Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5351
Peer-review started: April 5, 2023
First decision: May 19, 2023
Revised: May 30, 2023
Accepted: July 4, 2023
Article in press: July 4, 2023
Published online: August 6, 2023
Processing time: 119 Days and 22 Hours
Anti-melanoma differentiation-associated gene 5 antibody-positive (anti-MDA5 Ab+) dermatomyositis complicated with rapidly progressive interstitial lung disease (anti-MDA5 Ab+ DM-RP-ILD) has an unclear underlying mechanism with no recommended unified treatment plan. Herein, one of the cases that we report (Case 2) was successfully treated with tocilizumab despite having lung infection.
Case 1 was a 30-year-old woman who was admitted due to recurrent rash for 5 mo, fever and cough for 1 mo, and chest tightness for 3 d. She was diagnosed with non-myopathic dermatomyositis (anti-MDA5 Ab+) and interstitial pneumonia, and was treated with the combination of hormone therapy and cyclophosphamide followed by oral tacrolimus. Case 2 was a 31-year-old man admitted due to systemic rash accompanied by muscle weakness of limbs for more than 1 mo, and chest tightness and dry cough for 4 d. He was diagnosed with dermatomyositis (anti-MDA5 Ab+) and acute interstitial pneumonia with Pneumocystis jirovecii and Aspergillus fumigatus infections and was treated with hormone therapy (without cyclophosphamide) and the combination of tocilizumab and tacrolimus. The condition of both patients eventually improved and they were discharged and showed clinically stable condition at the latest follow-up.
Tocilizumab could be a salvage treatment for patients with anti-MDA5 Ab+ DM-RP-ILD who are refractory to intensive immunosuppression.
Core Tip: The early detection of myositis-related antibody profile and its concentration together with serum ferritin and cytokine levels is key to the clinical diagnosis and prognosis of anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis complicated with rapidly progressive interstitial lung disease (anti-MDA5 Ab+ DM-RP-ILD). For patients with anti-MDA5 Ab+ DM-RP-ILD refractory to intensive immunosuppression, tocilizumab could be a salvage treatment.
- Citation: Wang QH, Chen LH. Treatment of refractory anti-melanoma differentiation-associated gene 5 anbibody-positive dermatomyositis complicated by rapidly progressing interstitial pulmonary disease: Two case reports. World J Clin Cases 2023; 11(22): 5351-5357
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5351.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5351
At present, no consensus has been reached on the optimal treatment plan for patients with anti-melanoma differentiation-associated gene 5 antibody-positive (anti-MDA5 Ab+) dermatomyositis complicated with rapidly progressive interstitial lung disease (ILD) (anti-MDA5 Ab+ DM-RP-ILD), including on the dosage, course, and dose reduction plan of glucocorticoids that might be used for the treatment. Due to the severity and rapid progression of the disease, it is common to adopt a large dose of hormone therapy or even hormonal shock therapy in the early stage of the disease[1], but recent reports suggest that although high-dose hormone monotherapy may temporarily improve the general symptoms and oxygenation index of patients, it is usually ineffective in improving the prognosis of rapidly progressive interstitial lung disease (RP-ILD)[1,2], where there is also a significant risk of opportunistic infections, gastrointestinal bleeding, and even death. Therefore, moderate immunosuppressive therapy and intensive supportive therapy are preferred over hormonal shock therapy.
For the treatment of anti-MDA5 Ab+ clinically amyopathic dermatomyositis (CADM) complicated with RP-ILD, the most common regimen is the so-called three-drug combination regimen of high-dose hormone, calcineurin inhibitor, and intravenous cyclophosphamide; nevertheless, only a few observational studies have been conducted for this regimen[2,3]. The use of other immunosuppressants such as azathioprine and mycophenolate mofetil in CADM patients with ILD is mainly based on small-scale retrospective studies and case reports, which have used them as a second-line therapy[4]. Methotrexate is not generally recommended to treat this disease and is associated with the risk of drug-induced hypersensitivity pneumonia. In recent years, rituximab has been proven to improve muscle strength in refractory myositis[1], but the number of clinical studies on the use of this drug for the treatment of interstitial lung lesions is low, where it should also be used with caution when there is evidence of pulmonary infection[1]. Among other emerging options, one can point out the combination of multiple treatment methods with direct hemoperfusion of polymyxin B[5]. Report of refractory cases of anti-MDA5 Ab+-DM-RP-ILD from China is uncommon. Here, we report two cases of refractory anti-MDA5 Ab+ CADM presented by RP-ILD in our hospital from 2018 to 2021.
Case 1: A 30-year-old female designer was admitted to the hospital due to recurrent rash for 5 mo, fever and cough for 1 mo, and chest tightness for 3 d.
Case 2: A 31-year-old man was admitted for systemic rash accompanied by muscle weakness of limbs for more than 1 mo, and chest tightness and dry cough for 4 d.
Case 1: The patient was previously treated with methylprednisolone 40 mg BID twice a day plus cyclophosphamide 0.8 QM for 3 wk at a local hospital 1 mo ago, but her condition did not improve; she was transferred to our hospital complaining of acute chest tightness and aggravation.
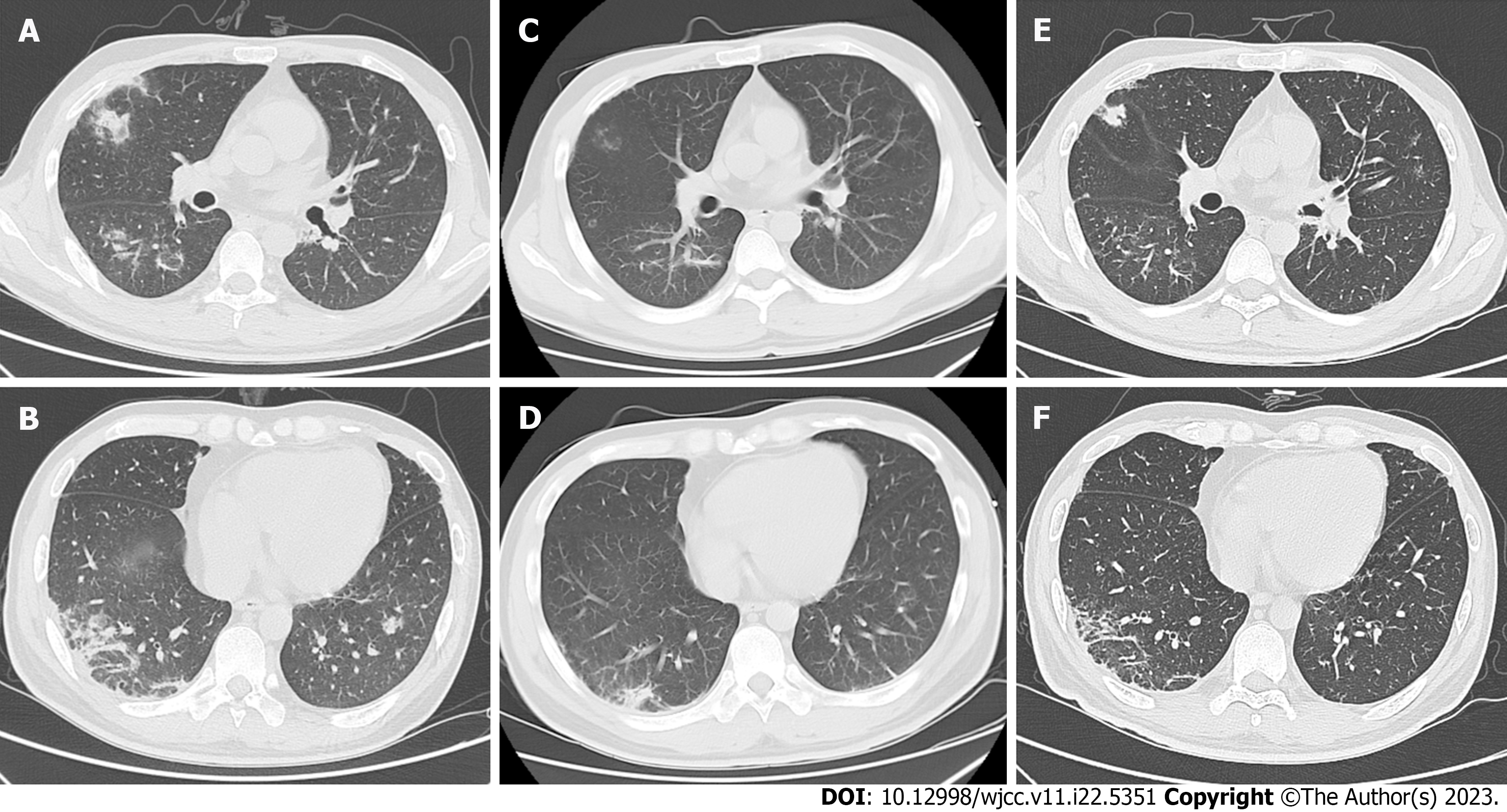
Case 2: One month before the patient’s admission, fatigue appeared. Chest tightness and dry cough occurred 4 d ago, and he was hospitalized in another hospital. Next-generation sequencing (NGS) of bronchoalveolar lavage fluid suggested pneumocystis and Aspergillus fumigatus infection. The myositis panel results were anti-MDA5 antibody IgG+++ and anti-Ro52 (+). Methylprednisolone 80 mg and cyclophosphamide 0.8 mg IV drops were given for 10 d. Given that chest computed tomography (CT) indicated a significant increase in interstitial changes in both lungs (Figure 1A and B), he was transferred to our hospital for further diagnosis and treatment.
Case 1: Multiple red skin rashes on the face (frontal and suborbital), anterior chest, bilateral elbows, interphalangeal joints, and metacarpophalangeal joints were observed, where no joint tenderness, deformity, or edema of lower limbs was identified. The patient's limbs exhibited grade IV muscle strength, and the muscle tone was normal.
Case 2: Examination showed difficulty in raising hands and slight difficulty in raising head, and the patient had limb pain; these were accompanied with purple red rashes on the upper eyelid, nose root, knuckles of both palms, and extension of both elbows. Physical examination suggested no superficial lymph node enlargement. A red rash could be seen on the nose, while the Gottron sign was observed on the elbow. The shoulder joint was tender with limited movement; the limbs exhibited grade V muscle strength and normal muscle tone.
Case 1: Blood examinations were performed after admission for the white blood cell count (6.7 × 109/L), hemoglobin (118.00 g/L), platelet count (2.1 × 1011/L), C-reactive protein (8.0 mg/L), immunoglobulin G (26.60 g/L), immunoglobulin A (2.97 g/L), immunoglobulin M (3.85 g/L), ferritin (1455.60 μg/L), creatine kinase-MB (27 U/L), aspartate aminotransferase (1207 U/L), lactate dehydrogenase (387 U/L), and alanine aminotransferase (226 U/L).
Case 2: The blood results were as follows: White blood cell, 1.6 × 1010/L; hemoglobin, 124 g/L; platelet count, 3.2 × 1011/L; erythrocyte sedimentation rate, 35.00 mm/h; hypersensitive C-reactive protein, 7.2 mg/L; interleukin-6 (IL-6), 305.8 pg/mL; serum ferritin, 3351 µg/L; erythrocyte creatine kinase-MB, 1553 U/L; and lactate dehydrogenase, 699 U/L. The myositis spectrum was anti-MDA5 Ab(+++), where electromyography suggested the presence of myogenic changes.
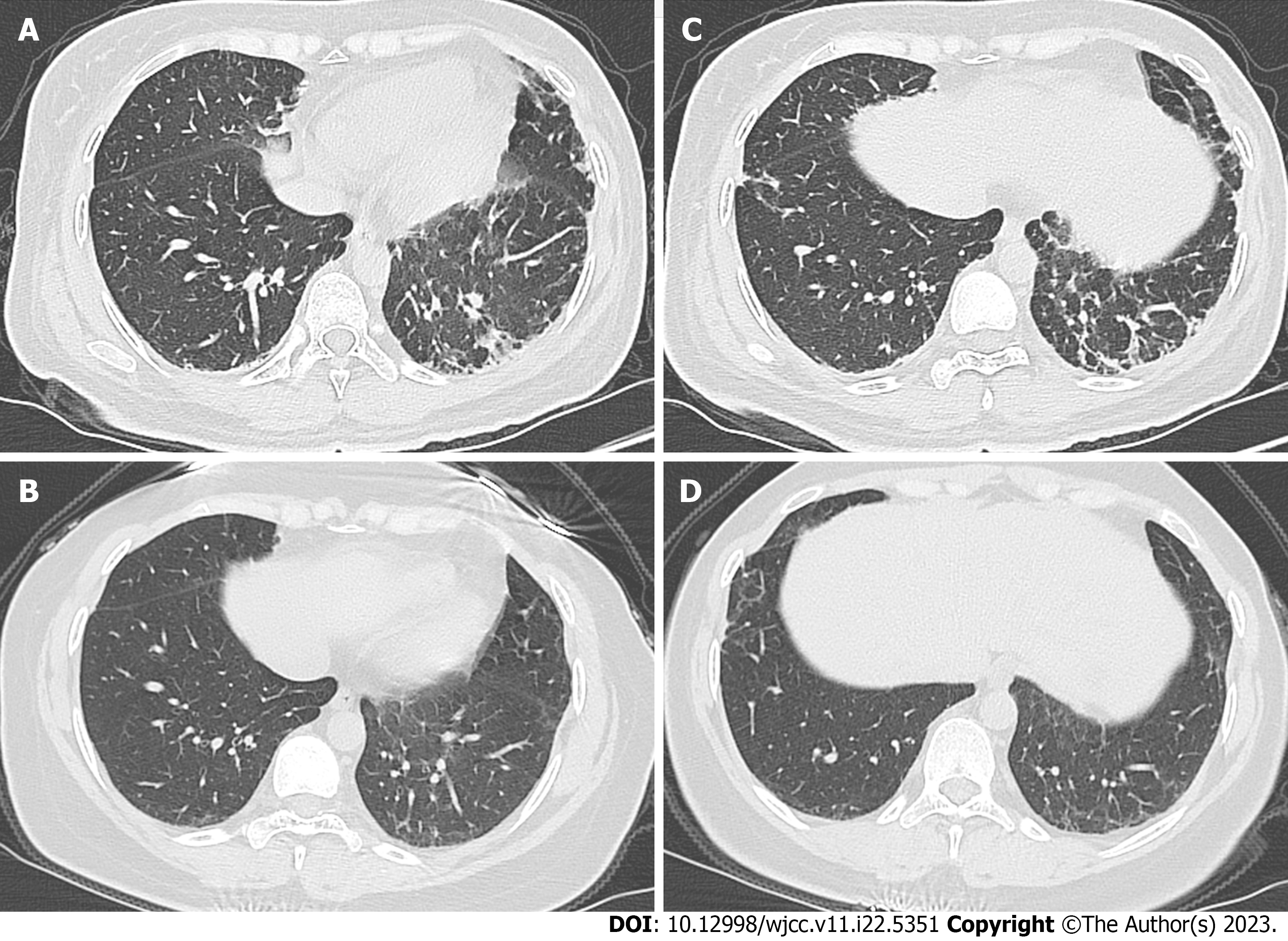
Case 1: The chest CT images are shown in Figure 2. Patchy exudation and interstitial changes were observed in both lungs on admission (Figure 2A and B). There was moderately restrictive ventilatory dysfunction and the pulmonary diffusing capacity was severely reduced. After 4 wk of hormone and cyclophosphamide treatment, only mild subpleural gridded changes were observed in both lungs (Figure 2C and D).
Case 2: The chest CT images are shown in Figure 1. On admission, the images showed scattered flaked ground-glass shadows in both lungs and multiple cords in the lower lungs (Figure 1C and D). Multiple patchy subpleural exudation, consolidation, and interstitial changes were also observed in both lungs together with multiple infectious legions (Figure 1A and B). After treatment with the combination of hormone therapy and tocilizumab, the images showed substantial improvement in subpleural exudation and consolidation (Figure 1E and F).
Case 1: The myositis panel results were anti-MDA5 Ab+ (+++) and anti-Ro52 IgG positive (++), which led to a diagnosis of non-myopathic dermatomyositis (anti-MDA5 Ab+) and interstitial pneumonia.
Case 2: The final diagnosis was dermatomyositis (anti-MDA5 Ab+) and acute interstitial pneumonia with Pneumocystis jirovecii and Aspergillus fumigatus infections.
Case 1: After admission, the patient was found to have a severe onset of acute disease, was monitored by electrocardiogram (ECG), and was given oxygen. Methylprednisolone 80 mg BID was given to relieve inflammation for 1 wk, and gamma globulin 20 g QD once a day was given for 5 d. Methylprednisolone dosage was reduced to 80 mg QD for a week and then was tapered to 60 mg QD for 7 d, with intermittent use of plasma, albumin, and other symptomatic treatment to improve the body's immune function. After excluding contraindications, immunotherapy with cyclophosphamide 0.8 g once a month was provided. During hospitalization, the patient also experienced dysphagia, subcutaneous emphysema, nausea, and vomiting. After 3 wk of treatment, her condition improved, with less facial periorbital rash, cough and sputum, and no chest tightness and acute breath. The serum ferritin was 733.90 μg/L, and the patient was discharged with 50 mg prednisone QD. She was followed for 2 years, and prednisone was tapered to 2 tablets gradually. Cyclophosphamide was changed from an intravenous drip of 0.8 g once a month for 6 mo to tacrolimus 1 mg BID. After discharge, the patient was followed up on a 2-3 mo basis.
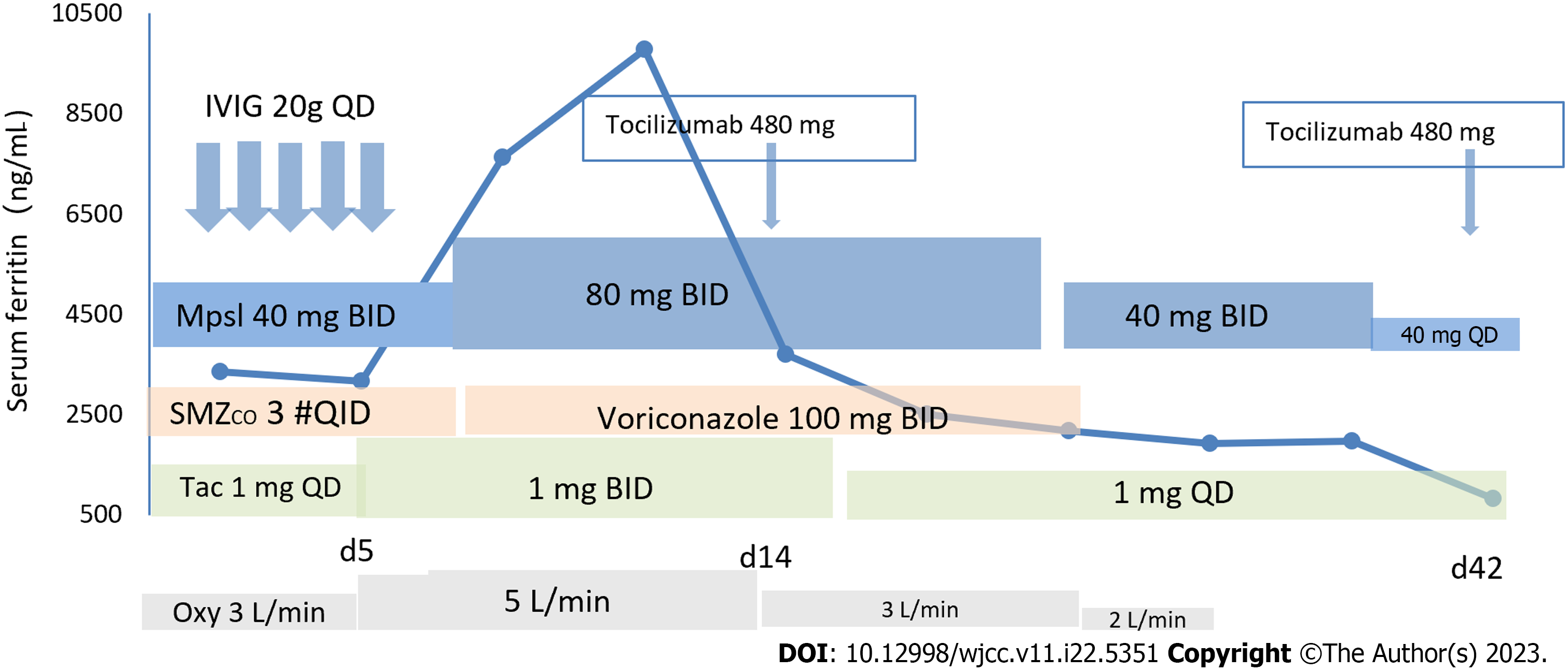
Case 2: Immediately after admission, the patient underwent ECG monitoring, oxygen inhalation, and nasogastric tube feeding. The treatment regimen consisted of methylprednisolone 80 mg per day followed by an additional intravenous drip twice a day to treat the primary disease, human blood gamma globulin 20 g for 5 d, intermittent support with albumin and plasma reinforcement therapy, and oral tacrolimus that was adjusted to 1 mg twice a day or once a day according to the blood concentration levels. The patient experienced high fever, dysphagia, sore throat, hoarseness, and irritative cough. For alveolar lavage NGS suggesting pneumocystis, sulfamethoxazole complex, voriconazole, and tocilizumab 480 mg once a month were added. The lung condition and liver injury were gradually alleviated. The repeated C-reactive protein was normal, the serum ferritin decreased to 1980 µg/L, creatine kinase index was normal (its value decreased from pre-treatment 1553 U/L to post-treatment 203 U/L), and the lung was substantially improved on repeated CT (Figure 1E and F). Hormone shock was contraindicated, because this patient had obvious concurrent infection.
Case 1: At the latest follow-up, the condition of the patient was stable.
Case 2: At the latest follow-up, the patient’s condition was stable, and his chest tightness and cough were relieved (see Figure 3 for the specific drugs used by the patient).
Idiopathic inflammatory myopathy (IIM) is a group of autoimmune myopathies with etiology not fully understood. MDA5 antibody is a myositis-specific antibody relatively common in CADM subtype in IIM. In CADM, muscle involvement is mostly mild. RP-ILD is highly correlated with CADM with an incidence of 24%-65%, which often leads to respiratory failure[6]. Half of patients with respiratory failure die due to respiratory failure progression, where the survival time between the appearance of respiratory symptoms and death is only 2 to 3 mo under the therapeutic effect of treatment using immunosuppressants[6].
The main clinical manifestations of anti-MDA-5 Ab+ CADM are rash, joint muscle soreness, muscle weakness, hoarseness, choking, mediastinal emphysema, and rapidly progressive ILD. It has been established that anti-MDA-5 Ab+ CADM with RP-ILD progresses rapidly and is more difficult to treat than classic dermatomyositis complicated with rapidly progressive interstitial lung disease (DM-RP-ILD)[6]. The acute disease onset in half of CADM patients may be accounted for by the overactivation of alveolar macrophages, leading to neutrophil activation, release of lymphocyte chemokines, pathological inflammation and, ultimately, lung tissue damage. It has been reported that approximately 50% of CADM with RP-ILD patients die during the early disease stages[7,8]. Studies on adverse factors suggest that ferritin and IL-18 in DM-RP-ILD patients with positive anti-MDA5 antibodies are significantly increased and are positively correlated with disease activity[9,10]. The fact that for our two patients serum ferritin decreased substantially after treatment suggests that a positive correlation exists between serum ferritin and disease activity.
Muro et al[11] documented that the serum anti-MDA5 antibody level of 11 newly treated CADM patients with ILD decreased after treatment and even turned negative in some cases, suggesting that anti-MDA5 antibody can be used to evaluate the efficacy of treatment in RP-ILD patients. Gan et al[12] proposed that cellular keratin 19 fragment (CyFRA21-1) is also a risk factor and a useful marker for detecting rapidly progressive ILD caused by MDA5-resistant CADM. Huang et al[13] found that the incidence of subcutaneous emphysema, hoarseness, and dysphagia in patients with positive anti-MDA5 and anti-Ro52 antibodies was significantly higher than that in patients with only positive anti-MDA5 antibodies; also the mortality rate of the former patients was as high as 54.55%. These findings suggest that the early detection of myositis-related antibody profile and their concentration together with serum ferritin and cytokine levels are key elements in clinical diagnosis and prognosis.
For the two cases of severely-ill patients successfully treated in our hospital, no large dose of hormonal shock therapy was applied; at the start of the treatment methylprednisolone was used at 80 mg (intravenous administration), which was later gradually reduced to oral treatment. Infection is one of the important causes of death in such patients, especially those treated with high-dose hormone combined with multiple immunosuppressants. Thus, it is essential that the patients are provided with supportive treatment, such as intermittent infusion of plasma and albumin, and attention should be paid to their 24-h intake of water and their electrolyte balance. In addition, there is no clear treatment guideline for immunosuppressants.
For treating one of the cases reported here (Case 1), the combination of hormone therapy and cyclophosphamide was used, which after half a year was changed to oral tacrolimus. For the other patient (Case 2), due to the accompanying pulmonary fungal infection, hormone therapy was not combined with cyclophosphamide; instead, the combination of tocilizumab and tacrolimus was used to address the primary disease. The patients' condition eventually improved and they were discharged. At the latest follow-up, the patients were found to be clinically stable. Thus, the relatively satisfactory outcome of treatment with tocilizumab in Case 2 may indicate that tocilizumab could be a salvage treatment for patients with RP-ILD who are refractory to intensive immunosuppression. To our knowledge, there is only one prior report of using tocilizumab for treatment of dermatomyositis, where tocilizumab was found to be effective for the treatment of six patients[14]. With respect to targeting other cytokines, such as tumor necrosis factor-alpha (TNF-α) and type 1 interferon (T1-IFN), we should point out the following. First, examining data of 122 cases of new-onset or exacerbation of ILD secondary to administration of biologic therapies revealed that in 97% of cases, the biologic agent used was blocking TNF-α[15], which discouraged us from considering targeting TNF-α in our patients. With respect to the significance of T1-IFN in the pathogenesis and treatment of anti-MDA5 Ab+ DM-RP-ILD, we should say that high T1-IFN signatures in serum and affected skin of anti-MDA5 Ab+ DM-RP-ILD patients have been reported, highlighting the potential of targeting T1-IFN as a treatment strategy in such patients[16]. Nevertheless, we are unaware of a report in the literature that has tested this treatment strategy in patients with anti-MDA5 Ab+ DM-RP-ILD. In this context, we should point out that a phase I b randomized, double-blind, controlled clinical study of sifalimumab found elevated T1-IFN signaling in 77% of patients with inflammatory myopathy[17]. Sifalimumab is currently not marketed in China and its efficacy in treating polymyositis/dermatomyositis-related pulmonary interstitial lesions remains to be observed. Thus, due to the very high mortality of anti-MDA5 Ab+ DM-RP-ILD and the circumstances around targeting TNF-α and T1-IFN, our decision to use tocilizumab was based on the above-mentioned prior report[14] and also the fact that IL-6 is closely related to the pathogenesis of anti-MDA5 Ab+ DM-RP-ILD[18]. Given that for patients with serious lung infections the combination of other biological agents and immunosuppressants is contraindicated, the relatively satisfactory outcome observed in Case 2 for tocilizumab is noteworthy. Of course, further follow-up is needed to clarify this potentially meaningful treatment.
In conclusion, the prognosis of RP-ILD in patients with anti-MDA5 Ab+ dermatomyositis is poor, and the fatality rate is high for such patients. The underlying mechanism of this disease remains poorly understood, and no consensus has been reached on the optimal treatment plan. Early detection of myositis-related antibody spectrum, their concentration, and levels of serum ferritin and cytokines are key for clinical diagnosis and prognosis assessment of this disease. For patients with high titers of anti-MDA5 antibodies, high serum ferritin, or adverse prognostic factors, clinicians should adopt more aggressive treatment regimens to improve patient survival rate. In addition to symptomatic therapy, the three-drug combination therapy scheme consisting of high doses of glucocorticoids, calcineurin inhibitors, and intravenous cyclophosphamide is recommended; tocilizumab represents a potential solution for enhancing the immune suppression of rescue medications in patients with refractory RP-ILD. Larger clinical studies are warranted to corroborate our findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Coutant F, France; Sharma D, India S-Editor: Lin C L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Tokunaga K, Hagino N. Dermatomyositis with Rapidly Progressive Interstitial Lung Disease Treated with Rituximab: A Report of 3 Cases in Japan. Intern Med. 2017;56:1399-1403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Barnes H, Holland AE, Westall GP, Goh NS, Glaspole IN. Cyclophosphamide for connective tissue disease-associated interstitial lung disease. Cochrane Database Syst Rev. 2018;1:CD010908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Yamada K, Asai K, Okamoto A, Watanabe T, Kanazawa H, Ohata M, Ohsawa M, Hirata K. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. BMC Res Notes. 2018;11:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Parperis K, Kiyani A. Clinically amyopathic dermatomyositis associated with anti-MDA5 antibody. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Ichiyasu H, Horio Y, Tsumura S, Hirosako S, Sakamoto Y, Sakata S, Nakashima K, Komatsu T, Kojima K, Masunaga A, Fujii K, Saita N, Kohrogi H. Favorable outcome with hemoperfusion of polymyxin B-immobilized fiber column for rapidly progressive interstitial pneumonia associated with clinically amyopathic dermatomyositis: report of three cases. Mod Rheumatol. 2014;24:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Sato S, Hoshino K, Satoh T, Fujita T, Kawakami Y, Kuwana M. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: Association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60:2193-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 460] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 7. | Mukae H, Ishimoto H, Sakamoto N, Hara S, Kakugawa T, Nakayama S, Ishimatsu Y, Kawakami A, Eguchi K, Kohno S. Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest. 2009;136:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Sun Y, Liu Y, Yan B, Shi G. Interstitial lung disease in clinically amyopathic dermatomyositis (CADM) patients: a retrospective study of 41 Chinese Han patients. Rheumatol Int. 2013;33:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Gono T, Sato S, Kawaguchi Y, Kuwana M, Hanaoka M, Katsumata Y, Takagi K, Baba S, Okamoto Y, Ota Y, Yamanaka H. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology (Oxford). 2012;51:1563-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 10. | Motegi SI, Sekiguchi A, Toki S, Kishi C, Endo Y, Yasuda M, Ikeuchi H, Sakairi T, Hara K, Yamaguchi K, Maeno T, Hiromura K, Ishikawa O. Clinical features and poor prognostic factors of anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis with rapid progressive interstitial lung disease. Eur J Dermatol. 2019;29:511-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 11. | Muro Y, Sugiura K, Hoshino K, Akiyama M. Disappearance of anti-MDA-5 autoantibodies in clinically amyopathic DM/interstitial lung disease during disease remission. Rheumatology (Oxford). 2012;51:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Gan YZ, Zhang LH, Ma L, Sun F, Li YH, An Y, Li ZG, Ye H. Risk factors of interstitial lung diseases in clinically amyopathic dermatomyositis. Chin Med J (Engl). 2020;133:644-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Huang W, Ren F, Wang Q, Luo L, Zhou J, Huang D, Pan Z, Tang L. Clinical features of thirty-two patients with anti-melanoma differentiation-associated gene 5 antibodies. Clin Exp Rheumatol. 2019;37:803-807. [PubMed] |
| 14. | Zhang X, Zhou S, Wu C, Li M, Wang Q, Zhao Y, Zeng X. Tocilizumab for refractory rapidly progressive interstitial lung disease related to anti-MDA5-positive dermatomyositis. Rheumatology (Oxford). 2021;60:e227-e228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Perez-Alvarez R, Perez-de-Lis M, Diaz-Lagares C, Pego-Reigosa JM, Retamozo S, Bove A, Brito-Zeron P, Bosch X, Ramos-Casals M. Interstitial lung disease induced or exacerbated by TNF-targeted therapies: analysis of 122 cases. Semin Arthritis Rheum. 2011;41:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | Ono N, Kai K, Maruyama A, Sakai M, Sadanaga Y, Koarada S, Inoue T, Tada Y. The relationship between type 1 IFN and vasculopathy in anti-MDA5 antibody-positive dermatomyositis patients. Rheumatology (Oxford). 2019;58:786-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Higgs BW, Zhu W, Morehouse C, White WI, Brohawn P, Guo X, Rebelatto M, Le C, Amato A, Fiorentino D, Greenberg SA, Drappa J, Richman L, Greth W, Jallal B, Yao Y. A phase 1b clinical trial evaluating sifalimumab, an anti-IFN-α monoclonal antibody, shows target neutralisation of a type I IFN signature in blood of dermatomyositis and polymyositis patients. Ann Rheum Dis. 2014;73:256-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Gono T, Kaneko H, Kawaguchi Y, Hanaoka M, Kataoka S, Kuwana M, Takagi K, Ichida H, Katsumata Y, Ota Y, Kawasumi H, Yamanaka H. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology (Oxford). 2014;53:2196-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |