Published online Aug 16, 2023. doi: 10.12998/wjcc.v11.i23.5567
Peer-review started: May 4, 2023
First decision: June 12, 2023
Revised: June 28, 2023
Accepted: July 27, 2023
Article in press: July 27, 2023
Published online: August 16, 2023
Processing time: 103 Days and 23.6 Hours
Townes–Brocks syndrome (TBS) is a rare autosomal dominant syndrome that is characterized by a triad of imperforate anus, dysplastic ears, and thumb malformations. Heterozygous variants of SALL1 are responsible for this syndrome. Renal structural abnormalities and functional impairments are often reported in TBS patients.
We report a case of TBS in a Chinese family. The index patients showed obvious renal atrophy and renal failure. TBS was suggested after a physical examination and pedigree analysis. Whole exome sequencing revealed a heterozygous variant of SALL1. The variant (NM_001127892 c.1289_c.1290 insC) led to a read-frame shift of the encoded protein, which was confirmed by Sanger sequencing. The variant cosegregated with the phenotype among affected members.
A novel variant in SALL1 gene may be the molecular pathogenic basis of this disorder.
Core Tip: Townes-Brocks syndrome (TBS) is a rare autosomal dominant syndrome, which is caused by the mutations of SALL1. We report a case of TBS with renal impairment in a Chinese family. TBS was suspected and a heterozygous variant of SALL1 was revealed by whole exome sequencing. The variant led to a read-frame shift of the encoded protein. The variant co-segregated with the phenotype among affected members, which suggested that the variant of SALL1 might be the molecular pathogenic basis of this disorder.
- Citation: Wu J, Zhang J, Xiao TL, He T. Townes–Brocks syndrome with adult renal impairment in a Chinese family: A case report. World J Clin Cases 2023; 11(23): 5567-5572
- URL: https://www.wjgnet.com/2307-8960/full/v11/i23/5567.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i23.5567
Townes–Brocks syndrome (TBS, OMIM 107480) is characterized by the triad of imperforate anus, dysplastic ears, and thumb malformations. Other features of the condition include hearing loss, foot malformations, renal impairment, genitourinary malformations, and congenital heart disease. This syndrome is an autosomal dominant syndrome caused by a pathogenic variant of SALL1 gene[1]. This syndrome was first described by Townes and Brocks in 1972, and the mutations in SALL1 responsible for TBS were first identified by Kohlhase in 1998[2,3]. Thereafter, many TBS cases were found with pathogenic variants of SALL1 to make a molecular diagnosis.
The clinical presentations of TBS are complicated. TBS should be suspected in individuals with the three major clinical features: imperforate anus or anal stenosis, dysplastic ears, and typical thumb malformations. As the disease-causing gene is clear, identification of a pathogenic variant of SALL1 by molecular genetic testing can establish the precise diagnosis if clinical features are inconclusive. The protein encoded by SALL1 is a zinc finger transcriptional repressor and may be part of the NuRD histone deacetylase complex. The encoded protein plays a crucial role in the development of multiple organs. Among the TBS patients, one third of cases have impaired renal functions. This category of patients is often treated by nephrologists. However, the clinical diagnosis and treatment are still a challenge for nephrologists. In this case report, we describe an adult TBS patient, whose genetic testing was performed by whole exon sequencing (WES). A pathogenic variant of SALL1 gene was found, which helped to make a precise diagnosis.
A 46-year-old Han Chinese male (height 158 cm, weight 51 kg) was admitted to our department because of renal failure.
He had a history of gout for approximately 18 years, and his serum creatinine level had been elevated for 5 years. The patient was referred to our hospital for better treatment.
Not special.
Pedigree analysis showed that his mother had renal impairment.
No obvious edema was noted, except for slight anemia at the admission check.
Routine blood tests showed decreased blood red cell counts and hemoglobin (Table 1). Serum chemistry showed elevated levels of serum creatinine, uric acid, parathyroid hormone, and cystatin C, as well as decreased levels of albumin and calcium. Urine tests revealed mild proteinuria and occult blood. Based on estimated glomerular filtration rate, the patient was categorized as having end-stage renal disease (ESRD) and was undergoing hemodialysis.
| Parameters | Proband | Mother | Reference range |
| Routine blood tests | |||
| RBC, 1012/L | 2.26 | 4.3-5.8 | |
| HGB, g/L | 70 | 91 | 115-150/130-175 |
| Routine urine tests | |||
| UP | 3+ | 1+ | Negative |
| Urinary occult blood | 1+ | Negative | |
| 24-h UP, g/d | 0.86 | 0-0.12 | |
| UP/urinary creatinine, g/g | 1.42 | 0.23 | |
| Serum chemistry | |||
| Creatinine, μmol/L | 610.5 | 309 | 45-105 |
| Uric acid, μmol/L | 486.0 | 140-420 | |
| Cystatin-C, mg/L | 5.22 | 3.62 | 0-1.16 |
| PTH, pg/mL | 816.1 | 258.0 | 12-65 |
| Albumin, g/L | 34.4 | 39.6 | 40-50 |
| Calcium, mmol/L | 1.88 | 2.02-2.6 | |
| Phosphorus, mmol/L | 1.39 | 0.74 | 0.9-1.34 |
| Thyroxine (T4), nmol/L | 58.42 | 62.68-150.84 | |
| Serum iron, μmol/L | 5.80 | 10.62-29.54 | |
| ESR, mm/h | 57.0 | 0-15 | |
| CPR, mg/L | 59.2 | 0-8 | |
| Immunology | |||
| IgG, g/L | 3.80 | 7-15 | |
| IgA, g/L | 0.23 | 0.7-4.0 | |
| C3, g/L | 0.69 | 0.69 | 0.9-2.1 |
| Lambda, mg/dL | 191.0 | 313-723 | |
| Kappa, mg/dL | 296.0 | 629-1350 |
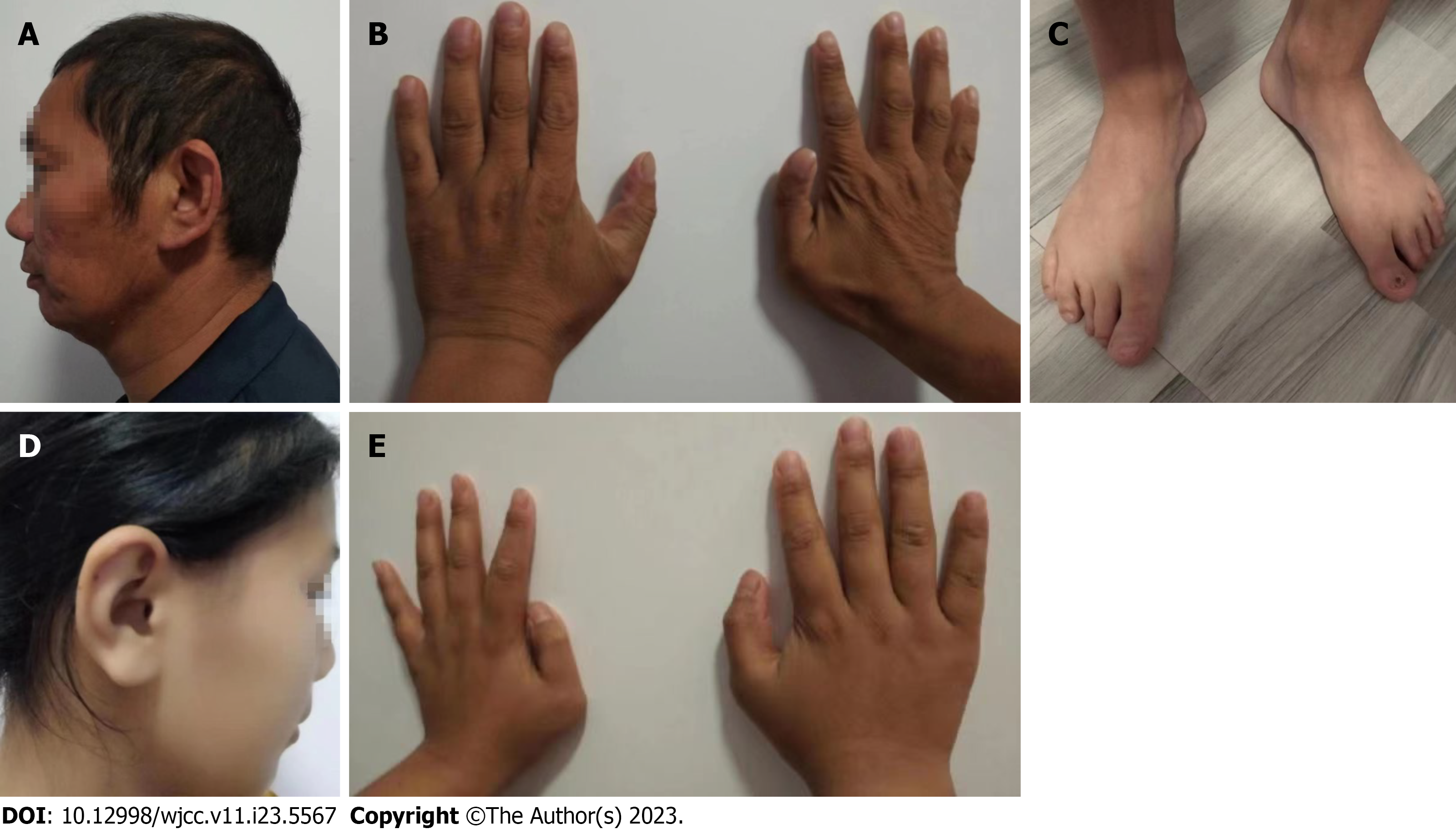
During hospitalization, an abnormality in his hand was noted by our nephrologist, and a more detailed physical examination was performed. Excessive earlobe and toe deformities were detected, and the preaxial polydactyly underwent surgical resection when he was a boy (Figure 1). He was born with an imperforate anus, and surgery was performed after birth to correct anal stenosis. Visual impairment was recognized on examination of the ocular fundus, but no hearing loss was evident.
Abdominal ultrasound examination revealed obvious renal atrophy (right 4.9 cm × 2.0 cm × 0.52 cm, left 5.3 cm × 2.0 cm × 0.59 cm), with thinned renal cortex and hyperechogenicity. No abnormalities were detected in the liver, pancreas or spleen on ultrasound examination.
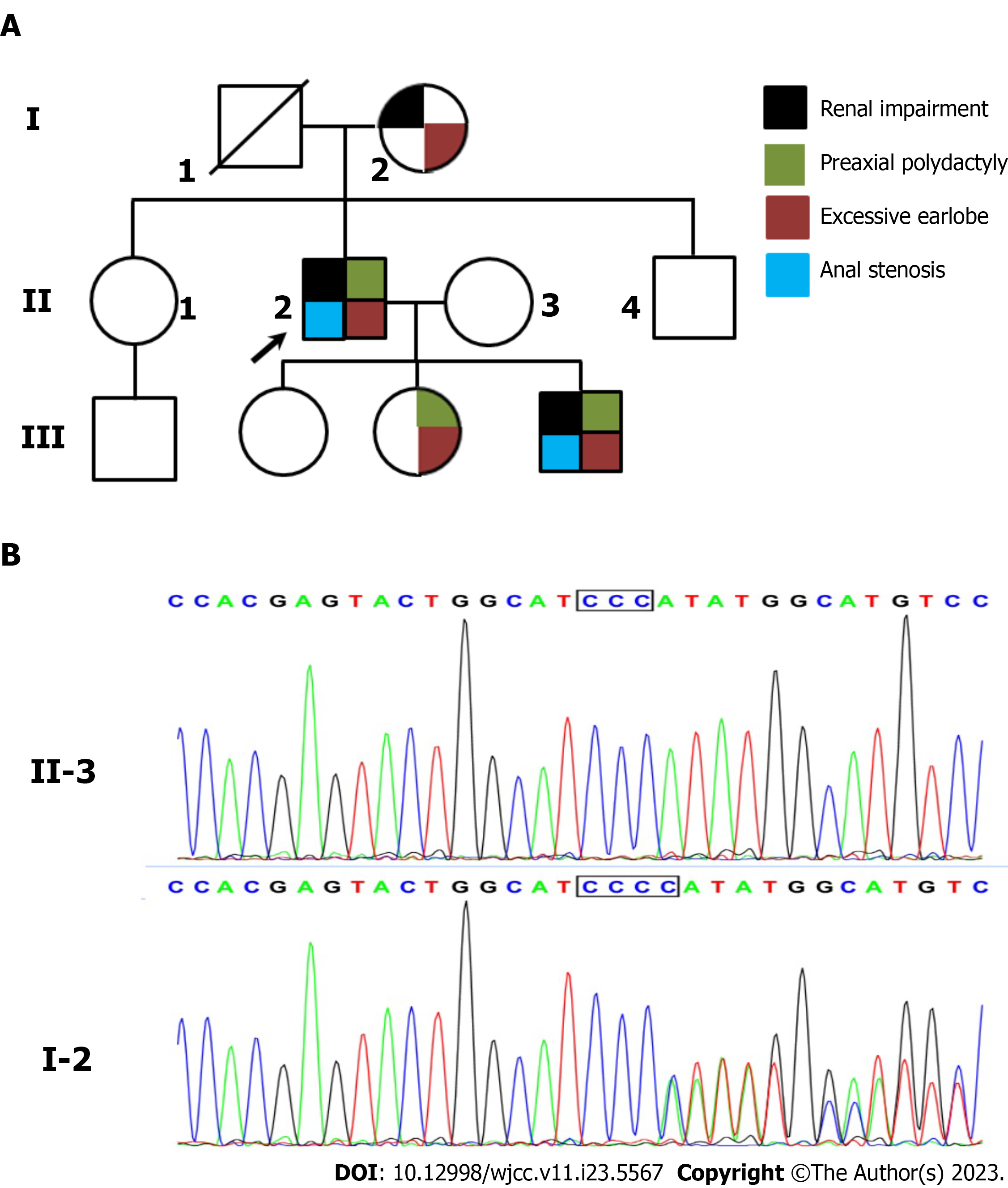
Pedigree analysis showed that his mother had the same appearance of ears and toes, and renal impairment was observed at the age of 68 years (Table 1 and Figure 2). His second daughter had the same symptoms, such as ears, preaxial polydactyly, and toes. She was diagnosed with leukemia at the age of 18 years without any renal impairment. The proband’s son had similar symptoms, but his renal function was normal, except for elevated uric acid (529.0 μmol/L). Based on these findings, TBS was highly suspected in this family.
Considering the diagnosis of TBS and the similar symptoms of the family members, the proband was recommended to undergo genetic testing to make a precise diagnosis. As his father had already died for unknown reasons, DNA samples were isolated from the peripheral blood cells of the proband, his mother, and children. WES was performed by the Chigene (Beijing) Translational Medical Research Center, as previously described[4]. Sequence analysis revealed a heterozygous variant of the SALL1 gene. The variant NM_001127892 c.1289_c.1290 insC led to a read-frame shift of the encoded protein. Sanger sequencing was performed to validate the identified variations (Figure 2). The variant cosegregated with the phenotype among affected members. The variant was excluded from the Single Nucleotide Polymorphism (dbSNP) and Human ClinVar databases. According to the American College of Medical Genetics and Genomics standards and guidelines, the variant was classified as pathogenic (PVS1+PM2+PP1).
TBS with renal impairment.
Due to the high level of serum creatinine, he was treated with temporary hemodialysis. In addition, he received oral nifedipine (30 mg/d) to control the blood pressure, and febuxostat (40 mg/d) to lower the uric acid. Recombinant human EPO was also administered for one week to correct anemia.
At a 1-year follow-up, the patient had stage 5 chronic kidney disease and was treated with regular hemodialysis.
In this case report, a diagnosis of TBS in this family was highly suspected, but uremia was the first symptom to prompt the proband to visit the hospital. After analyzing the family history and symptoms, genetic testing based on WES was performed and a final diagnosis was made. In clinical practice, uremic patients with TBS symptoms are rare, and nephrologists can hardly associate renal impairment with TBS. Therefore, misdiagnosis is often made.
It has been reported that renal anomalies, including functional impairment with or without structural abnormalities, were detected in 43% of patients with TBS. Recently, Beaudoux et al[3] reported two related TBS cases that exhibited kidney hypoplasia (focal and segmental glomerulosclerosis) and ESRD. Their literature review showed that 10 of 44 adult cases of TBS with genetic confirmation had kidney disease. Several studies have reported renal impairment in patients with TBS in the Chinese population. Fang et al[5] reported a novel heterozygous mutation in SALL1 in a TBS family in which the proband and his paternal aunt had a history of unexplained renal failure with hemodialysis. Another study reported a 40-day-old infant with renal failure, polycystic renal dysplasia, and other symptoms. Genetic testing revealed that the patient harbored an unreported frameshift variant of SALL1[6]. Lin et al[7] reported a male patient who presented with multiple bilateral cortical kidney cysts at the age of 4 years and end-stage renal failure at 16 years. As extrarenal involvement, including imperforate anus at birth, preaxial polydactyly, and dysplastic right ear, was evident, TBS was suspected and confirmed by WES. These studies showed that the age of onset of renal impairment varies greatly. In the family, one had no renal presentation and three members had renal impairment. In addition, the age of onset of renal impairment varied from 18 to 68 years. This indicated intrafamilial variability in the renal presentation.
SALL1 encodes a zinc finger transcriptional repressor, which contains 11 WT1-binding sites and one SIX1-binding site. Currently, approximately 300 variants have been deposited in the ClinVar database, and 58 variants are categorized as pathogenic or likely pathogenic. Most of them are nonsense mutations and frameshift mutations and are located in the hotspot of SALL1 mutations[8]. In our case, the pathogenic variant was also located in the hotspot, and the putative prematurely terminated protein lacked all the DZF domains. In a mouse model, the truncated mutant protein was capable of dominant-negative activity that resulted in the ectopic activation of two downstream genes. Therefore, TBS phenotypes are due to the expression of a truncated mutant protein and not haploinsufficiency[9].
In this case, the second daughter of the proband had leukemia at the age of 18 years. Besides similar symptoms, including excessive earlobe, preaxial polydactyly, and toe deformities, she was also diagnosed with leukemia at the age of 18 years. However, we cannot conclude an association between SALL1 mutations and leukemia.
In summary, we report a novel pathogenic variant of SALL1 in a Chinese family with TBS, and expand the spectrum of SALL1 mutations. Genetic testing based on WES benefits the clinical diagnosis and genetic counseling of patients with TBS.
We wish to thank the patient and her family for participation in the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Malik S, Pakistan; Mijwil MM, Iraq S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Kohlhase J. Townes-Brocks Syndrome. 2007 Jan 24. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–. [PubMed] |
| 2. | Miller EM, Hopkin R, Bao L, Ware SM. Implications for genotype-phenotype predictions in Townes-Brocks syndrome: case report of a novel SALL1 deletion and review of the literature. Am J Med Genet A. 2012;158A:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Beaudoux O, Lebre AS, Doco Fenzy M, Spodenkiewicz M, Canivet E, Colosio C, Poirsier C. Adult diagnosis of Townes-Brocks syndrome with renal failure: Two related cases and review of literature. Am J Med Genet A. 2021;185:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Zhang J, Dai LM, Li FR, Zhang B, Zhao JH, Cheng JB. A Chinese family of autosomal recessive polycystic kidney disease identified by whole exome sequencing. Medicine (Baltimore). 2020;99:e20413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Fang JX, Zhang JS, Wang MM, Liu L. Novel mutation in the SALL1 gene in a four-generation Chinese family with uraemia: A case report. World J Clin Cases. 2022;10:7068-7075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Wei H, Sun L, Li M, Chen H, Han W, Fu W, Zhong J. [Analysis of SALL1 gene variant in a boy with Townes-Brocks syndrome without anal atresia]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2022;39:401-404. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Lin FJ, Lu W, Gale D, Yao Y, Zou R, Bian F, Jiang GR. Delayed diagnosis of Townes-Brocks syndrome with multicystic kidneys and renal failure caused by a novel SALL1 nonsense mutation: A case report. Exp Ther Med. 2016;11:1249-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Kohlhase J, Taschner PE, Burfeind P, Pasche B, Newman B, Blanck C, Breuning MH, ten Kate LP, Maaswinkel-Mooy P, Mitulla B, Seidel J, Kirkpatrick SJ, Pauli RM, Wargowski DS, Devriendt K, Proesmans W, Gabrielli O, Coppa GV, Wesby-van Swaay E, Trembath RC, Schinzel AA, Reardon W, Seemanova E, Engel W. Molecular analysis of SALL1 mutations in Townes-Brocks syndrome. Am J Hum Genet. 1999;64:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Kiefer SM, Robbins L, Barina A, Zhang Z, Rauchman M. SALL1 truncated protein expression in Townes-Brocks syndrome leads to ectopic expression of downstream genes. Hum Mutat. 2008;29:1133-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |