Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5412
Peer-review started: April 30, 2023
First decision: June 19, 2023
Revised: July 4, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: August 6, 2023
Processing time: 95 Days and 5.3 Hours
Endocardial fibroelastosis (EFE) is commonly considered to be an inflammatory reactive lesion of hyperplasia and deposition of tissue fibers and collagen in the endocardium and/or subendocardium, which is strongly associated with endocardial sclerosis, ventricular remodeling and acute and chronic heart failure, and is one of the important causes for pediatric heart transplantation. Early diagnosis and treatment are the key factors in determining the prognosis of the children. In this paper, we would like to highlight the potential unintended consequences of the use of sedation and biopsy for pediatric acute heart failure caused by EFE and the comprehensive considerations prior to clinical diagnosis.
Core Tip: The high-risk medical operation for the clinical diagnosis of pediatric acute heart failure due to endocardial fibroelastosis remains debatable, and the medical management of sedation and biopsy requires a comprehensive assessment of the indications and contraindications in children.
- Citation: Xin XX, Se YY. Caution in the use of sedation and endomyocardial biopsy for the management of pediatric acute heart failure caused by endocardial fibroelastosis. World J Clin Cases 2023; 11(22): 5412-5415
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5412.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5412
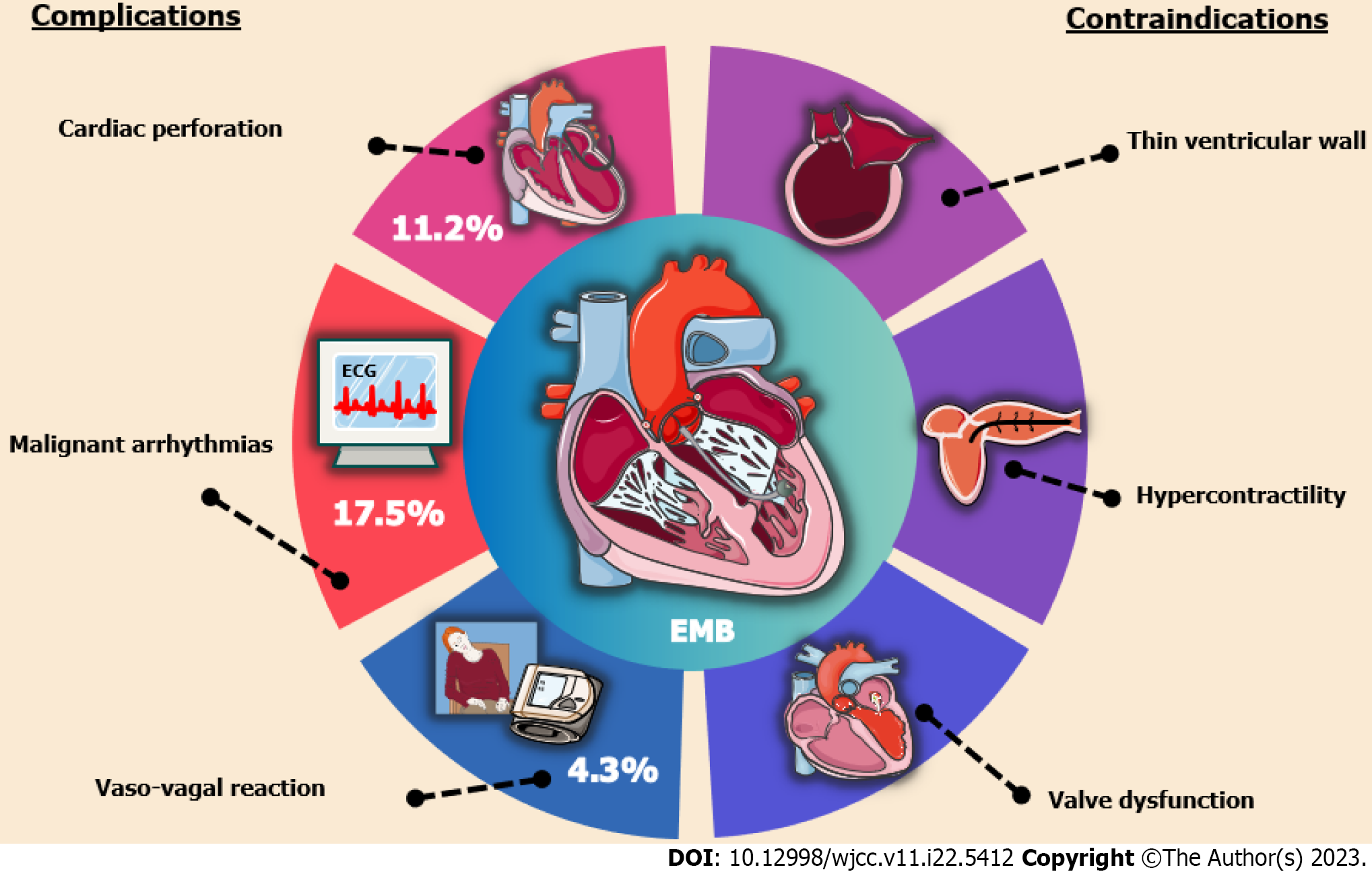
With great interest, we read a research report entitled “Pediatric acute heart failure caused by endocardial fibroelastosis mimicking dilated cardiomyopathy: A case report” by Xie et al[1], and also we congratulate and commend the authors for their excellent work and unremitting efforts on the comparison between differential diagnosis of endocardial fibroelastosis (EFE) and dilated cardiomyopathy (DCM) in infants and toddlers. However, sedation and endomyocardial biopsy (EMB) in children with EFE during the acute onset phase are worthy of further discussion. In some EFE cases, while performing a cardiac magnetic resonance imaging (MRI) in the acute period is informative seemingly, there are many times when the risks of sedating a child with acute heart failure to obtain an MRI outweigh the benefits of the information gained, such as leading to central inhibitory coma, apnea, hypotension and elusive arrhythmias[2-4]. In addition, the risks of biopsy in an infant with EFE confused with DCM are fairly high, particularly the risk of perforation, bleeding and the exacerbation of hemodynamic derangement and heart failure[5-7]. A latest joint position statement on EMB states that hemodynamically unstable patients with acute heart failure and ventricular dilatation are at relatively high risks of cardiac perforation, pericardial tamponade and malignant arrhythmias, while the development of these risks is strongly related to operator expertise in the subspecialty of cardiac catheterization[8]. In parallel, patients with thin ventricular wall and uncooperative posture have been included as contraindications for EMB[9,10], as illustrated in Figure 1. Despite the increasing maturity and popularity of EMB with advances in medical technology, the majority of myocardial biopsy samples and pathology reports related to EFE are obtained from autopsies and not directly from the children with EFE at the time of onset[11]. As such, for children with acute heart failure who are highly suspected both of EFE and DCM, seeking a high-risk medical test for an absolute clinical diagnosis is not a good alternative, and a meticulous echocardiography is sufficient to diagnose EFE rather than an EMB with trauma[12-15].
On top of that, previous EFE studies have been based on the endothelial-mesenchymal transition of the endocardium, but a 2017 genetic lineage tracing study by Zhang et al[16] indicated that neonatal endocardial endothelial cells did not make any contribution to fibroblasts in EFE-like tissues; instead, epicardium-derived mesenchymal cells were the major source of EFE fibroblasts, and demonstrated that TGF-β was a potential therapeutic target. Accordingly, there will be growing evidence to support the advantages of genetic lineage tests for the early identification of EFE, whether for the clinical diagnosis or effective treatment of EFE[17-20]. It is worth noting that, while fibrosis is also known to develop in association with secondary EFE as well as hypertrophic and restrictive cardiomyopathies, the pathophysiological mechanism of primary EFE is certainly distinct from the secondary EFE and traditional intramyocardial fibrosis as they share an incomplete overlapping genetic lineage[21,22]. Hence, improving physicians’ adequate appreciation of EFE lesions and sorting out comprehensive information considerations prior to clinical diagnosis will not only beneficial to improve the medical management of the children, but reduce the harm caused by unnecessary high-risk interventions and invasive inspections in children with EFE.
In summary, sedation and EMB should be used with caution in the management of pediatric acute heart failure caused by EFE, while EFE with a fuller understanding and a more comprehensive consideration prior to clinical diagnosis will facilitate the subsequent early treatment of the children, also further genetic testing is expected to provide more valuable information for the differential diagnosis of the children, relative to biopsy.
A huge thanks to my girlfriend Xiao-Xuan Xin for her constant companionship and support. I wish her happiness and joy forever and hope from the bottom of my heart that we can move on to a more distant future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Govindarajan KK, India; Ong H, Malaysia S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
| 1. | Xie YY, Li QL, Li XL, Yang F. Pediatric acute heart failure caused by endocardial fibroelastosis mimicking dilated cardiomyopathy: A case report. World J Clin Cases. 2023;11:1771-1781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 2. | Suzuki Y, Watanabe J, Ono S, Fukui N, Sugai T, Tsuneyama N, Someya T. Increase in the risk of chlorpromazine-induced QT prolongation during nighttime: is a short-period ECG during daytime sufficient? Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1122-1123. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Bradley BD, Green G, Ramsay T, Seely AJ. Impact of sedation and organ failure on continuous heart and respiratory rate variability monitoring in critically ill patients: a pilot study. Crit Care Med. 2013;41:433-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Qishuo T, Youyou Z, Jie Z, Yalei Y, Wei Z, Quan L, Liang L, Liang R. Role of the heat shock protein family in chlorpromazine-induced cardiotoxicity. J Appl Toxicol. 2023;43:1064-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Brighenti M, Donti A, Giulia Gagliardi M, Maschietto N, Marini D, Lombardi M, Vairo U, Agnoletti G, Milanesi O, Pongiglione G, Bonvicini M; Italian Society of Pediatric Cardiology. Endomyocardial biopsy safety and clinical yield in pediatric myocarditis: An Italian perspective. Catheter Cardiovasc Interv. 2016;87:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Wu LA, Lapeyre AC 3rd, Cooper LT. Current role of endomyocardial biopsy in the management of dilated cardiomyopathy and myocarditis. Mayo Clin Proc. 2001;76:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Wang M, Chen K, Chen X, Chen L, Song J, Hu S. Endomyocardial biopsy in differential diagnosis between arrhythmogenic right ventricular cardiomyopathy and dilated cardiomyopathy: an in vitro simulated study. Cardiovasc Pathol. 2018;34:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Seferović PM, Tsutsui H, McNamara DM, Ristić AD, Basso C, Bozkurt B, Cooper LT Jr, Filippatos G, Ide T, Inomata T, Klingel K, Linhart A, Lyon AR, Mehra MR, Polovina M, Milinković I, Nakamura K, Anker SD, Veljić I, Ohtani T, Okumura T, Thum T, Tschöpe C, Rosano G, Coats AJS, Starling RC. Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society Position statement on endomyocardial biopsy. Eur J Heart Fail. 2021;23:854-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 9. | Xu XQ, Tian Z, Fang Q, Jing ZC, Zhang SY. Standard Operation Procedure of Percutaneous Endomyocardial Biopsyin Peking Union Medical College Hospital. Medical Journal of Peking Union Medical College Hospital. 2021;12:322-327. [DOI] [Full Text] |
| 10. | Mueller GC, Michel-Behnke I, Knirsch W, Haas NA, Abdul-Khaliq H, Gitter R, Dittrich S, Dähnert I, Uhlemann F, Schubert S, Tarusinov G, Happel C, Bertram H, Sieverding L, Eicken A, Kozlik-Feldmann R, Weil J. Feasibility, safety and diagnostic impact of endomyocardial biopsies for the diagnosis of myocardial disease in children and adolescents. EuroIntervention. 2018;14:1089-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Xu J, Han YY, Sun JH. [Advance in research on endocardial fiborelastosis]. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14:475-480. [PubMed] |
| 12. | Yoshida Y, Sato T, Kano I, Fukuda M, Sasaki T, Hoshino H, Tanaka M, Terasawa Y. Ultrasonic studies on endocardial fibroelastosis. Tohoku J Exp Med. 1977;123:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, He L, Cai J, Lv T, Yi Q, Xu Y, Liu L, Zhu J, Tian J. Measurements in Pediatric Patients with Cardiomyopathies: Comparison of Cardiac Magnetic Resonance Imaging and Echocardiography. Cardiology. 2015;131:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Avegliano GP, Costabel JP, Asch FM, Sciancalepore A, Kuschnir P, Huguet M, Tobon-Gomez C, Frangi AF, Ronderos R. Utility of Real Time 3D Echocardiography for the Assessment of Left Ventricular Mass in Patients with Hypertrophic Cardiomyopathy: Comparison with Cardiac Magnetic Resonance. Echocardiography. 2016;33:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL, Nihoyannopoulos P, Edvardsen T, Lancellotti P; EACVI Scientific Documents Committee for 2014–16 and 2016–18; EACVI Scientific Documents Committee for 2014–16 and 2016–18. Clinical practice of contrast echocardiography: recommendation by the European Association of Cardiovascular Imaging (EACVI) 2017. Eur Heart J Cardiovasc Imaging. 2017;18:1205-1205af. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 16. | Zhang H, Huang X, Liu K, Tang J, He L, Pu W, Liu Q, Li Y, Tian X, Wang Y, Zhang L, Yu Y, Wang H, Hu R, Wang F, Chen T, Wang QD, Qiao Z, Lui KO, Zhou B. Fibroblasts in an endocardial fibroelastosis disease model mainly originate from mesenchymal derivatives of epicardium. Cell Res. 2017;27:1157-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Teekakirikul P, Kelly MA, Rehm HL, Lakdawala NK, Funke BH. Inherited cardiomyopathies: molecular genetics and clinical genetic testing in the postgenomic era. J Mol Diagn. 2013;15:158-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Kindel SJ, Miller EM, Gupta R, Cripe LH, Hinton RB, Spicer RL, Towbin JA, Ware SM. Pediatric cardiomyopathy: importance of genetic and metabolic evaluation. J Card Fail. 2012;18:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Ellepola CD, Knight LM, Fischbach P, Deshpande SR. Genetic Testing in Pediatric Cardiomyopathy. Pediatr Cardiol. 2018;39:491-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Hershberger RE, Lindenfeld J, Mestroni L, Seidman CE, Taylor MR, Towbin JA; Heart Failure Society of America. Genetic evaluation of cardiomyopathy--a Heart Failure Society of America practice guideline. J Card Fail. 2009;15:83-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 377] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 21. | Luca AC, Lozneanu L, Miron IC, Trandafir LM, Cojocaru E, Pădureţ IA, Mihăilă D, Leon-Constantin MM, Chiriac Ş, Iordache AC, Ţarcă E. Endocardial fibroelastosis and dilated cardiomyopathy - the past and future of the interface between histology and genetics. Rom J Morphol Embryol. 2020;61:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Zhao Y, Wang LK, Eskin A, Kang X, Fajardo VM, Mehta Z, Pineles S, Schmidt RJ, Nagiel A, Satou G, Garg M, Federman M, Reardon LC, Lee SL, Biniwale R, Grody WW, Halnon N, Khanlou N, Quintero-Rivera F, Alejos JC, Nakano A, Fishbein GA, Van Arsdell GS, Nelson SF, Touma M. Recessive ciliopathy mutations in primary endocardial fibroelastosis: a rare neonatal cardiomyopathy in a case of Alstrom syndrome. J Mol Med (Berl). 2021;99:1623-1638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |