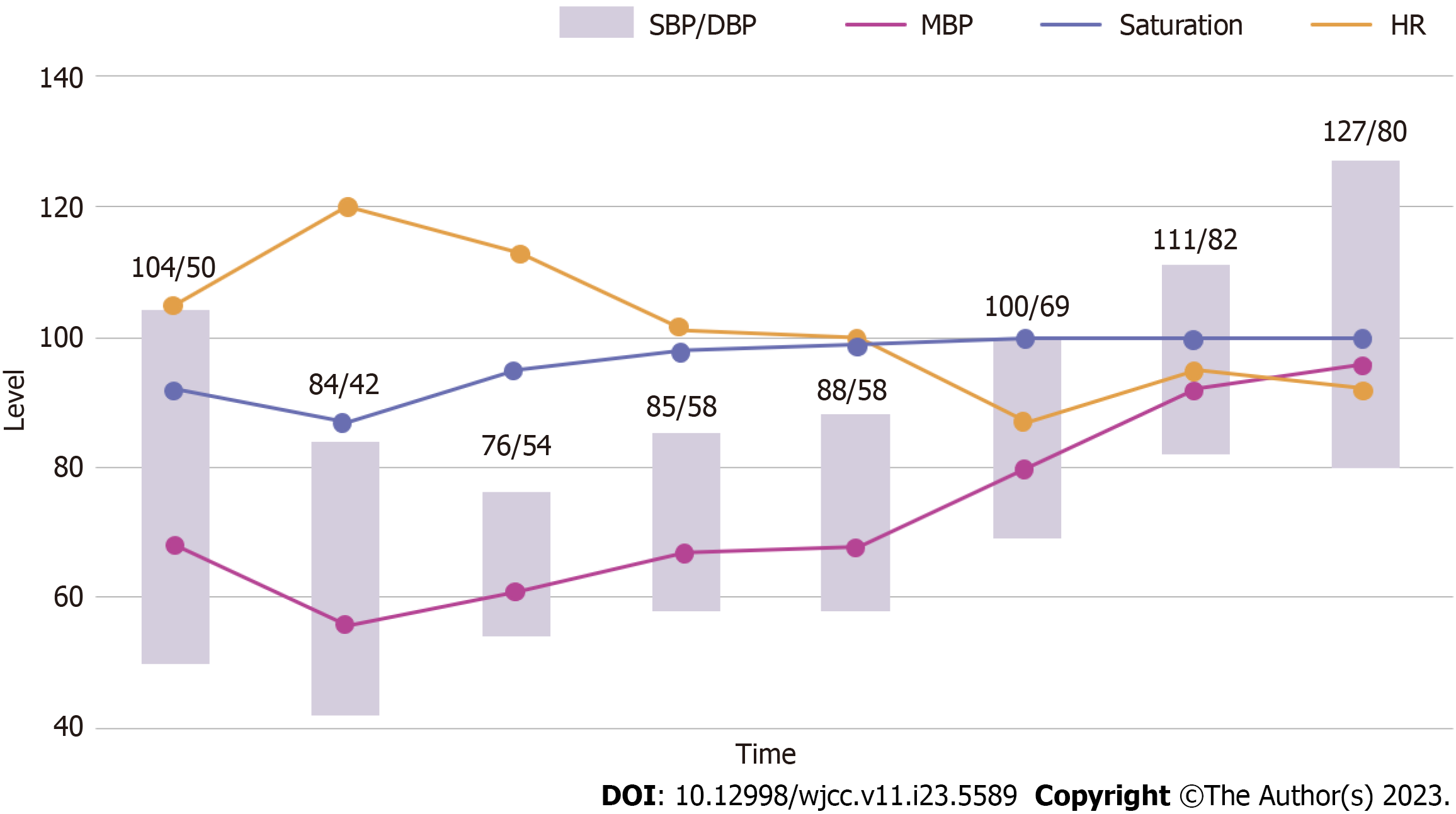
His blood pressure (BP) was 104/50 mmHg, and his heart rate was 105 beats per minute. Despite receiving intravenous injections of chlorpheniramine 4 mg and dexamethasone 5 mg, as well as hydration with 0.9% normal saline, his BP dropped to 84/42 mmHg with oxygen saturation of 87% on room air, indicating signs of anaphylactic shock. He received 0.3 mg of intramuscular epinephrine and supplemental oxygen via nasal cannula at 5 liters per minute. His oxygen saturation improved to 95%, but his BP remained low at 76/54 mmHg (Figure 1).
Lactic acid was elevated to 5.2 mmol/L. CK-MB and Troponin T were normal at first, but increased to 118.2 ng/mL and 1.770 ng/mL. To check the recurrence of myocardial infarction, the underlying disease of the patient, further evaluations were performed.
Chest X-rays were performed, and there were no specific findings.
Comprehensively considering patient progress and imaging examinations, PEG-induced anaphylactic shock was diagnosed.
It was decided to transfer him to the intensive care unit for further resuscitation with norepinephrine infusion and close monitoring. To maintain hemodynamic stability, norepinephrine infusion was initiated at 0.1 mcg/kg/min. The patient’s hemodynamics were continuously monitored and resuscitated by placing right internal jugular central and right brachial arterial lines. His BP was stabilized within the normal range and eventually the norepinephrine infusion was discontinued. After he had fully recovered and stabilized, he underwent the scheduled surgery and was discharged on the 7th post-operative day.
After he had fully recovered and stabilized, he underwent the scheduled surgery and was discharged on the 7th post-operative day.
PEGs, also known as macrogols, are hydrophilic polymers of ethylene. Depending on their MW, there are various types of PEGs ranging from 400 to 20000. Each type of PEG with different MW is used in various fields. Notably, the higher the MW, the less absorption occurs in the gastrointestinal tract, making it an ideal bowel cleansing agent before colon surgery or diagnostic colonoscopy[5].
Colyte®, the bowel preparation agent taken by the patient in this case, contains 29.5 g of PEG 3350, 0.37 g of potassium chloride, 0.84 g of sodium bicarbonate, 0.73 g of sodium chloride and 2.85 g of anhydrous sodium sulfate. This formu
PEG is generally considered a non-allergenic substance due to its chemical inertness and poor absorption in the gastrointestinal tract. However, it can trigger an allergic reaction if the larger MW of PEG is absorbed and sensitized[6]. A study has shown that PEG is excreted in urine during gut lavage, suggesting that it can be absorbed and trigger an immune response[7].
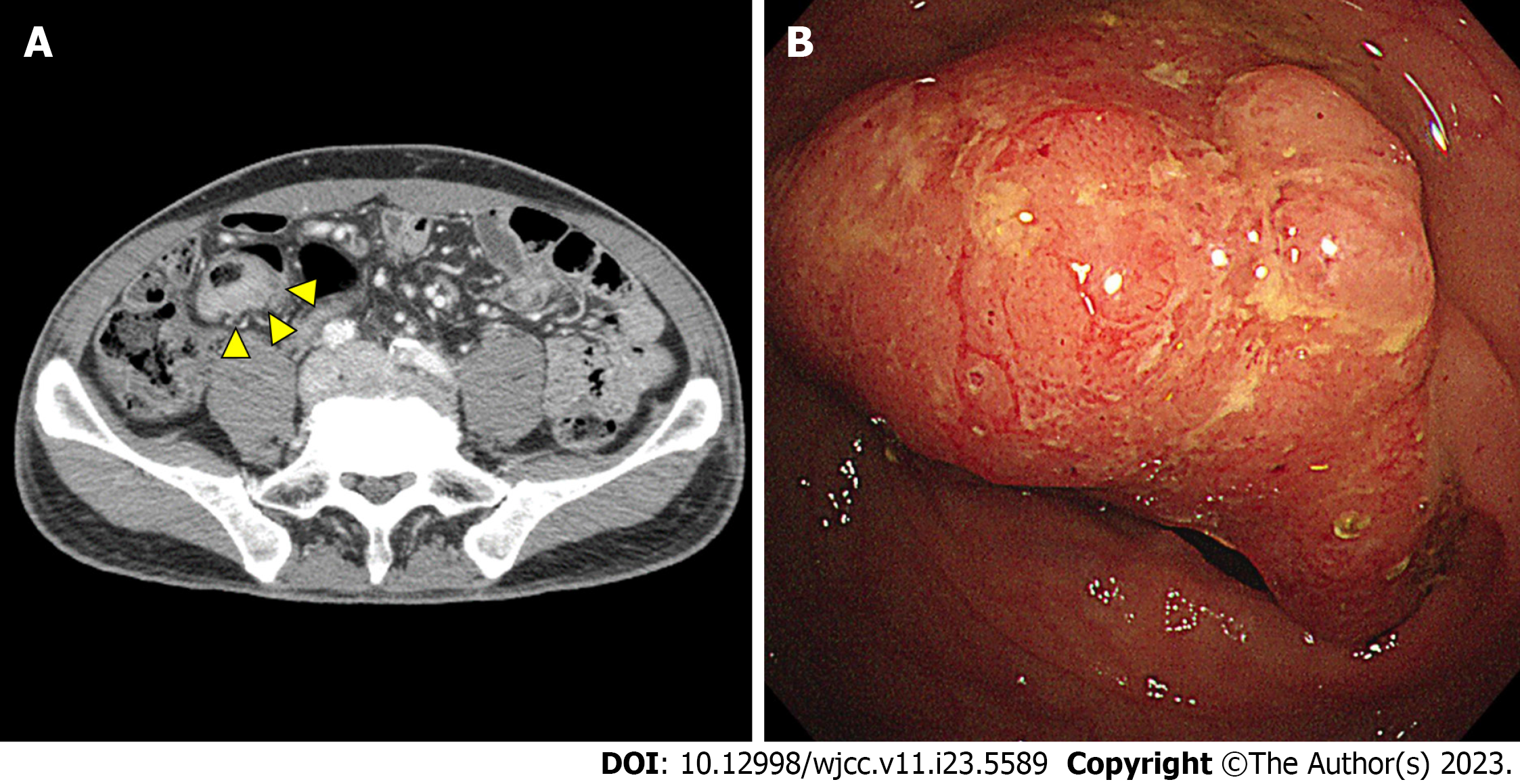
After ingestion, PEG is typically not absorbed by the normal intestinal mucosa. However, if the mucosal barrier is impaired for any reason, PEG can be absorbed through mucosal breaks. Gastrointestinal diseases such as diverticulitis and ulcerative colitis can cause a loss of mucosal integrity. In one case, a 39-year-old man with a history of diverticulitis experienced an anaphylactic reaction after taking Colyte®[8]. A study has shown that patients with active ulcerative colitis have a higher rate of PEG absorption than those in remission, and the absorption rate during remission is similar to that of those without the disease[9]. The patient in this case had the eccentric enhancing wall thickening of the sigmoid colon with an infiltrated polypoid lesion accompanying ulceration, which may have been a predisposing factor for anaphylaxis to Colyte® (Figure 2).
A review article described 37 reported cases of PEG hypersensitivity, with symptoms ranging from skin irritation, such as urticaria, pruritus, and erythema, to life-threatening cardiovascular collapse, such as hypotension and respiratory distress[10]. In more than half of the cases, bowel cleanser was the cause of the allergic reactions, which is thought to be due to the high concentration of PEG and predisposing factor of colonoscopy or surgery, inflammation or damage to the gastrointestinal tract[10]. Additional cases of the allergic reactions caused by PEG-containing bowel cleanser are listed in Table 1.
| Age | Sex | Exposure | Symptoms | Ref. |
| 39 | Male | Colyte® (PEG 3350) | Hypotension, dyspnea, urticaria, itching | [15] |
| 74 | Male | HalfLytely® (PEG 3350) | Hypotension, erythema, hoarseness, choking sensation | [5] |
| 30 | Female | Golytely® (PEG 3350) | Urticaria, pruritus, erythema, chest tightness | [8] |
| 52 | Male | PEG-containing bowel cleansing agent (PEG 3350) | Hypotension, tachycardia, flushing, urticaria, wheezing, unconsciousness | [16] |
| 36 | Male | Bohm® (PEG 4000) | Urticaria, angioedema | [17] |
| 44 | Male | Bohm® (PEG 4000) | Urticaria, angioedema | [17] |
| 52 | Female | Golytely® (PEG 3350) | Dyspnea, angioedema, pruritus | [18] |
| 70 | Male | Golytely® (PEG 3350) | Oral tingling, tong swelling, angioedema, edema of lower extremity | [19] |
| 44 | Female | Golytely® (PEG 3350) | Unconsciousness, seizures | [20] |
It is worth noting that the MW of PEG determines whether an allergic reaction occurs. For instance, a 42-year-old woman who tolerated tablets containing PEG 4000 and PEG 8000 developed generalized urticaria and angioedema after taking tablets with PEG 20000[11]. Similarly, a 20-year-old man who was regularly prescribed Mesalazine MR (PEG 6000) without any allergic reaction developed near-fatal anaphylaxis after taking Gaviscon Double Action tablets (PEG 20000)[11]. PEG is used as a tablet coating agent, and it is believed to be very small, so it can be seen allergic reactions occurred depending on the MW.
The amount of ingested PEG is a critical factor in determining an individual’s allergic reaction. For example, a 44-year-old woman diagnosed with PEG allergy underwent an oral challenge test with PEG 4000. The patient had previously taken oral medications containing PEG 4000 without any allergic reactions. The oral test started with a dose of 1 mg of PEG 4000 and reached a cumulative dose of 7.1 g, which point the patient developed pruritus followed by edema of the lips, eyelids, feet, and hands[12]. Similarly, the patient in this case had taken Plavix® (PEG 6000) for myocardial infarction, Gasmotin® (PEG 6000), and Gaster® (PEG 20000) for dyspepsia without any allergic reactions. Additionally, after surgery for sigmoid colon cancer, this patient received wound care with a Mepilex® bandage (PEG-containing film) without any skin irritation. Although Colyte® (PEG 3350) has a relatively small MW, one package contains 29.5 g of PEG, and this patient had taken more than two packages of Colyte®. Thus, patients may have an individual MW threshold in com
PEG 2000 is an excipient used in the production of severe acute respiratory syndrome coronavirus 2 mRNA vaccines by Moderna and Pfizer-BioNTech. However, the incidence of anaphylaxis cases related to these vaccines is higher than expected, with 5.15 and 4.80 cases per million doses reported for Moderna and Pfizer-BioNTech respectively. This suggests that PEG may be a possible culprit of allergic reactions[13,14]. The patient in this case received three doses of the Moderna vaccine without any other complications but undergone allergy testing. So it is unclear whether the MW of the PEG contained in the vaccine is insufficient to sensitize, whether it is sufficient to sensitize but not enough to cause an allergic reaction, or whether the amount of PEG was insufficient to cause an allergic reaction. Further evaluation is necessary to determine the patient’s threshold and prevent potential allergic reactions.
At our medical center, the typical length of stay after sigmoid colon cancer surgery is seven days. However, due to this patient’s allergic reaction and subsequent elevation of CK-MB and Troponin I levels, the hospital stay was extended to 21 d to ensure that there were no signs of recurrent myocardial infarction before proceeding with surgery. Allergic reactions not only cause significant discomfort for the patient but can also be life-threatening and prolong hospitalization, even if the patient ultimately recovers.
PEG is widely used across various fields and is generally considered to cause few allergic reactions. However, in rare cases, it can trigger severe allergic reactions that may be life-threatening. Due to the low awareness of the allergenic potential of PEG and the challenges associated with avoiding its widespread use, patients with PEG allergy are at risk of under-diagnosis and repeated exposure. Therefore, it is important for clinicians to be aware of the potential for PEG which is used to bowel preparation before the colorectal surgery to cause allergic reactions, promptly diagnose cases of PEG allergy, and provide appropriate management to prevent future episodes of allergic reactions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dimofte GM, Romania; Seow-Choen F, Singapore; Spadaccini M, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Wong-On-Wing A, Ruth K, Hinerth K, Deng A, Woldekiros M, Ellenbogen RG, Crowder CM. Severe Polyethylene Glycol Allergy Considerations for Perioperative Management: A Case Report. A A Pract. 2022;16:e01619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Henning T. Polyethylene glycols (PEGs) and the pharmaceutical industry. Fharma Chem. 2001;1:57-59. |
| 3. | Chadwick VS, Phillips SF, Hofmann AF. Measurements of intestinal permeability using low molecular weight polyethylene glycols (PEG 400). II. Application to normal and abnormal permeability states in man and animals. Gastroenterology. 1977;73:247-251. [PubMed] |
| 4. | Cox F, Khalib K, Conlon N. PEG That Reaction: A Case Series of Allergy to Polyethylene Glycol. J Clin Pharmacol. 2021;61:832-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Gachoka D. Polyethylene Glycol (PEG)-Induced Anaphylactic Reaction During Bowel Preparation. ACG Case Rep J. 2015;2:216-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49:6288-6308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2443] [Cited by in RCA: 2515] [Article Influence: 167.7] [Reference Citation Analysis (0)] |
| 7. | Brady CE 3rd, DiPalma JA, Morawski SG, Santa Ana CA, Fordtran JS. Urinary excretion of polyethylene glycol 3350 and sulfate after gut lavage with a polyethylene glycol electrolyte lavage solution. Gastroenterology. 1986;90:1914-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Lee SH, Hwang SH, Park JS, Park HS, Shin YS. Anaphylaxis to Polyethylene Glycol (Colyte®) in a Patient with Diverticulitis. J Korean Med Sci. 2016;31:1662-1663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Almer S, Franzén L, Olaison G, Smedh K, Ström M. Increased absorption of polyethylene glycol 600 deposited in the colon in active ulcerative colitis. Gut. 1993;34:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46:907-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 251] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 11. | Sellaturay P, Nasser S, Ewan P. Polyethylene Glycol-Induced Systemic Allergic Reactions (Anaphylaxis). J Allergy Clin Immunol Pract. 2021;9:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 12. | Sohy C, Vandenplas O, Sibille Y. Usefulness of oral macrogol challenge in anaphylaxis after intra-articular injection of corticosteroid preparation. Allergy. 2008;63:478-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, Donahue JG, Kharbanda EO, Naleway A, Nelson JC, Xu S, Yih WK, Glanz JM, Williams JTB, Hambidge SJ, Lewin BJ, Shimabukuro TT, DeStefano F, Weintraub ES. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA. 2021;326:1390-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 461] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 14. | McNeil MM, DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol. 2018;141:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 15. | Lee SH, Cha JM, Lee JI, Joo KR, Shin HP, Baek IH, Jeon JW, Lim JU, Lee JL, Lee HM, Cho YH. Anaphylactic shock caused by ingestion of polyethylene glycol. Intest Res. 2015;13:90-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Shah S, Prematta T, Adkinson NF, Ishmael FT. Hypersensitivity to polyethylene glycols. J Clin Pharmacol. 2013;53:352-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Antón Gironés M, Roan Roan J, de la Hoz B, Sánchez Cano M. Immediate allergic reactions by polyethylene glycol 4000: two cases. Allergol Immunopathol (Madr). 2008;36:110-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Assal C, Watson PY. Angioedema as a hypersensitivity reaction to polyethylene glycol oral electrolyte solution. Gastrointest Endosc. 2006;64:294-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Stollman N, Manten HD. Angioedema from oral polyethylene glycol electrolyte lavage solution. Gastrointest Endosc. 1996;44:209-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Denham DDJ. Polyethylene glycol (Peg 3350)-induced anaphylaxis: a case report. South Med J. 1992;44:209-210. |