Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5316
Peer-review started: April 4, 2023
First decision: June 12, 2023
Revised: June 23, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: August 6, 2023
Processing time: 120 Days and 23.5 Hours
The deterioration of thyroid health is involved in the progression of heart failure (HF). This is usually a lengthy process, so there are almost no reports on its rapid development. Here we report a case of a young male who rapidly developed hypothyroid cardiomyopathy secondary to radioactive iodine treatment, suggesting that severe HF might occur even after a short period of hypo
A 26-year-old man was referred to our hospital for HF presenting with dyspnea on exertion and chest discomfort lasting for 1 mo. He received radioactive iodine treatment for hyperthyroidism 1 year ago and had an almost normal echocardiogram 6 mo ago. Admission echocardiogram and cardiac magnetic resonance (CMR) revealed left ventricle (LV) global hypokinesia and severely depressed systolic function. In addition, late gadolinium enhancement indicated no obvious changes in the myocardium. Thyroid function tests showed decreased serum levels of thyroid hormone (TH) and elevated thyroid-stimulating hormone. Based on an exclusionary examination, the patient was diagnosed with hypothyroid cardiomyopathy and was started on replacement therapy. His HF symptoms were completely relieved during the six-month follow-up, and echocardiogram and CMR revealed recovered LV size and ejection fraction.
This report demonstrates that severe fluctuations in TH levels may lead to acute HF, which can completely recover with timely thyroid hormone replacement. In addition, our findings highlight the importance of routinely detecting cardiac function in patients treated with radioactive iodine.
Core Tip: We present the case of a young man who had clinical manifestations of heart failure but an almost normal echocardiogram some 6 mo ago. His condition progressed rapidly, and auxiliary examinations ruled out other diseases. The diagnosis of hypothyroid cardiomyopathy was retrospective and exclusive. We must pay attention to changes in cardiac function after radioactive iodine treatment and consider the possibility of hypothyroid cardiomyopathy when the symptoms of heart failure appear.
- Citation: Li ZH, Ni LJ, Liu YQ, Si DY. Rapid progression of heart failure secondary to radioactive iodine treatment of hyperthyroidism: A case report. World J Clin Cases 2023; 11(22): 5316-5321
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5316.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5316
Thyroid hormones have important physiological effects. The lack of thyroid hormone (TH) can affect almost every organ system in the body, resulting in various clinical manifestations[1]. In the cardiovascular system, hypothyroidism may result in reduced cardiac output, arterial hypertension, sinus bradycardia, QT interval prolongation and pericardial effusion, and it can be associated with many diseases, especially heart failure (HF)[2,3]. Conversely, HF itself can lead to reduced serum levels of active TH[4]. The deterioration of thyroid health can be involved in the progression of HF, though this is usually a lengthy process, and there are almost no reports on its rapid development. Here we report a case of a young male who rapidly developed hypothyroid cardiomyopathy secondary to radioactive iodine treatment, suggesting that severe HF might occur even after a short period of hypothyroidism.
A 26-year-old man was admitted to our hospital due to dyspnea on exertion and chest discomfort, with no inducing factors, 1 mo ago.
The patient’s symptoms gradually worsened within 1 mo. Additional symptoms included a weight gain of 20 kg in the prior 3 mo and paroxysmal nocturnal dyspnea. He denied fever, upper respiratory tract infection, chest pain, palpitations or a history of drug use.
His medical records were notable for radioactive iodine treatment for hyperthyroidism 1 year ago, and he had an almost normal echocardiogram 6 mo ago, which revealed a left ventricle (LV) with a diameter of 48.8 mm and a normal left ventricular ejection fraction (LVEF) of 65%. He did not frequently control his cardiac or thyroid function in the past 6 mo.
He denied any family history of sudden cardiac death or primary cardiomyopathy.
On physical examination at admission, his blood pressure was 105/81 mmHg, and his pulse rate was 92 beats/min. Additionally, an early diastolic gallop rhythm could be noticed at the cardiac apex.
His myocardial enzymes were in the normal range, which ruled out myocarditis. No obvious abnormalities were found in routine blood tests, routine coagulation tests, liver function tests, kidney function tests, etc. Notable laboratory findings on presentation included high total cholesterol and triglyceride levels of 5.81 mmol/L and 4.72 mmol/L, respectively, and an elevated brain natriuretic peptide level of 502 pg/mL. Thyroid function tests revealed thyroid-stimulating hormone of 41.08 mIU/L (0.37-4.94), free triiodothyronine (T3) of 2.17 pmol/L (3.1-6.8), free thyroxine (T4) of 8.0 pmol/L (12-22), T3 of 0.79 nmol/L (1.35-3.15), T4 of 46.9 nmol/L (70-156), anti-thyroglobulin antibody of 758.3 IU/mL (0-115), antithyroid peroxidase autoantibody of 215.8 IU/mL, and antithyroid microsome antibody of 69.6% (0-25).
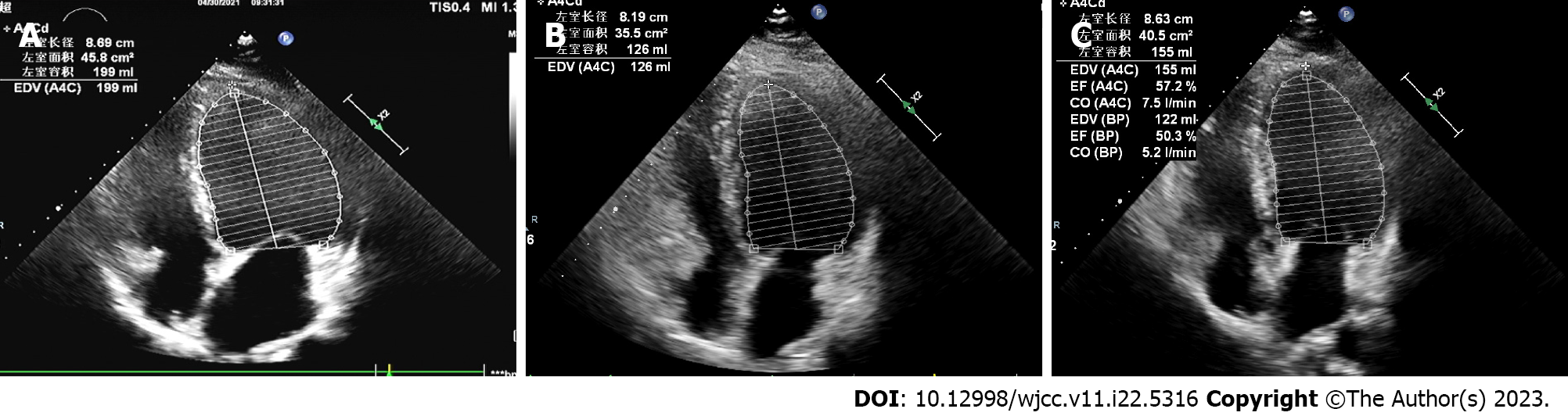
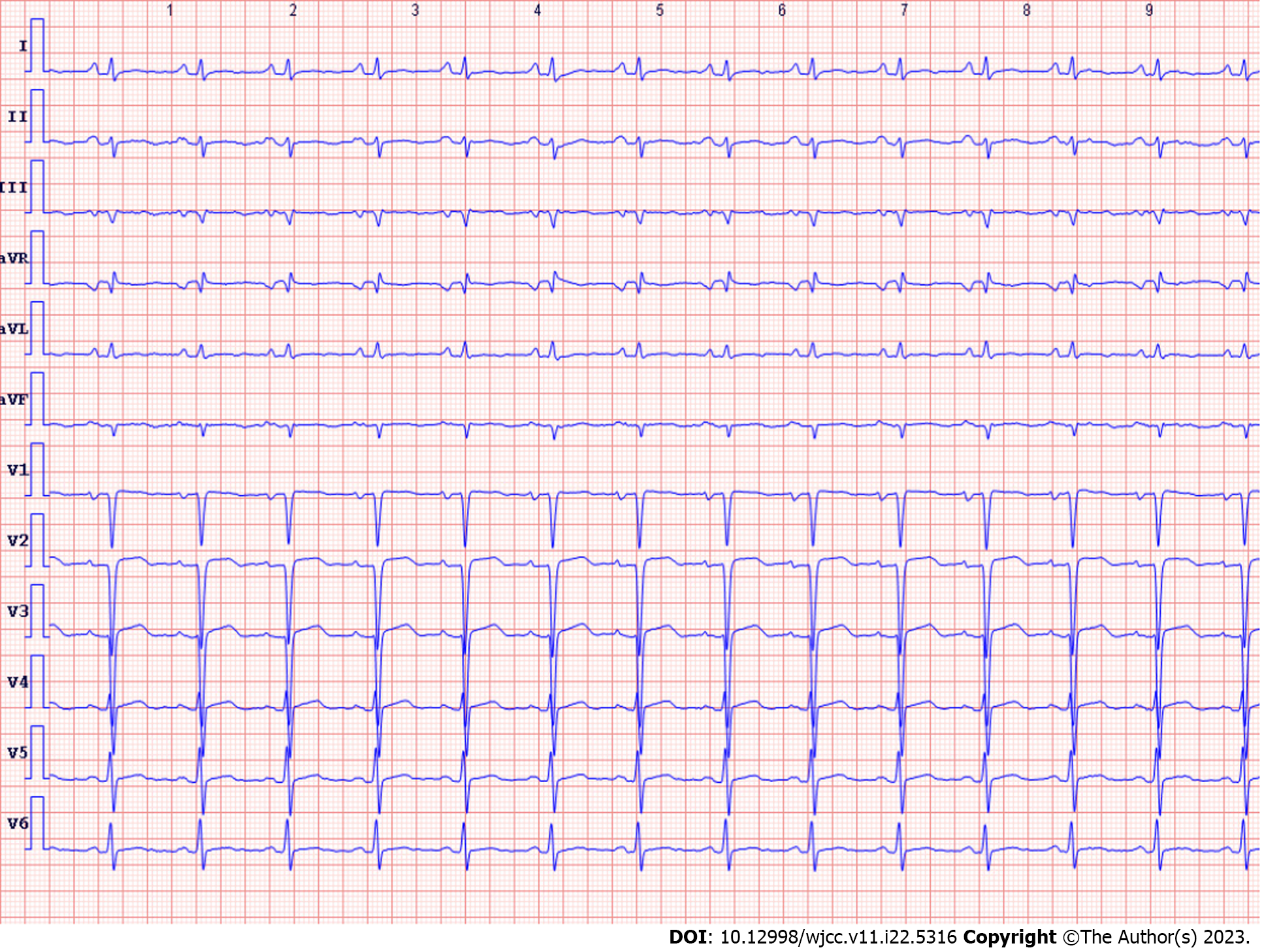
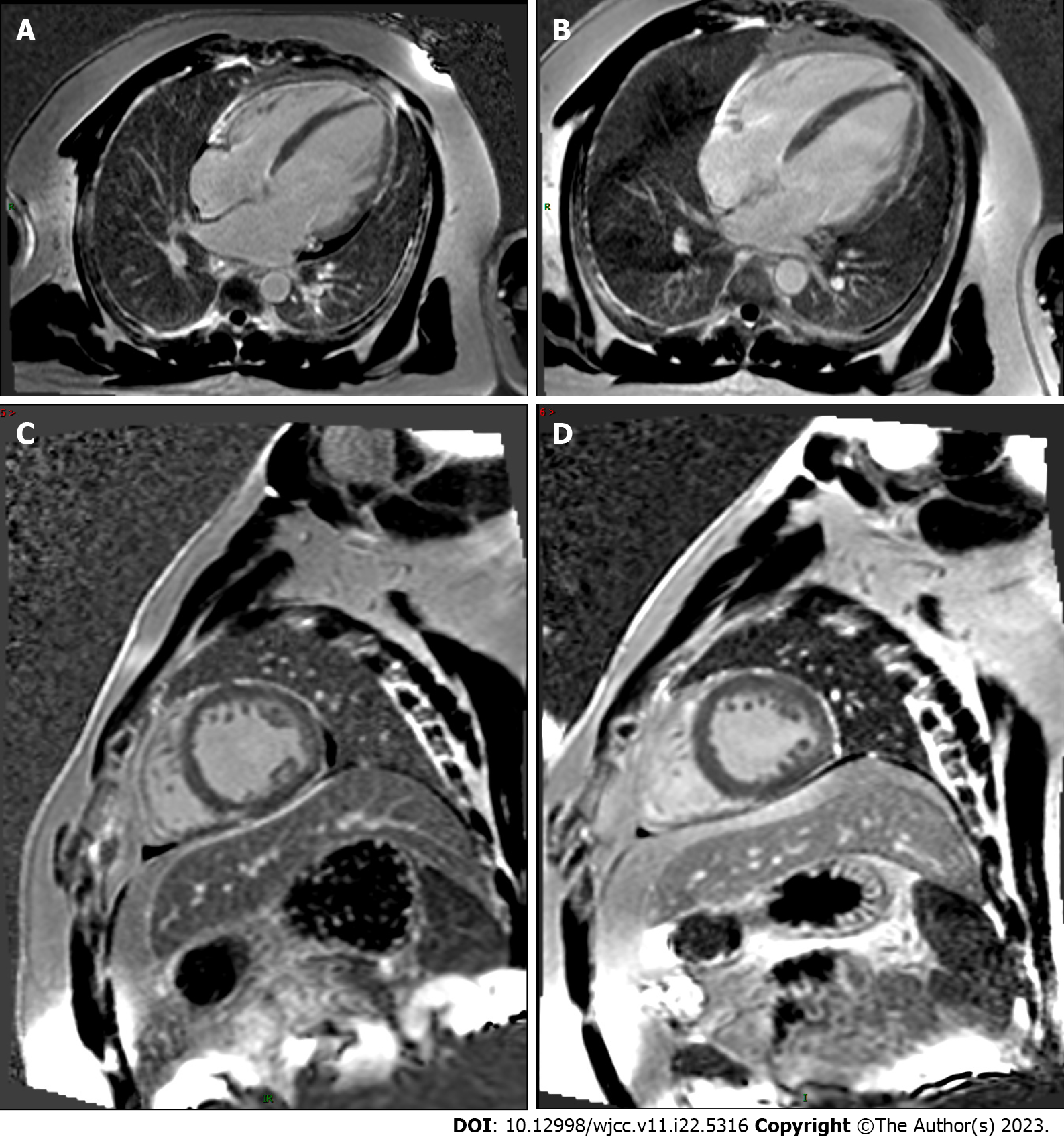
His echocardiogram (Figure 1A) showed significant enlargement of the LV (diameter of 54 mm), severe LV global hypokinesis, minimal pericardial effusion and severe systolic dysfunction, with LVEF of 35%. We did not see any valve damage. The electrocardiogram (Figure 2) revealed obvious Q waves in leads III, aVF, and V1-V3. As coronary computed tomography angiography detected no coronary artery stenosis, we ruled out coronary heart disease. Cardiac magnetic resonance (CMR) (Figure 3A and C) also indicated an enlarged LV and decreased LVEF without abnormal myocardial perfusion of the left ventricular wall. Late gadolinium enhancement showed no obvious changes in the myocardium. Based on the above results, certain special types of cardiomyopathies were ruled out. Thyroid ultrasonography showed no diffuse mass.
After ruling out other possibilities, the patient was diagnosed with hypothyroid cardiomyopathy.
The patient was given TH replacement therapy with levothyroxine 25 µg/d. He was given anti-heart failure treatment with furosemide 20 mg/d, spironolactone 25 mg/d, metoprolol 47.5 mg/d, and Entresto 50 mg/d twice a day. One week later, he had responded well and was discharged because of the resolution of clinical symptoms. He continued the treatment with the oral drugs.
One month later, the patient showed no obvious clinical symptoms, and the heart did not have murmurs or extra heart sounds. Thyroid function had returned to normal. Echocardiogram (Figure 1B) showed the improvement of LVEF from 35% to 44%, the reduction of left atrium from 49.4 mm to 41.4 mm, the reduction of LV from 53.6 mm to 52.0 mm and pericardial effusion had resolved compared with that during admission. At the 6-mo follow-up appointment, he remained asymptomatic. Physical examination showed no obvious positive signs. A repeat echocardiogram (Figure 1C) revealed a diminishment of the enlarged LV chamber and a continuous increase in LVEF to 54% and the LV continued to shrink to 51.2 mm. The follow-up CMR (Figure 3B and D) also showed that the dilated LV decreased, the overall systolic function recovered, and pericardial effusion decreased compared with the CMR findings during admission. These objective data corresponded to significant symptom alleviation and return to normal functional status.
Our patient had rapidly progressive HF secondary to hypothyroidism after radioactive iodine therapy. LV global hypokinesia and severely depressed systolic function were found on his echocardiogram. Although hypothyroidism is common after iodine-131 treatment, it is rare to see such a drastic change in cardiac function that may be related to rapid changes in thyroid hormones. This suggests that severe fluctuations in TH levels may lead to acute changes in the myocardium, eventually resulting in HF. The patient responded well to TH replacement therapy and had a gradual shrinkage of his enlarged LV chamber and markedly improved LV systolic function after treatment. He was diagnosed with hypothyroid cardiomyopathy because of the strong temporal relationship between thyroid hormone supplementation and his reversed symptoms and other exclusionary examinations. To the best of our knowledge, there are no similar reports in the literature. Hypothyroid cardiomyopathy has been reported in previous cases, most of which were secondary to Hashimoto’s thyroiditis[5], and the remaining cases were complications of Sheehan’s syndrome or hypopituitarism[6,7]. In addition, they were often the result of a long period of untreated hypothyroidism. However, our patient's HF symptoms developed rapidly, in less than 6 mo after radioactive iodine therapy. It is worth mentioning that all these symptoms were improved or even completely recovered by timely supplementation with thyroid hormones. The present results are consistent with previous reports concerning the potential reversibility of HF after levothyroxine replacement, emphasizing the potential relationship between rapid changes in thyroid hormones and the development of HF.
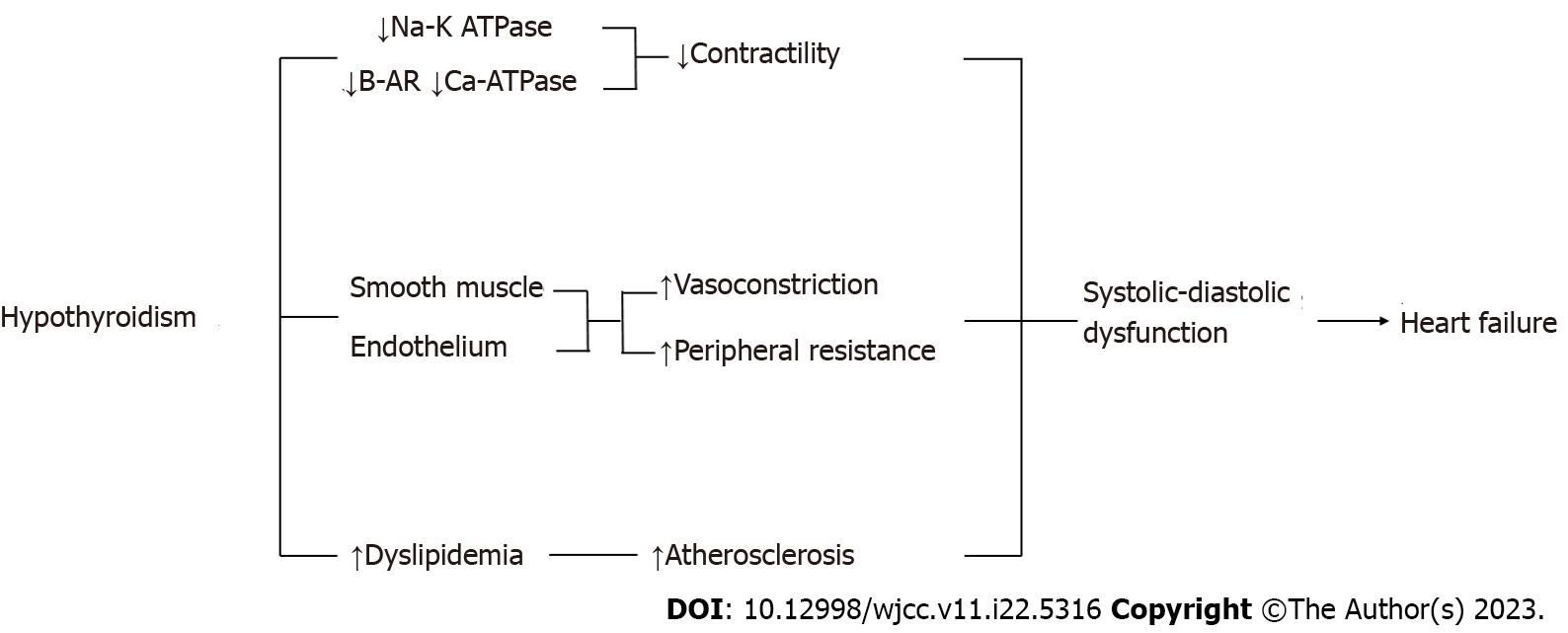
Hypothyroidism can lead to HF. On the one hand, it reduces contractility due to reduced activity of the Sodium-Potassium ATPase and CA2 + ATP-ase pumps. On the other hand, it increases peripheral resistance by acting on the endothelial cells and smooth muscle cells to cause vasoconstriction. It also determines the increase in serum cholesterol levels, which can lead to ischemic disorders. All of these result lead to systolic-diastolic dysfunction that may favor the onset or progression of heart failure (Figure 4)[8].
Animal and clinical trials have also demonstrated the relationship between heart failure and hypothyroidism. Previous animal experiments suggested that chronic hypothyroidism alone could produce dilated HF, characterized by impaired coronary blood flow and increased myocyte length/width ratio[9]. Low TH levels have also been independently associated with a worse prognosis in HF patients without a history of hypothyroidism[10]. A growing body of evidence suggests that a mutual cause-and-effect relationship might exist between HF and the hypothyroid state. Several cross-sectional case-control studies have shown that approximately 30% of patients with CHF have low T3 levels and that T3 in serum decreases proportionately to the severity of heart disease[11]. Although controversial, some HF patients may benefit from TH supplementation[12]. Our case shows that a sudden decrease in TH can cause HF in a short time and can be recovered by TH replacement, which may provide a new perspective to guide the treatment of hypothyroidism with HF.
Herein, we introduced a case of a young male who rapidly developed hypothyroid cardiomyopathy secondary to radioactive iodine treatment, thus demonstrating the importance of periodic monitoring of cardiac function after iodine-131 treatment. Moreover, we showed the importance of timely supplementation with thyroid hormones, which may completely reverse the disease process.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Barik R, India; Gupta P, United States S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Martinez F. Thyroid hormones and heart failure. Heart Fail Rev. 2016;21:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Delitala AP, Scuteri A, Fiorillo E, Orrù V, Lakatta EG, Schlessinger D, Cucca F. Carotid Beta Stiffness Association with Thyroid Function. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Zimmerman J, Yahalom J, Bar-On H. Clinical spectrum of pericardial effusion as the presenting feature of hypothyroidism. Am Heart J. 1983;106:770-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Danzi S, Klein I. Thyroid Abnormalities in Heart Failure. Heart Fail Clin. 2020;16:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Seol MD, Lee YS, Kim DK, Choi YH, Kim DJ, Park SH, Cho HJ, Cho WH. Dilated cardiomyopathy secondary to hypothyroidism: case report with a review of literatures. J Cardiovasc Ultrasound. 2014;22:32-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Mushtaq Z, Mushtaq Z, Kanwal A, Fiazuddin F. Hypothyroidism Induced Cardiomyopathy. J Am Coll Cardiol. 2020;75. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Verhoestraete P, Carpentier M, Donck J, Vandekerckhove H. Dilated cardiomyopathy and rhabdomyolysis caused by hypopituitarism: a challenging diagnosis. Acta Cardiol. 2020;75:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Rizzo C, Gioia MI, Parisi G, Triggiani V, Iacoviello M. Dysthyroidism and Chronic Heart Failure: Pathophysiological Mechanisms and Therapeutic Approaches. Adv Exp Med Biol. 2018;1067:239-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Tang YD, Kuzman JA, Said S, Anderson BE, Wang X, Gerdes AM. Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation. 2005;112:3122-3130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Brenta G, Thierer J, Sutton M, Acosta A, Vainstein N, Brites F, Boero L, Gómez Rosso L, Anker S. Low plasma triiodothyronine levels in heart failure are associated with a reduced anabolic state and membrane damage. Eur J Endocrinol. 2011;164:937-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Pingitore A, Landi P, Taddei MC, Ripoli A, L'Abbate A, Iervasi G. Triiodothyronine levels for risk stratification of patients with chronic heart failure. Am J Med. 2005;118:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Badran HM, Faheem N, Zidan A, Yacoub MH, Soltan G. Effect of Short-Term L-Thyroxine Therapy on Left Ventricular Mechanics in Idiopathic Dilated Cardiomyopathy. J Am Soc Echocardiogr. 2020;33:1234-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |