Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5329
Peer-review started: April 25, 2023
First decision: June 12, 2023
Revised: June 24, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: August 6, 2023
Processing time: 100 Days and 8.4 Hours
Both hepatoid adenocarcinoma of the stomach (HAS) and neuroendocrine differentiation (NED) are rare histological subtypes of gastric cancer with unique clinicopathological features and unfavorable outcomes. HAS with NED is even rarer.
Here, we report a 61-year-old man with HAS with NED, as detected by gastric wall thickening by positron emission tomography/computed tomography for a pulmonary nodule. Distal gastrectomy was performed, and pathological exa
We treated a patient with HAS with NED who underwent adjuvant chemo
Core Tip: Hepatoid adenocarcinoma of the stomach (HAS) with neuroendocrine differentiation (NED) is rare histological subtype. We first reported the detailed processes of surgery and chemotherapy of HAS with NED and the survival time was 27 mo combined with postoperative chemotherapy, which provided an important reference for clinical diagnosis and treatment of this condition.
- Citation: Fei H, Li ZF, Chen YT, Zhao DB. Hepatoid adenocarcinoma of the stomach with neuroendocrine differentiation: A case report and review of literature. World J Clin Cases 2023; 11(22): 5329-5337
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5329.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5329
Hepatoid adenocarcinoma of the stomach (HAS) accounts for approximately 0.3% to 1.0% of all gastric cancers and is an extremely rare subtype with tissue morphology similar to hepatocellular carcinoma (HCC)[1]. Most, but not all, HAS cases produce alpha-fetoprotein (AFP)[2], and increased serum AFP is mainly due to hepatoid cells[3-5]. Neuroendocrine neoplasms (NENs) can be divided into neuroendocrine tumors and neuroendocrine carcinomas (NECs). NEC is characterized by neuroendocrine differentiation (NED) and divided into small-cell NEC (SCNEC) and large-cell NEC (LCNEC); the latter has better survival prognosis than the former[6]. Ninety percent of SCNECs originate from the lung. The incidence of LCNEC is 1.8/100000, with only 3% occurring in the stomach[7]. Nonneuroendocrine components (ade
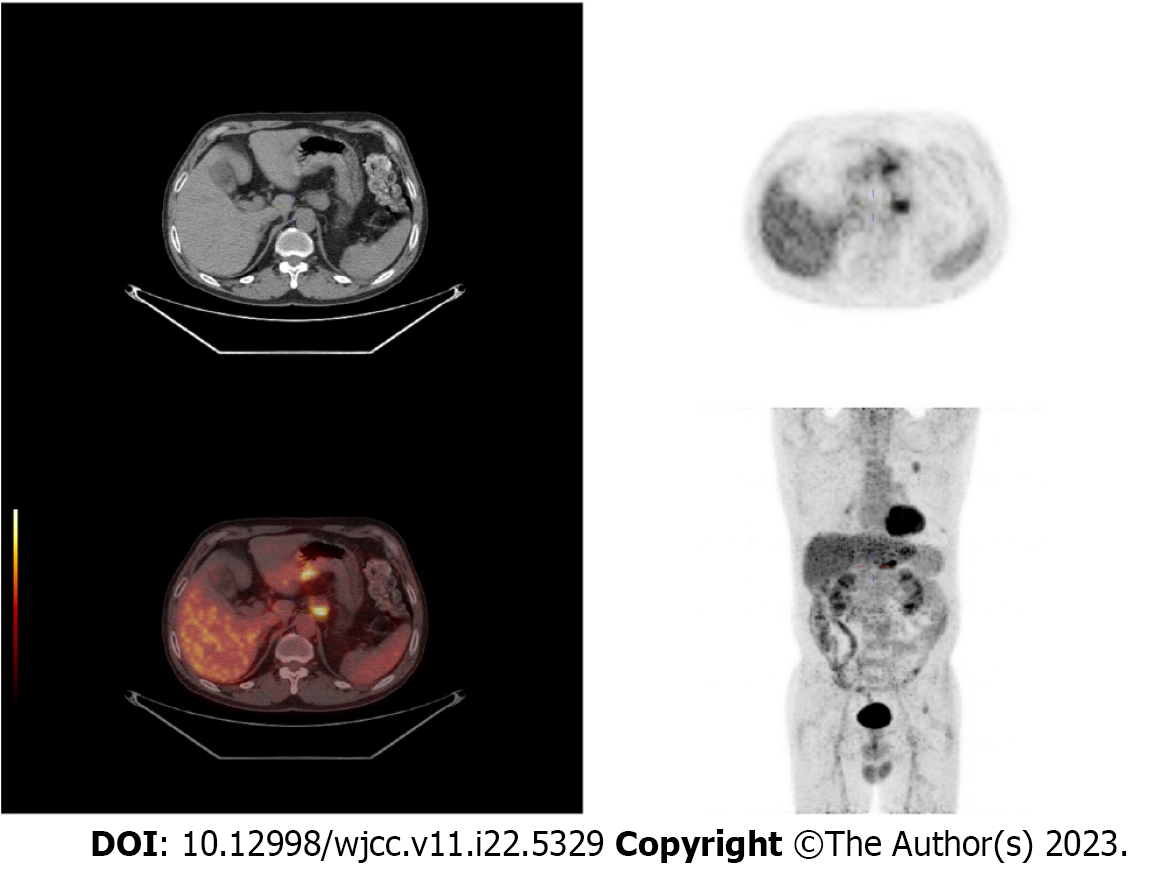
Positron emission tomography/computed tomography (PET/CT) revealed thickening of the gastric lesser curvature at 1 wk.
A 61-year-old man underwent PET/CT for pulmonary nodules. PET/CT revealed thickening of the gastric lesser curvature with metabolic hyperplasia.
In addition, he was diagnosed with hypertension 5 years prior and took nifedipine daily. He had been drinking alcohol at approximately 250 g/day and smoking 20 cigarettes/day for over 40 years.
The patient denied any family history of malignant tumors.
Physical assessment revealed no abnormalities.
Laboratory examinations, including tumor marker levels, revealed no abnormalities.
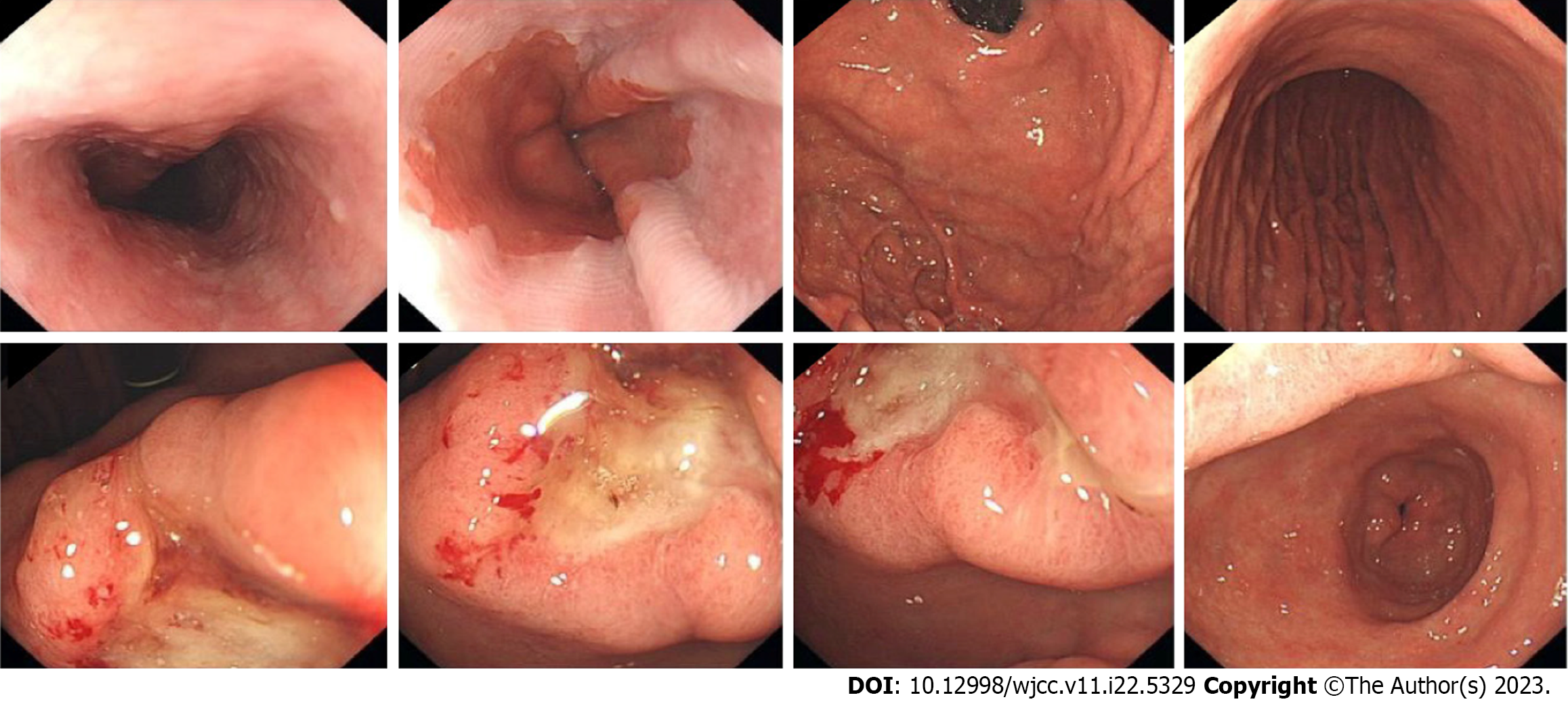
PET/CT (Figure 1) revealed thickening of the gastric lesser curvature with metabolic hyperplasia. Gastroscopy (Figure 2) showed a localized ulcerative lesion extending from the angle to the antrum of the stomach that was mainly located in the mucosal layer and submucosal layer. The lesion was diagnosed as poorly differentiated carcinoma based on biopsy pathology.
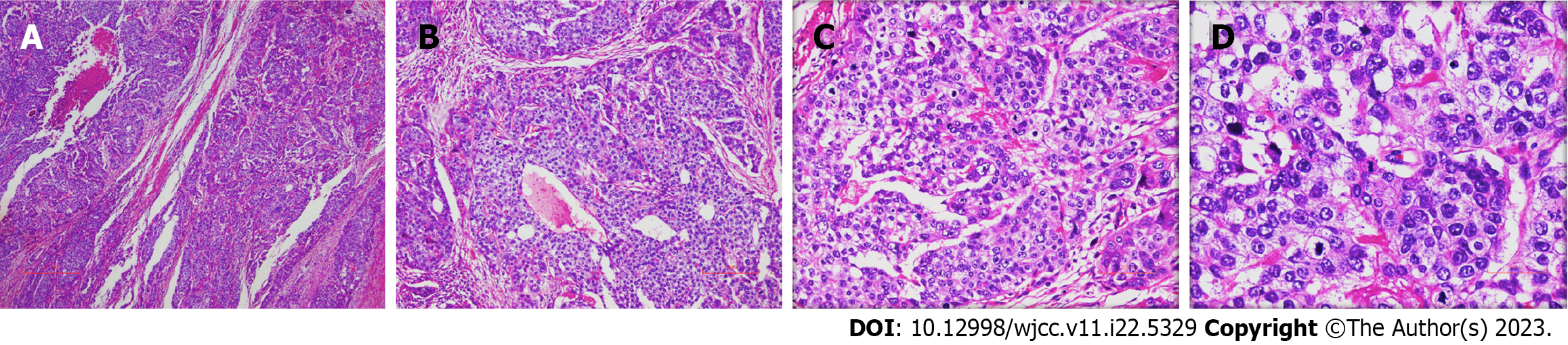
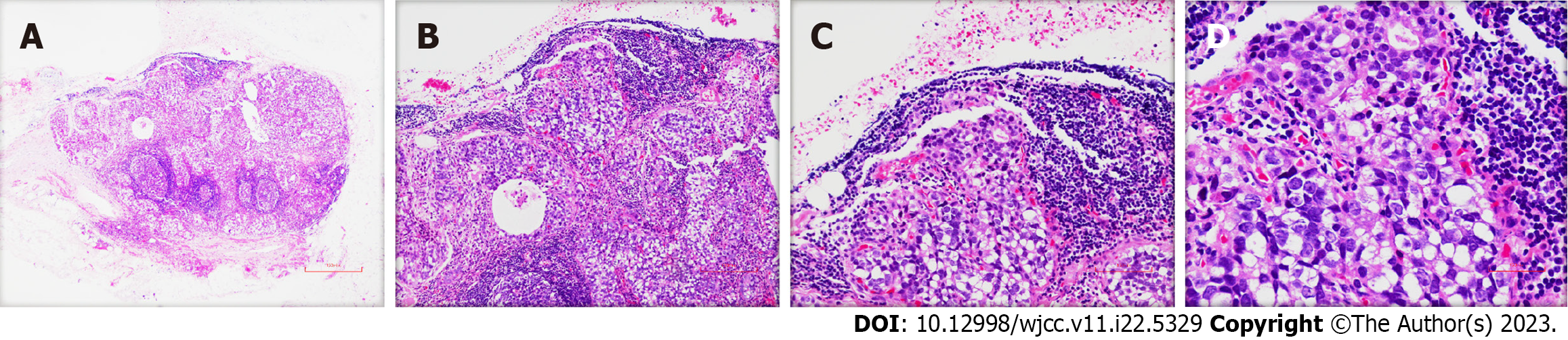
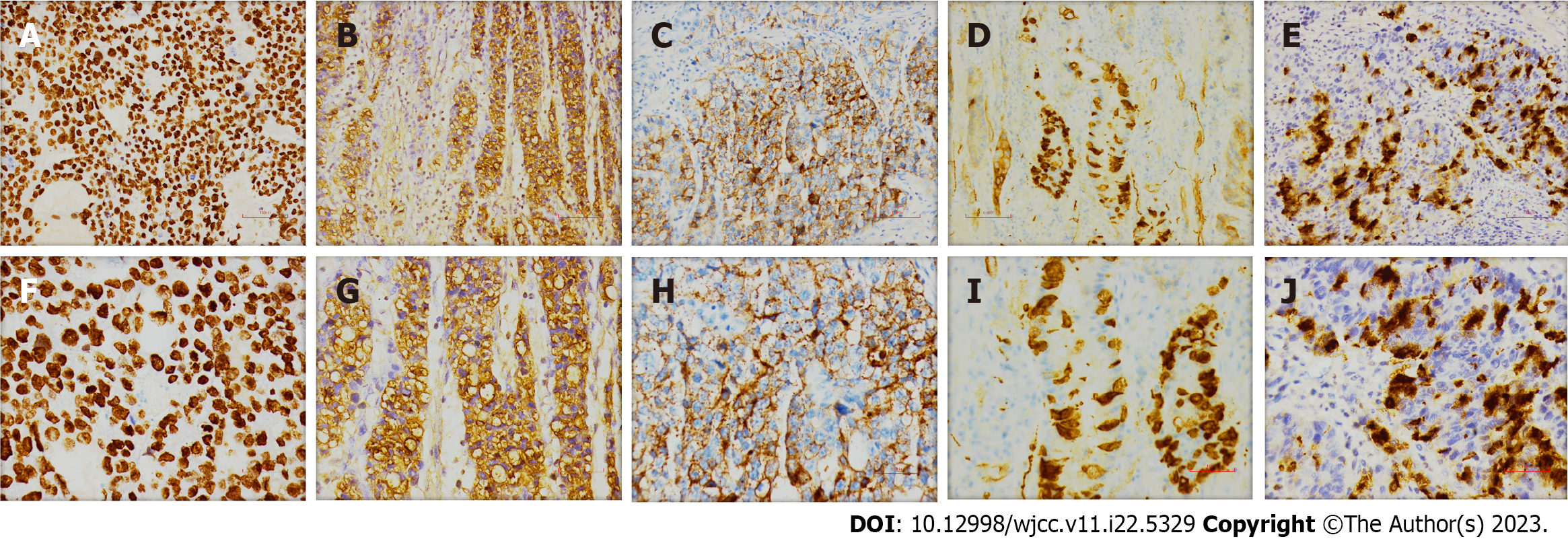
The surgically resected specimen showed an ulcer-type tumor with a size of 2 cm × 1.5 cm × 0.5 cm in the lesser curvature of the gastric antrum. Postoperative pathology revealed HAS with NED. Histological examination showed that the tumor invaded the submucosal layer and subserous fat with multifocal growth. There was angiolymphatic invasion, but no nerve invasion was noted. The surrounding gastric mucosa showed chronic active inflammation with massive Helicobacter Pylori infection (Figure 3). Some lymph nodes were found to have metastatic carcinoma (4/29). One lymph node on the greater curvature (1/8) was positive, and two lymph nodes on the lesser curvature (2/16) were positive. The tumor node metastasis classification was T3N2M0 (stage III) (Figure 4). Immunohistochemical staining showed SALL4 (+), AFP (+), Glypican-3 (GPC-3) (+), Synaptophysin (Syn) (+), and Chromogranin A (CgA) (+) (Figure 5). Hepatoid components produced SALL4, AFP and GPC-3, and the neuroendocrine markers Syn and CgA revealed the presence of NED.
The patient was diagnosed with HAS with NED pT3N2M0 (stage III), accompanied by hypertension.
The patient underwent distal gastrectomy with D2 lymphadenectomy at our hospital. He was discharged from the hospital with satisfactory recovery. The patient then received ten cycles of systemic chemotherapy (regimen: 60 mg docetaxel on day 1, 140 mg oxaliplatin on day 2, and 1.5 g capecitabine twice a day on days 1-8, half a month on each course). CT scanning revealed lymph node metastasis in the cardia and peritoneum at 4 mo postsurgery and multiple liver metastases at 6 mo postsurgery. In addition, he underwent thoracentesis and intrapleural injection chemotherapy (regimen: 40 mg cisplatin four times, 60 mg Endostar twice, and 2.3 million units interleukin-2 twice) for malignant pleural effusion. He then received three cycles of second-line chemotherapy treatment (280 mg irinotecan on day 1, 60 mg S-1 twice a day on days 1-10, and 500 mg apatinib once a day, two weeks on each course). S-1 is a combination product of tegafur, gimeracil, and oteracil potassium. Unfortunately, the liver metastases continued to progress, and he experienced grade 3 neutropenia, causing him to refuse further treatment.
He died at 27 mo after the operation due to the tumor multiple metastases. We think that aggressive surgical resection with postoperative chemotherapy to control tumor progression may improve patients’ outcome.
We retrieved 6 patients with stomach cancer including hepatoid adenocarcinoma and neuroendocrine Components. The clinicopathologic features of these cases are summarized in Table 1. The average age of the patients was 65 years (range: 48–83 years). Four of 6 patients were men. All of them developed lymph node metastases, which indicated the aggressive nature of these components. AFP and CgA expression was detected in the carcinomatous elements. Six patients under
| Ref. | Rassidakis et al[60] | Okamoto et al[61] | Suzuki et al[62] | Lipi et al[63] | Wincewicz et al[64] | Li et al[65] | Current case |
| Age | 48 | 78 | 83 | 50 | 73 | 60 | 61 |
| Sex | Man | Woman | Man | Man | Woman | Man | Man |
| TNM stage | N3 | T3N2M0 | T4N2M1 | N3 | T3N3M1 | T2N1Mx | T3N2M0 |
| Tumor location | Anterior wall of the gastric body | Pyloric antrum | Upper-third of the stomach | Cardia | Gastric antrum | Gastric antrum | |
| Tumor size | 70 mm × 55 mm | 90 mm × 60 mm × 30 mm | 75 mm × 110 mm | 85 mm × 65 mm × 45 mm | 60 mm × 40 mm | 16 mm | 20 mm × 15 mm × 5 mm |
| Histologic patterns | HAC, NED | HAC, NEC, TAC | HAC, NEC, TAC | HAC, LCNEC, TAC | HAC, NED, OGCs | HAC, NED | HAC, NED |
| Immunohistochemistry | CGA, AFP | CK8, AE1/AE3, AFP, CGA | CGA, AFP, SP | AFP, Syn, CGA, CK | CGA, AFP, AE1/AE3, CK | AFP, Syn, CGA | SALL4, AFP, GPC-3, Syn, CgA |
| Surgery | Total gastrectomy | Subtotal gastrectomy with lymphadenectomy | Gastrectomy | Total gastrectomy | R2 radical gastrectomy | Distal gastrectomy with D2 lymphadenectomy | |
| Treatment | Doxorubicin, mitomycin-C, 5-fluorouracil, octreotide (4 cycles) | Cisplatin + VP 16 (2 cycles) | Docetaxel + oxaliplatin + capecitabine (10 cycles), irinotecan + S-1 + apatinib (3 cycles) | ||||
| AFP, ng/mL | 800 (post-op) | 168 (pre-op) | 1683 (pre-op) | ||||
| Outcome | Alive, 12 mo | Died, 53 mo, liver recurrence | Died, 6 mo | Liver recurrence | Alive, 6 mo | Died, 27 mo, liver recurrence |
Although hepatoid adenocarcinoma can occur in various organs, the stomach is the most common site. HAS mixed with common adenocarcinoma components is frequently observed[9], but the origin remains obscure. Previous studies have indicated that adenocarcinoma cells can switch from the intestinal type to the hepatoid phenotype[10], with the two components possibly arising from pluripotent precursor cells[11]. Pathological diagnosis is still the gold standard for HAS. In our case, gastric lesions were detected by PET, which can diagnose and stage HAS accurately. Immunohistochemistry staining for AFP, SALL4, and GCP3 indicated hepatoid differentiation[12,13]. All three markers were detected in this case. HAS is highly aggressive, and patients with high serum AFP levels are more likely to have lymph nodes and liver metastases[14]. LIN28 combined with SALL4 shows 98% specificity in discriminating HAS from HCC[15]. In summary, the availability of various auxiliary tests assists in accurate diagnosis.
The clinical manifestations of HAS lack specificity, and are were no significant differences from gastric cancer with regard to symptoms. In most cases, the tumor is at an advanced stage when diagnosed. In general, HAS is aggressive and has a high recurrence rate[15]. Current research on HAS is controversial. The median OS was reported to be 11 mo (range 0.1-102), with a one-year survival rate of 55%[16]. The five-year disease-free survival was only 20.7%[17-19]. However, Zhou et al[18] found that the prognosis of HAS is not as poor as previously believed[18], and the 5-year survival reached 41.1% after radical surgery[5]. One recent study showed that the independent prognostic factors of OS include the serum AFP level[20,21] in gastric cancer; another study showed that preoperative carcinoembryonic antigen levels of 5 ng/mL or more can be used to predict worse prognosis[9].
Radical surgery combined with adjuvant chemotherapy is considered the primary choice for these patients, but no consensus has been reached regarding therapy[22]. Adjuvant chemotherapy is an independent favorable prognostic factor of HAS[23,24]. Retrospectively, more than half of cases are at advanced stages at diagnosis, and the recurrence rate is quite high (47%)[25,26]. Metastatic HAS lacks standard therapy; therefore, determining a suitable treatment regimen is a clinically urgent issue to be solved. Cisplatin-based chemotherapy is considered the mainstay of therapy[27]. Two patients who received cisplatin and etoposide regimens achieved complete responses[28,29]. FOLFOX might be a the
The stomach is the most common organ of mixed adeno-neuroendocrine carcinoma[35], and NED is usually the dominant component[36]. NED represents a special type of tumor that can express various polypeptide hormones, such as synaptophysin and chromogranin A[37], and the Ki67 index is always more than 20%. Our case was mixed with two distinct components, and the etiopathogenesis of this phenomenon is still controversial. Domori and colleagues found that nearly 70% of gastric NECs presented with an adenocarcinoma component, and a previous report indicated that NECs originate from a preceding adenocarcinoma[38]. Conversely, Fujimoto et al[39] considered that the adenocarcinoma component might arise from the NEC component[39]. Sun et al[40] found that the NED component in gastric mixed adeno-neuroendocrine carcinoma (MANEC) showed marked genetic heterogenicity because the NED components of different cases were not clustered in hierarchical clustering analysis[40]. Similar to gastric adenocarcinoma, TP53 is the most commonly mutated gene in gastric MANEC[41]. Scardoni et al[42] considered a monoclonal origin of gastric MANECs with the same TP53 mutation and level of p53 protein expression in two cases, as detected by next-generation sequencing[42].
G-NEC is a highly aggressive neoplasm with a large proportion of metastasis at diagnosis, and NED is the principal component of the metastatic foci in MANECs[43]. Moreover, the presence of liver metastases correlates with poor prognosis in G-NEC patients[44,45]. Because of its rare occurrence, systemic treatment options are limited, and currently, chemotherapy is still the main therapeutic approach. Cisplatin or carboplatin combined with etoposide is the standard chemotherapeutic regimen for the treatment of G-NEC according to the standard systemic therapy of pulmonary small-cell lung cancer (SCLC)[46,47]. A multicenter retrospective analysis reported a median overall survival (OS) of 13.3 mo for GNEC[48]. No evident difference was apparent between platinum-based chemotherapy regimens[49]. The choice of treatment options should be selected based on the toxicity profile[50]. Nevertheless, the prognosis of gastric NEC remains dismal[46]. There are limited data on the efficacy of second-line therapy. The FOLFIRI regimen has the potential to improve outcomes of patients for whom first-line therapy fails[51]. Peptide receptor radionuclide therapy should be considered an alternative to existing treatment options, and more research is needed[52,53]. Immune checkpoint inhibitors offer new hope for treatment of NECs. Gastric tumor tissues express higher levels of PD-L1 mRNA than respective controls[54]. Kim et al[55] found significantly increased expression of PD-L1 in high-grade tumors, and PD-L1-positive tumors were associated with decreased OS[55]. Yang and colleagues confirmed that high expression of PD-L1 in G-NECs correlates with poor prognosis, providing a basis for immunotherapy targeting the PD-1/PD-L1 pathway in G-NECs[56,57]. After combination immunotherapy with ipilimumab and nivolumab, 43% of patients with pancreatic NENs[58] and 19% of SCLC patients[59] achieve an objective response. Further research is necessary to investigate the therapeutic efficacy of immune checkpoint inhibitors.
Mixed carcinomas usually raise a clinical dilemma with respect to diagnosis and treatment decisions. Only a few cases of HAS with NED have been reported, and we first report the detailed processes of treatment and development, we thought that aggressive surgical resection with postoperative chemotherapy to control tumor progression may improve patients’ outcome, providing an important reference for clinical diagnosis and treatment of this condition. We hope that our report provides valuable experience to other clinicians.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hegazy AA, Egypt; Koganti SB, United States S-Editor: Fan JR L-Editor: A P-Editor: Zhang XD
| 1. | Su JS, Chen YT, Wang RC, Wu CY, Lee SW, Lee TY. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: a literature review. World J Gastroenterol. 2013;19:321-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 118] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Zhang ZR, Wu J, Li HW, Wang T. Hepatoid adenocarcinoma of the stomach: Thirteen case reports and review of literature. World J Clin Cases. 2020;8:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Inagawa S, Shimazaki J, Hori M, Yoshimi F, Adachi S, Kawamoto T, Fukao K, Itabashi M. Hepatoid adenocarcinoma of the stomach. Gastric Cancer. 2001;4:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Yu C. Comment on: "Hepatoid adenocarcinoma of the stomach: a unique subgroup with distinct clinicopathological and molecular features. et al Gastric Cancer. 2019;22:1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Wang Y, Sun L, Li Z, Gao J, Ge S, Zhang C, Yuan J, Wang X, Li J, Lu Z, Gong J, Lu M, Zhou J, Peng Z, Shen L, Zhang X. Hepatoid adenocarcinoma of the stomach: a unique subgroup with distinct clinicopathological and molecular features. Gastric Cancer. 2019;22:1183-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 6. | Abdel-Rahman O, Fazio N. Outcomes of small-cell vs large-cell gastroenteropancreatic neuroendocrine carcinomas: A population-based study. J Neuroendocrinol. 2021;33:e12971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Korse CM, Taal BG, van Velthuysen ML, Visser O. Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: experience of two decades of cancer registry. Eur J Cancer. 2013;49:1975-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 8. | Yamamoto K, Itoi T, Sofuni A, Tsuchiya T, Tanaka R, Tonozuka R, Honjo M, Mukai S, Fujita M, Asai Y, Matsunami Y, Kurosawa T, Yamaguchi H, Nagakawa Y. Expanding the indication of endoscopic papillectomy for T1a ampullary carcinoma. Dig Endosc. 2019;31:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Lin JX, Wang ZK, Hong QQ, Zhang P, Zhang ZZ, He L, Wang Q, Shang L, Wang LJ, Sun YF, Li ZX, Liu JJ, Ding FH, Lin ED, Fu YA, Lin SM, Xie JW, Li P, Zheng CH, Huang CM. Assessment of Clinicopathological Characteristics and Development of an Individualized Prognostic Model for Patients With Hepatoid Adenocarcinoma of the Stomach. JAMA Netw Open. 2021;4:e2128217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Akiyama S, Tamura G, Endoh Y, Fukushima N, Ichihara Y, Aizawa K, Kawata S, Motoyama T. Histogenesis of hepatoid adenocarcinoma of the stomach: molecular evidence of identical origin with coexistent tubular adenocarcinoma. Int J Cancer. 2003;106:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Liu Z, Wang A, Pu Y, Li Z, Xue R, Zhang C, Xiang X, E JY, Bu Z, Bai F, Ji J. Genomic and transcriptomic profiling of hepatoid adenocarcinoma of the stomach. Oncogene. 2021;40:5705-5717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Zhao M, Sun L, Lai JZ, Shi H, Mei K, He X, Jin X, Lai J, Cao D. Expression of RNA-binding protein LIN28 in classic gastric hepatoid carcinomas, gastric fetal type gastrointestinal adenocarcinomas, and hepatocellular carcinomas: An immunohistochemical study with comparison to SALL4, alpha-fetoprotein, glypican-3, and Hep Par1. Pathol Res Pract. 2018;214:1707-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Ushiku T, Shinozaki A, Shibahara J, Iwasaki Y, Tateishi Y, Funata N, Fukayama M. SALL4 represents fetal gut differentiation of gastric cancer, and is diagnostically useful in distinguishing hepatoid gastric carcinoma from hepatocellular carcinoma. Am J Surg Pathol. 2010;34:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Li W, Li Q, Yu Y, Wang Y, Chen E, Chen L, Wang Z, Cui Y, Liu T. Effect of Immune Checkpoint Inhibitors Plus Chemotherapy on Advanced Gastric Cancer Patients with Elevated Serum AFP or Hepatoid Adenocarcinoma. Cancer Manag Res. 2020;12:11113-11119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Xia R, Zhou Y, Wang Y, Yuan J, Ma X. Hepatoid Adenocarcinoma of the Stomach: Current Perspectives and New Developments. Front Oncol. 2021;11:633916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Metzgeroth G, Ströbel P, Baumbusch T, Reiter A, Hastka J. Hepatoid adenocarcinoma - review of the literature illustrated by a rare case originating in the peritoneal cavity. Onkologie. 2010;33:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Zeng XY, Yin YP, Xiao H, Zhang P, He J, Liu WZ, Gao JB, Shuai XM, Wang GB, Wu XL, Tao KX. Clinicopathological Characteristics and Prognosis of Hepatoid Adenocarcinoma of the Stomach: Evaluation of a Pooled Case Series. Curr Med Sci. 2018;38:1054-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Zhou K, Wang A, Ao S, Chen J, Ji K, He Q, Ji X, Wu X, Zhang J, Li Z, Bu Z, Ji J. The prognosis of hepatoid adenocarcinoma of the stomach: a propensity score-based analysis. BMC Cancer. 2020;20:671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Liu X, Cheng Y, Sheng W, Lu H, Xu X, Xu Y, Long Z, Zhu H, Wang Y. Analysis of clinicopathologic features and prognostic factors in hepatoid adenocarcinoma of the stomach. Am J Surg Pathol. 2010;34:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Wang B, Xie Y, Zheng L, Zheng X, Gao J, Liu X, Yuan Y, Li Z, Lu N, Xue L. Both the serum AFP test and AFP/GPC3/SALL4 immunohistochemistry are beneficial for predicting the prognosis of gastric adenocarcinoma. BMC Gastroenterol. 2021;21:408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long Z, Zhu H, Wang Y. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol. 2010;102:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Lin CY, Yeh HC, Hsu CM, Lin WR, Chiu CT. Clinicopathologial features of gastric hepatoid adenocarcinoma. Biomed J. 2015;38:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Zhang JF, Shi SS, Shao YF, Zhang HZ. Clinicopathological and prognostic features of hepatoid adenocarcinoma of the stomach. Chin Med J (Engl). 2011;124:1470-1476. [PubMed] |
| 24. | Xiao C, Wu F, Jiang H, Teng L, Song F, Wang Q, Yang H. Hepatoid adenocarcinoma of the stomach: Nine case reports and treatment outcomes. Oncol Lett. 2015;10:1605-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Baek SK, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Clinicopathologic characteristics and treatment outcomes of hepatoid adenocarcinoma of the stomach, a rare but unique subtype of gastric cancer. BMC Gastroenterol. 2011;11:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Kumashiro Y, Yao T, Aishima S, Hirahashi M, Nishiyama K, Yamada T, Takayanagi R, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach: histogenesis and progression in association with intestinal phenotype. Hum Pathol. 2007;38:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Yoshizawa J, Ishizone S, Ikeyama M, Nakayama J. Gastric hepatoid adenocarcinoma resulting in a spontaneous gastric perforation: a case report and review of the literature. BMC Cancer. 2017;17:368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Søreide JA. Therapeutic Approaches to Gastric Hepatoid Adenocarcinoma: Current Perspectives. Ther Clin Risk Manag. 2019;15:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Simmet V, Noblecourt M, Lizée T, Morvant B, Girault S, Soulié P, Capitain O. Chemotherapy of metastatic hepatoid adenocarcinoma: Literature review and two case reports with cisplatin etoposide. Oncol Lett. 2018;15:48-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Velut G, Mary F, Wind P, Aparicio T. Adjuvant chemotherapy by FOLFOX for gastric hepatoid adenocarcinoma. Dig Liver Dis. 2014;46:1135-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Doi Y, Takii Y, Mitsugi K, Kimura K, Mihara Y. The Effectiveness of Hepatic Arterial Infusion Chemotherapy with 5-Fluorouracil/Cisplatin and Systemic Chemotherapy with Ramucirumab in Alpha-Fetoprotein-Producing Gastric Cancer with Multiple Liver Metastases. Case Rep Oncol Med. 2018;2018:5402313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Sun Y, Chang W, Yao J, Liu H, Zhang X, Wang W, Zhao K. Effect of immune checkpoint inhibitors in patients with gastric hepatoid adenocarcinoma: a case report and literature review. J Int Med Res. 2022;50:3000605221091095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Zou M, Li Y, Dai Y, Sun L, Huang T, Yuan X, Qiu H. AFP-producing hepatoid adenocarcinoma (HAC) of peritoneum and omentum: a case report and literature review. Onco Targets Ther. 2019;12:7649-7654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Tsuruta S, Ohishi Y, Fujiwara M, Ihara E, Ogawa Y, Oki E, Nakamura M, Oda Y. Gastric hepatoid adenocarcinomas are a genetically heterogenous group; most tumors show chromosomal instability, but MSI tumors do exist. Hum Pathol. 2019;88:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Chang CY, Wei CY, Chen PH, Hou MC, Chao Y, Chau GY, Lee RC, Huang YH, Su YH, Wu JC, Su CW. The role of albumin-bilirubin grade in determining the outcomes of patients with very early-stage hepatocellular carcinoma. J Chin Med Assoc. 2021;84:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Düzköylü Y, Aras O, Bostancı EB, Keklik Temuçin T, Ulaş M. Mixed Adeno-Neuroendocrine Carcinoma; Case Series of Ten Patients with Review of the Literature. Balkan Med J. 2018;35:263-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Verbeek WH, Korse CM, Tesselaar ME. GEP-NETs UPDATE: Secreting gastro-enteropancreatic neuroendocrine tumours and biomarkers. Eur J Endocrinol. 2016;174:R1-R7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Domori K, Nishikura K, Ajioka Y, Aoyagi Y. Mucin phenotype expression of gastric neuroendocrine neoplasms: analysis of histopathology and carcinogenesis. Gastric Cancer. 2014;17:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Fujimoto M, Matsuzaki I, Nishino M, Iwahashi Y, Warigaya K, Kojima F, Ono K, Murata SI. HER2 is frequently overexpressed in hepatoid adenocarcinoma and gastric carcinoma with enteroblastic differentiation: a comparison of 35 cases to 334 gastric carcinomas of other histological types. J Clin Pathol. 2018;71:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Sun L, Zhang J, Wang C, Zhao S, Shao B, Guo Y, Liu Y, Sun Y. Chromosomal and molecular pathway alterations in the neuroendocrine carcinoma and adenocarcinoma components of gastric mixed neuroendocrine-nonneuroendocrine neoplasm. Mod Pathol. 2020;33:2602-2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Toyomasu Y, Mochiki E, Ishiguro T, Ito T, Suzuki O, Ogata K, Kumagai Y, Ishibashi K, Saeki H, Shirabe K, Ishida H. Clinical outcomes of gastric tube reconstruction following laparoscopic proximal gastrectomy for early gastric cancer in the upper third of the stomach: experience with 100 consecutive cases. Langenbecks Arch Surg. 2021;406:659-666. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 42. | Scardoni M, Vittoria E, Volante M, Rusev B, Bersani S, Mafficini A, Gottardi M, Giandomenico V, Malleo G, Butturini G, Cingarlini S, Fassan M, Scarpa A. Mixed adenoneuroendocrine carcinomas of the gastrointestinal tract: targeted next-generation sequencing suggests a monoclonal origin of the two components. Neuroendocrinology. 2014;100:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 43. | Gurzu S, Fetyko A, Bara T, Banias L, Butiurca VO, Bara T Jr, Tudorache V, Jung I. Gastrointestinal mixed adenoneuroendocrine carcinoma (MANEC): An immunohistochemistry study of 13 microsatellite stable cases. Pathol Res Pract. 2019;215:152697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Ahmed A, Turner G, King B, Jones L, Culliford D, McCance D, Ardill J, Johnston BT, Poston G, Rees M, Buxton-Thomas M, Caplin M, Ramage JK. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer. 2009;16:885-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 220] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 45. | Pape UF, Berndt U, Müller-Nordhorn J, Böhmig M, Roll S, Koch M, Willich SN, Wiedenmann B. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2008;15:1083-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 270] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 46. | Fazio N, Spada F, Giovannini M. Chemotherapy in gastroenteropancreatic (GEP) neuroendocrine carcinomas (NEC): a critical view. Cancer Treat Rev. 2013;39:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Shah MH, Goldner WS, Halfdanarson TR, Bergsland E, Berlin JD, Halperin D, Chan J, Kulke MH, Benson AB, Blaszkowsky LS, Eads J, Engstrom PF, Fanta P, Giordano T, He J, Heslin MJ, Kalemkerian GP, Kandeel F, Khan SA, Kidwai WZ, Kunz PL, Kuvshinoff BW, Lieu C, Pillarisetty VG, Saltz L, Sosa JA, Strosberg JR, Sussman CA, Trikalinos NA, Uboha NA, Whisenant J, Wong T, Yao JC, Burns JL, Ogba N, Zuccarino-Catania G. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018. J Natl Compr Canc Netw. 2018;16:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 270] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 48. | Yamaguchi T, Machida N, Morizane C, Kasuga A, Takahashi H, Sudo K, Nishina T, Tobimatsu K, Ishido K, Furuse J, Boku N, Okusaka T. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci. 2014;105:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 49. | Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, Birkemeyer E, Thiis-Evensen E, Biagini M, Gronbaek H, Soveri LM, Olsen IH, Federspiel B, Assmus J, Janson ET, Knigge U. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 712] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 50. | Thomas KEH, Voros BA, Boudreaux JP, Thiagarajan R, Woltering EA, Ramirez RA. Current Treatment Options in Gastroenteropancreatic Neuroendocrine Carcinoma. Oncologist. 2019;24:1076-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Hentic O, Hammel P, Couvelard A, Rebours V, Zappa M, Palazzo M, Maire F, Goujon G, Gillet A, Lévy P, Ruszniewski P. FOLFIRI regimen: an effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr Relat Cancer. 2012;19:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 52. | Ezziddin S, Opitz M, Attassi M, Biermann K, Sabet A, Guhlke S, Brockmann H, Willinek W, Wardelmann E, Biersack HJ, Ahmadzadehfar H. Impact of the Ki-67 proliferation index on response to peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging. 2011;38:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Montanier N, Joubert-Zakeyh J, Pétorin C, Montoriol PF, Maqdasy S, Kelly A. The prognostic influence of the proliferative discordance in metastatic pancreatic neuroendocrine carcinoma revealed by peptide receptor radionuclide therapy: Case report and review of literature. Medicine (Baltimore). 2017;96:e6062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Oktay E, Yalcin GD, Ekmekci S, Kahraman DS, Yalcin A, Degirmenci M, Dirican A, Altin Z, Ozdemir O, Surmeli Z, Diniz G, Ayhan S, Bulut G, Erdogan A, Uslu R. Programmed cell death ligand-1 expression in gastroenteropancreatic neuroendocrine tumors. J BUON. 2019;24:779-790. [PubMed] |
| 55. | Kim ST, Ha SY, Lee S, Ahn S, Lee J, Park SH, Park JO, Lim HY, Kang WK, Kim KM, Park YS. The Impact of PD-L1 Expression in Patients with Metastatic GEP-NETs. J Cancer. 2016;7:484-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 56. | Yang MW, Fu XL, Jiang YS, Chen XJ, Tao LY, Yang JY, Huo YM, Liu W, Zhang JF, Liu PF, Liu Q, Hua R, Zhang ZG, Sun YW, Liu DJ. Clinical significance of programmed death 1/programmed death ligand 1 pathway in gastric neuroendocrine carcinomas. World J Gastroenterol. 2019;25:1684-1696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Yamashita S, Abe H, Kunita A, Yamashita H, Seto Y, Ushiku T. Programmed cell death protein 1/programmed death ligand 1 but not HER2 is a potential therapeutic target in gastric neuroendocrine carcinoma. Histopathology. 2021;78:381-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Klein O, Kee D, Markman B, Michael M, Underhill C, Carlino MS, Jackett L, Lum C, Scott C, Nagrial A, Behren A, So JY, Palmer J, Cebon J. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin Cancer Res. 2020;26:4454-4459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 59. | Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F, Morse MA, Ascierto PA, Horn L, Amin A, Pillai RN, Evans J, Chau I, Bono P, Atmaca A, Sharma P, Harbison CT, Lin CS, Christensen O, Calvo E. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 999] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 60. | Rassidakis GZ, Delladetsima JK, Letsos SP, Polyzos A, Yannopoulos A. Hepatoid adenocarcinoma of the stomach with extensive neuroendocrine differentiation and a coexisting carcinoid tumour. Histopathology. 1998;33:186-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Okamoto T, Ogasahara K, Fujiki M, Takagi H, Ikeda H, Makino T, Moriga T, Kawamoto K, Sano K, Yoshida Y, Itoh T, Takasan H, Wani Y, Kono Y. Primary coexistent neuroendocrine carcinoma, hepatoid adenocarcinoma, and tubular adenocarcinoma of the stomach with focal trophoblastic differentiation in metastatic lymph nodes. J Gastroenterol. 2003;38:608-610. [PubMed] |
| 62. | Suzuki A, Koide N, Kitazawa M, Mochizuka A, Ota H, Miyagawa S. Gastric composite tumor of alpha fetoprotein-producing carcinoma/hepatoid adenocarcinoma and endocrine carcinoma with reference to cellular phenotypes. Patholog Res Int. 2012;2012:201375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Lipi L, Sachdev R, Gautam D, Singh J, Mohapatra I. Triple composite tumor of stomach: a rare combination of alpha fetoprotein positive hepatoid adenocarcinoma, tubular adenocarcinoma and large cell neuroendocrine carcinoma. Indian J Pathol Microbiol. 2014;57:98-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Wincewicz A, Kowalik A, Zięba S, Lewitowicz P, Góźdź S, Sulkowski S. α-Fetoprotein-Producing Hepatoid Gastric Adenocarcinoma With Osteoclast-Like Giant Cells and Neuroendocrine Differentiation: A Case Study With Molecular Profiling. Int J Surg Pathol. 2015;23:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Li T, Liu T, Wang M, Zhang M. Α-fetoprotein producing hepatoid gastric adenocarcinoma with neuroendocrine differentiation: A case report. Medicine (Baltimore). 2018;97:e12359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |