Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5344
Peer-review started: April 24, 2023
First decision: May 31, 2023
Revised: June 9, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: August 6, 2023
Processing time: 101 Days and 2.4 Hours
Meningitis is a possible complication of pneumococcal infection concerning acute otitis media and sinusitis. It might compromise cognitive function, both for the infection itself and the vascular events that sometimes follow the acute phase.
Here we describe the case of a 32-year-old female patient admitted to the emer
The case is discussed in light of scientific knowledge of the long-term outcomes of this pathology in order to potentially improve diagnosis and promote better out
Core Tip: Meningitis is a possible complication of pneumococcal infection concerning acute otitis media and sinusitis. We describe the case of a 32-year-old female patient with extensive pneumococcal meningitis as a consequence of sinus outbreak. She presented with extensive laminar ischemic damage in the acute phase, resulting in severe cognitive and behavioural impairment. Four years of follow-up, through neuropsychological assessments and neuroradiological investigations, demonstrated the presence of subsequent vascular events, 3 months and 2 years after the onset. The global functional outcome was unfavorable.
- Citation: Abbruzzese L, Martinelli G, Salti G, Basagni B, Damora A, Scarselli C, Peppoloni G, Podgorska A, Rosso G, Bacci M, Alfano AR, MANCUSO M. Persistent dysexecutive syndrome after pneumococcal meningitis complicated by recurrent ischemic strokes: A case report. World J Clin Cases 2023; 11(22): 5344-5350
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5344.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5344
According to the National Health Surveillance Institute, the incidence of acute community bacterial meningitis (BM) was, in 2006, 23 per 100000 inhabitants, adding together all ages and all types of bacteria. In adults over the age of 24, the bacteria involved are: Pneumococcus, meningococcus, and, more rarely, listeria mono cytogenes, haemophilus influenzae and group B streptococcus[1]. In developing countries, the annual incidence of pneumococcal meningitis ranges from 1-2 to 20/100000. Sites of infection may concern acute otitis media, mastoiditis, sinusitis, pneumonia, and endocarditis. Clinical manifestations include sudden high fever, headache, nausea, vomiting, confusion, drowsiness, convulsion, and in more severe cases, loss of consciousness. Mortality is about 20%.
Long-term studies on survivors have demonstrated that adults after pneumococcal meningitis are at high risk of neurological and neuropsychological deficits impairing daily life activities and quality of life. In the Kloek et al[2] study, a cohort of 79 patients was evaluated 1 to 5 years after acute illness. After a median of 2 years, 34% of the sample had persistent neurologic sequelae, most commonly hearing loss (27%). On overall neuropsychological evaluation, patients performed worse than the controls. Primary cognitive deficits as cognitive slowness, associated with longer reaction times, were described[3,4]. In addition, alterations in executive function have also been reported, with prevalent working memory deficits, difficulty in inhibitory control of automatic responses, visuospatial reasoning, non-verbal learning, and verbal abstraction[3-6]. The alternating subcomponent of attention may also be compromised, together with impairments in the visual-constructive domain and in the amnestic visuospatial domain[3,4,6].
Neurological complications are frequent after pneumococcal meningitis[7]. Arterial stroke occurs in up to 30% of patients, cerebral venous thrombosis in up to 9%, and intracerebral hemorrhage in up to 9%[8]. In the study by Schmidt et al[5], brain swelling was observed in 24% of patients, small vessel vasculitis in 8.5%, impairment of cerebrospinal fluid circulation in 7%, and sinus thrombosis in 3%.
Cerebral infarction typically develops within the first few days of the disease when the central nervous system inflammation is most severe. Indeed, meningeal inflammation can lead to profound vascular alterations on the meningeal vessels, creating a vasculitis that participates in cerebral anoxia and alterations in blood flow. Delayed cerebral vasculo
We hereby report a case of a young woman affected by severe and persistent cognitive sequelae after pneumococcal meningitis due to extensive sinusitis, who presented repeated ischemic damages. We describe the clinical history, the cognitive-behavioural profile, and the functional outcome, during the four years following onset. Written informed consent was obtained from the patient for publication of this case report.
A 32-year-old female presented to the emergency department complaining hyperpyrexia, headache, and neck pain.
The patients’ symptoms started a few days earlier and had worsened in the last 12 h. The patient was pregnant at 39 + 1 wk of gestation and underwent emergency cesarean delivery due to the severity of the clinical condition.
The patient was completely independent in everyday activities and she had no previous neurological disorders.
The patient was a right-handed married female educated to 16 years of age, an artisan by profession. The patient had a three-year-old son born from a eutocic delivery and was pregnant with a second child upon admission to the hospital.
Physical examination showed symptoms consistent with a clinical pattern of meningitis.
The lumbar puncture was positive for meningitis. The patient also underwent hematological tests to assess thrombotic risk and was assessed with computed tomography-angiography of the cerebral circulation to exclude potential atheromatous plaques. In order to exclude potential artery/venous shunts, a transcranial doppler with bubble test was conducted. The results showed the absence of potential causes for stroke. Additional hematologic exams to exclude autoimmunity pathologies were conducted and were negative.
The brain magnetic resonance imaging (MRI) performed revealed bilateral T2 hyperintensity of the cortex signal in large brain areas. The fronto-polar, insular, fronto-medial and cingulate bilateral regions were affected, with a prevalence of damage on the right side. On this side, the frontal operculum and the inferior lobule, and, to a lesser extent, the superior parietal one, were more clearly involved. The neuroimaging was compatible with extensive laminar ischemic damage in the aforementioned areas.
The final diagnosis of the presented case is pneumococcal meningitis, with sinus outbreak.
The patient underwent pharmacological treatment with antibiotics and cortisone.
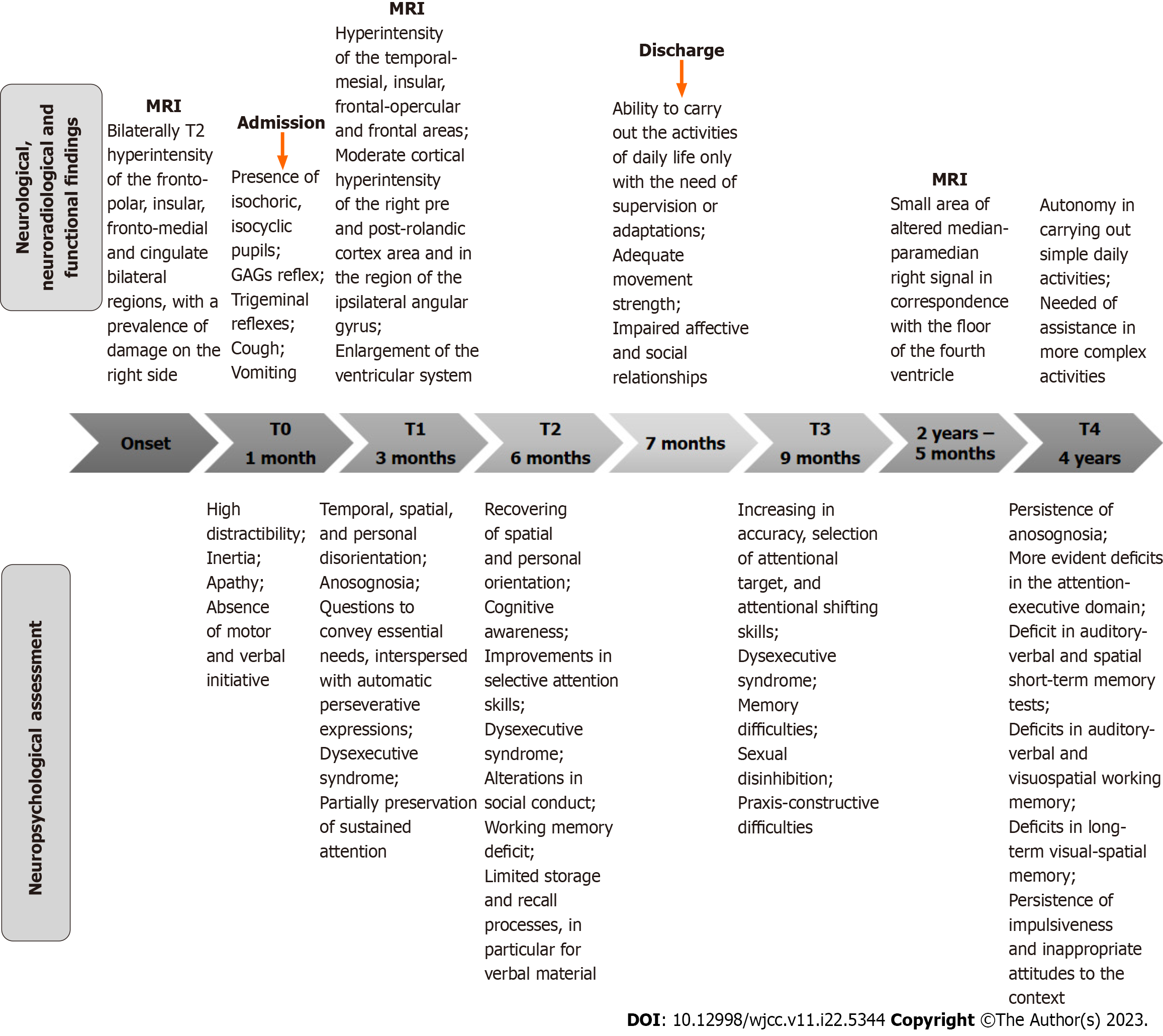
One month after onset, the patient was transferred to a neurorehabilitation unit for motor and cognitive rehabilitation. The results of the assessments carried out, both during this period of hospitalization and after discharge, are described below. Figure 1 shows the timeline of the main stages of the rehabilitative training, as well as the different neurological and neuropsychological assessments and neuroradiological examinations, conducted over the range of 4 years. The results of each neuropsychological test performed over time are presented in Table 1. Some tests were found to be inexecutable, due to the lack of attention skills.
| Cluster | Behavioural impairment | T0 | T1 | T2 | T3 | T4 |
| Motivation disorders | Apathy | |||||
| Reduced finalistic cognitiveness | + | + | + | + | + | |
| Reduced emotional reactivity | + | + | + | + | + | |
| Acinetic mutism | + | - | - | - | - | |
| Disinhibition disorders | ||||||
| Impulsiveness | ||||||
| Motor | + | + | + | + | + | |
| Cognitive | + | + | + | + | + | |
| Social/personal behaviour impaiments | ||||||
| Bizarre/inadequate behaviour | + | + | + | + | + | |
| Invasive behaviour | - | + | + | - | - | |
| Hyperorality | - | + | + | - | - | |
| Hyperactivity | - | + | + | - | - | |
| Afinalistic movements | + | + | + | + | + | |
| Dromomania | - | - | + | + | + | |
| Negligence in personal care | + | + | + | + | + | |
| Environmental dependency | + | + | + | - | - | |
| Aggressiveness | - | - | - | - | - | |
| Acquired sociopathy | ||||||
| Difficulty in recognising emotional expressions | - | - | - | - | - | |
| Difficulty in inferring other people's emotions | - | - | - | - | - | |
| Reduced theory of mind | + | + | + | + | + | |
| Perseverative disorders | ||||||
| Motor | + | + | + | + | + | |
| Cognitive | + | + | + | + | + | |
| Anosognosia | + | + | - | + | + |
The neurological examination (T0) performed upon admission to the rehabilitation ward showed the presence of isochoric pupils, isocyclic pupils, reactive to direct and consensual light stimulus as well as present pharyngeal reflex, trigeminal reflexes, coughing and vomiting. The patient showed symmetrical tendon reflexes, plantar reflex in bilateral flexion, non-elicitable Hoffmann, and bilaterally mentioned prehension reflex. The motor skills were preserved, with the execution of even complex activities, even if there were also automatic and aimless movements. The patient was not self-sufficient and required total assistance in most daily activities [functional independence measure (FIM)[10], FIM total: 25, motor FIM: 18, cognitive FIM: 7].
The patient showed herself to be alert, but high distractibility was present as irrelevant stimuli caught her attention. She visually engaged the examiner in all spatial positions and showed occasional imitative responses towards significant others in a framework of inertia, apathy, and the absence of motor and verbal initiative. Due to insufficient attentional resources, the neuropsychological evaluation was not yet administrable in this initial phase.
Three months after the onset and during the rehabilitation process, due to the sudden worsening of neurological conditions, the patient underwent a new MRI which showed the known hyperintensity of the signal in fluid attenuated inversion recovery (FLAIR) at the level of the cortex bilaterally. In particular, the temporal-mesial, insular, frontal-opercular and frontal areas were affected, with a slight right prevalence present. A new moderate cortical hyperintensity was found at the level of the right pre and post-rolandic cortex area and in the region of the ipsilateral angular gyrus. MRI also showed a net enlargement of the ventricular system, specifically the frontal and temporal horns, and of the periencephalic liquor sulci. In particular, the Sylvian fissures were enlarged bilaterally, due to pronounced atrophic outcomes (medial temporal lobe atrophy, score 3-4). Additionally, the corpus callosum was thinned.
Due to a significant behavioural change, a neuropsychological assessment was performed (T1). The profile was characterized by decontrolled behaviour, with distractibility, inability to inhibit irrelevant stimuli concerning environmental demands, disinhibition, hyperactivity leading to impulsive agitation, and automatic utilization behaviours. In addition, we observed temporal, spatial, and personal disorientation. Anosognosia was also observed. Communicative abilities began to convey essential needs, interspersed with automatic perseverative expressions, such as “Can we go outside?”, “Is my husband there?”, “Can you give me some food?”. Neuropsychological tests showed a severe dysexecutive syndrome with difficulty in controlling automatic responses and marked disturbance of attentional function, particularly for the selective and alternating subcomponents. The sustained attention was partially preserved. In the praxis-constructive domain, the performance qualified as pathological due to difficulties in the executive planning of the realization of the graphic elements.
Six months after the onset (T2), spatial and personal orientation was recovered. The awareness was classifiable in the cognitive level of Crossons’ model[11]: The patient verbally reported the neurological event that occurred as a result of the continuous exposure to that information during the rehabilitation training. Using a structured assessment, improve
Seven months after the onset, the patient was discharged for rehabilitation at the residence area. Upon returning home, the patient was able to carry out the activities of daily life only with the need of supervision or adaptations (total FIM: 98, motor FIM: 79, cognitive FIM: 19). From the motor point of view, she had regained adequate movement strength. Daily life management was significantly affected by the stability of the behavioural profile, which considerably compromised affective and social relationships.
The neuropsychological evaluation, performed at the follow-up 9 months after the neurological event (T3), showed an increase in accuracy, selection of attentional target, and attentional shifting skills. However, there was a regression of awareness aspects leading to anosognosia. The dysexecutive aspects and the memory difficulties previously noted were substantially stable. However, the loss of inhibitory control began to affect the sexual sphere, resulting in significant behavioural management difficulties. In the praxis-constructive domain, although a greater number of elements have been copied, a pathological performance persisted due to difficulties in the executive planning of the realization of the graphic elements.
At that time, the patient followed attending a recreational center, for people with disabilities where she undertook cognitive stimulation training, with ecological objectives aimed at recovering autonomy. With respect to her functional profile, she never went back to work and the parental role was delegated to her husband.
Two years and five months from onset, the patient suffered a new ischemic event in the pontine region. The MRI showed a small area of altered median-paramedian right signal in correspondence with the floor of the fourth ventricle, hyperintensity in the FLAIR, and long TR images, characterized by signs of restrictive edema in diffusion-weighted imaging and by nuanced impregnation after administration of contrast medium. The result was compatible with a recent pontine ischemic lesion. Apart from this new lesion, the remaining findings were compatible with the expected evolution of the previous brain damage.
At that time the patient started an antithrombotic therapy. Four years and two months after the onset, the patient underwent a new neuropsychological assessment (T4), which showed a substantially stable cognitive profile compared to the previous evaluation which was characterized by multi-domain difficulties. In particular, deficits in the attention-executive domain were more evident (distractibility due to interference from irrelevant stimuli, slowdown in information processing, deficit of attentional switching capacity, sensitivity to interference, and loss of inhibitory control and of cognitive flexibility). Regarding the memory system, scores at the lower limit of the norm were observed in both auditory-verbal and spatial short-term memory tests, as well as deficits in auditory-verbal and visuospatial working memory and in long-term visual-spatial memory. Relative to behaviour, compared to the previous evaluation, a slight improvement was observed (a decrease in sexual disinhibition and in-use behaviour, and the absence of hyperphagia). Instead, impulsiveness and sometimes inappropriate attitudes to the context remained. The patient was completely autonomous in carrying out simple daily activities but needed assistance with regard to more complex activities (Activities of Daily Living[12]: 6/6; Instrumental Activities of Daily Living[13]: 2/8). The patient was still anosognosic.
Regarding the rehabilitative interventions, the severity of the cognitive-behavioural framework had not always allowed the setting of structured training. In the initial phase, cognitive rehabilitation sessions focused on attentional exercises aimed at inhibiting the interference of irrelevant information and increasing the ability of sustained attention. Then, with the increasing of attentive skills, the rehabilitation focused on the reorganization of awareness and behaviour through cognitive-behavioural methods aimed at reorienting and monitoring impulsivity and verbal perseverations. The patient had also undergone selective and alternating attentional training.
Over time, the objectives have shifted from the restorative plan to the compensatory and functional efficiency plan, up to the inclusion in the recreation center.
Here we have described the case of a woman with severe cognitive sequelae, after BM starting from sinusitis and, consistent with the anatomical contiguity, prevalent damage in the frontal lobe, and consequently in executive functions and behaviour control. The patient presented dysexecutive syndrome with a severe behavioural disturbance. The profile has always been highly variable over time whereas it still remains serious. Symptoms are only partially alleviated, and significantly affect the resumption of independent life. Due to the cognitive and behavioural profile, the clinical observation was more informative than the neuropsychological assessment. Indeed, neuropsychological tests were greatly affected by attention impairment, compromising their administration and reliability. Overall, the patient mostly failed in all attentional and executive function tasks.
With respect to behavioural profile, in the post-acute phase, a Kluver-Bucy syndrome was even supposed. The syndrome follows bilateral lesions of the limbic system and manifests with personality alterations, hyperorality, hyperphagia, exhibitionism, compulsive hypersexuality, affective indifference, and aggression[14]. The patient presented with almost all the symptoms, except for aggression.
Compared to behavioural disorders of frontal origin often described in the literature[15], the patient presented a broad range of symptoms: Motivation disorders (reduced purposeful cognition, reduced emotional reactivity, and, in the initial phase, akinetic mutism), disinhibition disorders (motor and cognitive impulsivity, alterations in social conduct such as bizarre attitudes, sexual disinhibition, personhood, hyperactivity, dromomania, environmental dependency), perseverative behaviour and anosognosia.
After the severe first neurological damage, the patient had two more strokes in the following months and years which complicated the outcome. Stroke in young patients may be the manifestation of a specific haematological disease or may appear as a complication in the course of haematological disorders[16].
Nevertheless, thanks to the tests we conducted we tend to exclude this possibility, favoring the hypothesis of complications related to the infection. Indeed, it's known that infections affecting the central nervous system represent a rare cause of cerebral ischaemia. In the retrospective study of Arboix et al[17] of the 70 patients with cerebral ischemia from an unusual cause, 11 (15%) presented with central nervous system infections, such as syphilitic meningitis, infective endocarditis, meningococcal meningitis, pneumococcal meningitis, and human immunodeficiency virus infection. In our patient, the presence of vascular events repeated over time demonstrates how the suffering of the cerebral vessels on an inflammatory basis can involve a greater risk of occurring in new ischemic events, even at a distance from the acute phase.
The prognosis of our patient appears to have been particularly negative due to the sum of various factors. As already reported in the literature, BM, even in the absence of vascular complications, can lead to long-term alterations in cognitive functions[2-5]. A recent study hypothesized that macrophage migration inhibitory factor (MIF) upregulation, a proinflammatory cytokine, and a neuro-endocrine mediator, contributes to the cognitive impairments of survivors of pneumococcal meningitis[18]. Several studies showed the association between increased MIF production and Alzheimer disease suggesting MIF involvement in the neuro-inflammatory process occurring in mild cognitive impairment and in cognitive decline[19].
The case we reported, probably sums up the effects of primary inflammatory damage with those of secondary, repeated, vascular damage. Furthermore, despite undergoing many intensive cognitive rehabilitation training sessions, the improvements obtained over time were reduced. It is likely that the severe deficit of behavioural control has compromised the possibility of benefiting from rehabilitation.
Our case report presents some limitations. The lack of a neuropsychological battery sensitive to the severity of the patient’s profile and administered at regular time intervals may have influenced the case description.
In summary, this case report shows a negative functional outcome in a young patient affected by BM, due to a severe cognitive and behavioural disturbance.
Understanding and evaluating cognitive profile in BM is a critical component of diagnosis and rehabilitative treatment, as it can cause severe complications and a poor prognosis for the patient. The description of this case report would highlight some of the clinical and neuropsychological features of BM with cerebrovascular complications to improve diagnosis and potentially promote better outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arboix A, Spain; Tang ZP, China S-Editor: Qu XL L-Editor: A P-Editor: Ju JL
| 1. | Stahl JP. Meningiti acute. EMC-Neurologia. 2013;13:1-11. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Kloek AT, Brouwer MC, Schmand B, Tanck MWT, van de Beek D. Long-term neurologic and cognitive outcome and quality of life in adults after pneumococcal meningitis. Clin Microbiol Infect. 2020;26:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 3. | van de Beek D, Schmand B, de Gans J, Weisfelt M, Vaessen H, Dankert J, Vermeulen M. Cognitive impairment in adults with good recovery after bacterial meningitis. J Infect Dis. 2002;186:1047-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Hoogman M, van de Beek D, Weisfelt M, de Gans J, Schmand B. Cognitive outcome in adults after bacterial meningitis. J Neurol Neurosurg Psychiatry. 2007;78:1092-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Schmidt H, Heimann B, Djukic M, Mazurek C, Fels C, Wallesch CW, Nau R. Neuropsychological sequelae of bacterial and viral meningitis. Brain. 2006;129:333-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Merkelbach S, Sittinger H, Schweizer I, Müller M. Cognitive outcome after bacterial meningitis. Acta Neurol Scand. 2000;102:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Kastenbauer S, Pfister HW. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain. 2003;126:1015-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 309] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | van de Beek D, Weisfelt M, de Gans J, Tunkel AR, Wijdicks EF. Drug Insight: adjunctive therapies in adults with bacterial meningitis. Nat Clin Pract Neurol. 2006;2:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Kato Y, Takeda H, Dembo T, Tanahashi N. Delayed recurrent ischemic stroke after initial good recovery from pneumococcal meningitis. Intern Med. 2012;51:647-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Hamilton BB, Laughlin JA, Fiedler RC, Granger CV. Interrater reliability of the 7-level functional independence measure (FIM). Scand J Rehabil Med. 1994;26:115-119. [PubMed] |
| 11. | Crosson B, Barco PP, Velozo CA, Bolesta MM, Cooper PV, Werts D, Brobeck TC. Awareness and compensation in postacute head injury rehabilitation. J Head Trauma Rehabil. 1989;4:46-54. |
| 12. | Meghan MG. Activities of daily living evaluation. ed. Encyclopedia of Nursing & Allied Health. Kristine Krapp. Gale Group, 2002. |
| 13. | Cromwell DA, Eagar K, Poulos RG. The performance of instrumental activities of daily living scale in screening for cognitive impairment in elderly community residents. J Clin Epidemiol. 2003;56:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Lanska DJ. The Klüver-Bucy Syndrome. Front Neurol Neurosci. 2018;41:77-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Reber J, Tranel D. Frontal lobe syndromes. Handb Clin Neurol. 2019;163:147-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Arboix A, Bechich S, Oliveres M, García-Eroles L, Massons J, Targa C. Ischemic stroke of unusual cause: clinical features, etiology and outcome. Eur J Neurol. 2001;8:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Arboix A, Jiménez C, Massons J, Parra O, Besses C. Hematological disorders: a commonly unrecognized cause of acute stroke. Expert Rev Hematol. 2016;9:891-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Kloek AT, Seron MV, Schmand B, Tanck MWT, van der Ende A, Brouwer MC, van de Beek D. Individual responsiveness of macrophage migration inhibitory factor predicts long-term cognitive impairment after bacterial meningitis. Acta Neuropathol Commun. 2021;9:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Popp J, Bacher M, Kölsch H, Noelker C, Deuster O, Dodel R, Jessen F. Macrophage migration inhibitory factor in mild cognitive impairment and Alzheimer's disease. J Psychiatr Res. 2009;43:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |