Published online Aug 6, 2023. doi: 10.12998/wjcc.v11.i22.5309
Peer-review started: March 2, 2023
First decision: June 12, 2023
Revised: July 7, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: August 6, 2023
Processing time: 153 Days and 20.4 Hours
The co-occurrence of Anti-phospholipase A2 receptor-associated membranous nephropathy (anti-PLA2R-MN) and human immunodeficiency virus (HIV) in
A 32-year-old Chinese male diagnosed with HIV infection presented with a clinical history of proteinuria persisting for over two years. A kidney biopsy demonstrated subepithelial immune complex deposition and a thickened glo
Telitacicept might offer a potential therapeutic avenue for patients diagnosed with anti-PLA2R-MN concomitant with HIV infection.
Core Tip: The clinical management of anti-phospholipase A2 receptor-associated membranous nephropathy concurrent with human immunodeficiency virus infection presents significant challenges, primarily due to potential unregulated immune responses. In this report, we demonstrated that a weekly subcutaneous injection of telitacicept (160 mg) could ameliorate the 24-h urinary protein levels and enhance serum albumin concentrations, with no discernible impact on T cell counts, showcasing its safety profile.
- Citation: Wang JL, Sun YL, Kang Z, Zhang SK, Yu CX, Zhang W, Xie H, Lin HL. Anti-phospholipase A2 receptor-associated membranous nephropathy with human immunodeficiency virus infection treated with telitacicept: A case report. World J Clin Cases 2023; 11(22): 5309-5315
- URL: https://www.wjgnet.com/2307-8960/full/v11/i22/5309.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i22.5309
Anti-phospholipase A2 receptor-associated membranous nephropathy (anti-PLA2R-MN) is a form of primary mem
A 32-year-old Chinese male was admitted to our hospital with a progressive increase in urine protein levels to 4000-5000 mg/24 h.
Roughly 2.5 years prior, the patient sought a nephrology consultation due to fever and low back pain. He exhibited 2+ proteinuria. Serum creatinine levels of 83 μmol/L and a 24-h urinary protein quantification of 1835 mg. Antinuclear antibody, anti-Sm, anti-dsDNA, anti-PLA2R antibodies, and complement tests were all negative. The patient has been under regular follow-up since then.
He had been living with HIV for six years and had a prior history of successfully treated pulmonary tuberculosis five years prior.
No relevant personal or family history was provided.
Vital signs, including body temperature, blood pressure, heart rate, and respiratory rate were within normal limits.
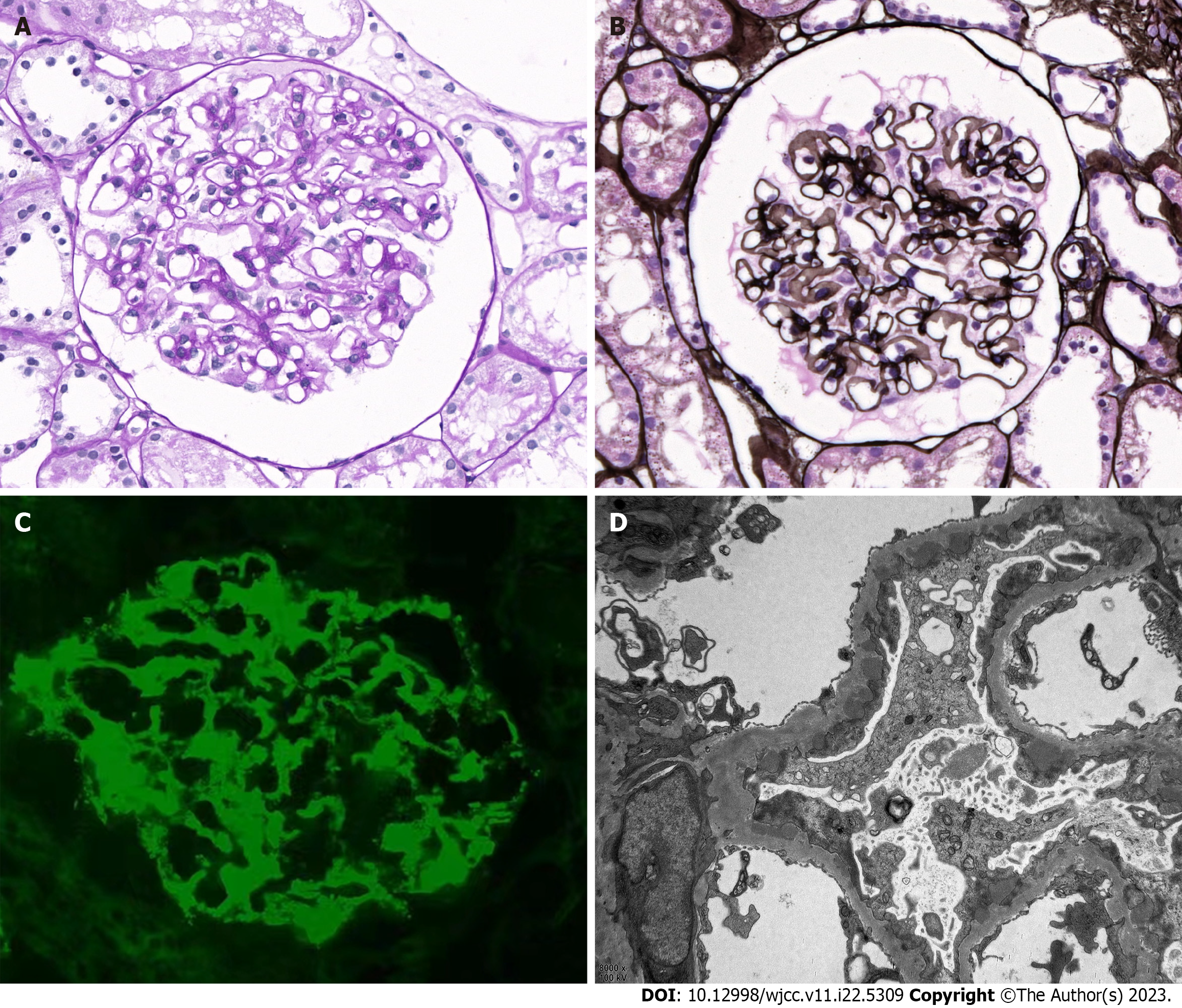
Upon admission, a kidney biopsy showed that all 22 glomeruli had neither global nor segment sclerosis, and the capillary loops were patent. No glomerular cell proliferation or inflammatory cell infiltration were observed. Subepithelial immune complex deposition and glomerular basement membrane thickening were noted, but no spikes or double contours were seen. The renal tubular epithelial cells exhibited granular changes without significant atrophy. Mild protein casts were found in the tubules. Mild inflammatory cell infiltration was noted in the renal interstitium, but there was no significant fibrosis or edema. The arterioles walls showed thickening (Figure 1). Congo red staining was negative. Immunofluorescence staining demonstrated granular deposition of PLA2R (3+) and immunoglobulin G (IgG) (1+) (weak positive of IgG4 and negative of IgG1) along the capillary loops. The findings of electron microscopy were compatible with membranous nephropathy (stage I-II).
No imaging studies were performed.
Based on the above clinical history and findings, the patient was diagnosed with anti-PLA2R-MN.
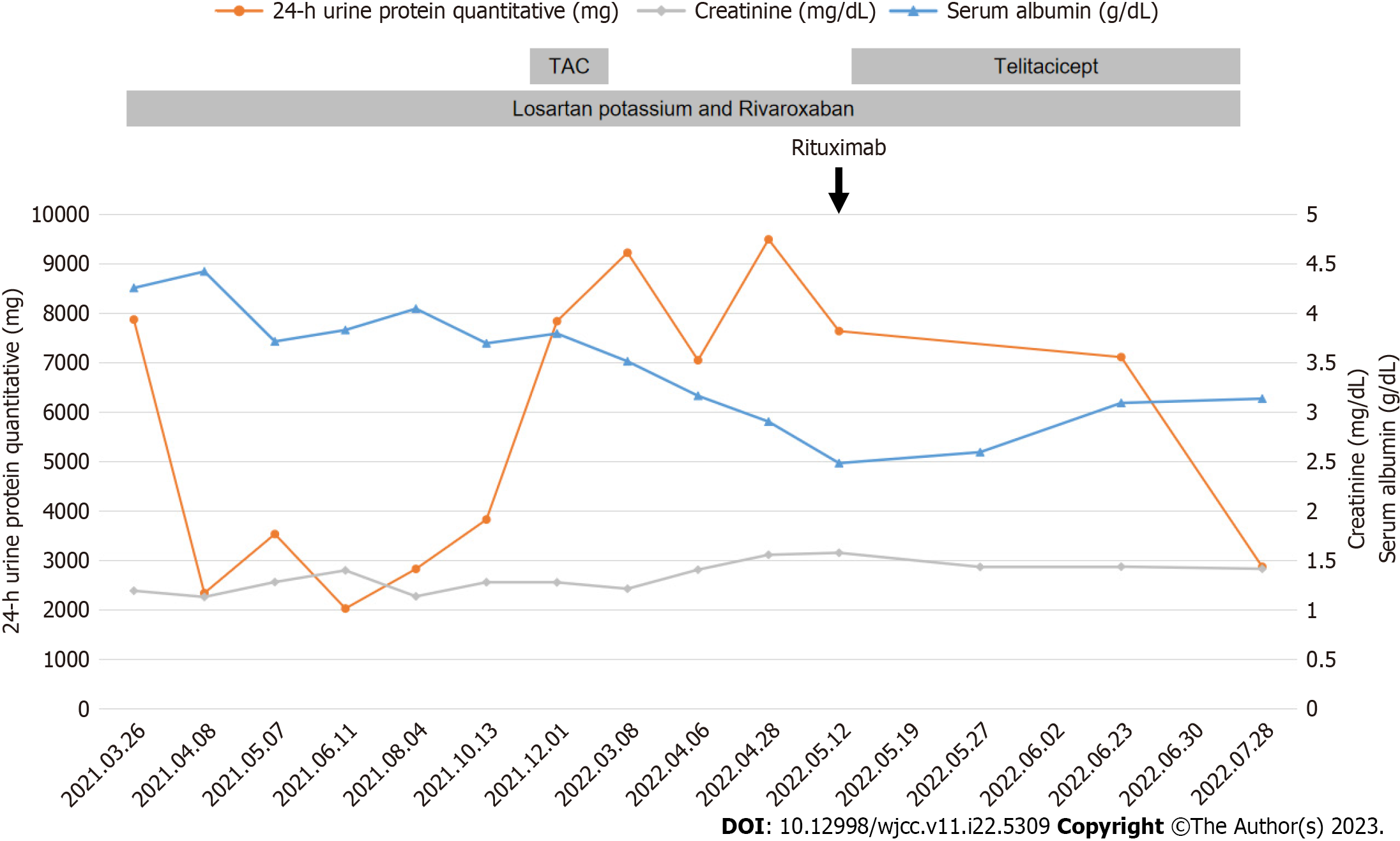
The patient’s initial treatment included losartan potassium and rivaroxaban, which corresponded with 24-h urinary protein levels of 2338-3826 mg and serum albumin concentrations of 33.3-39.8 g/L. However, a sudden surge in 24-h urinary protein level to 7838 mg led to the addition of tacrolimus (0.5 mg bid po). Plasma concentration level of tacrolimus was sub-therapeutic (1.8 ng/mL) two weeks later. Unfortunately, after three months on tacrolimus, 24-h urinary protein level still rose to 9224.17 mg, while fasting blood glucose level rose to 6.82 mmol/L.
Owing to side effects and suboptimal efficacy of tacrolimus, the treatment was discontinued and replaced with intravenous rituximab 2 mo later because his 24-h urine protein quantitative level increased to 9493.50 mg, serum albumin decreased to 26.13 g/L and anti-PLA2R antibody titer of 1:320. At that moment, his serum creatinine was 125.57 µmol/L; total T cell count was 828/µL; total B cell count was 158/µL; CD3+CD4+T cell count was 436/µL; and CD3+CD8+T cell count was 348/µL.
Unfortunately, an allergic reaction occurred during rituximab administration, including body-wide itching, scattered small red papules on the back, and an elevated heart rate (100-110 times/min), leading to its discontinuation after 200 mg. Subsequently, treatment with subcutaneous injections of telitacicept (160 mg once a week) was initiated.
After three months of telitacicept treatment, the patient’s 24-h urinary protein levels declined to 2869.76 mg, his serum albumin level rosed from 22.35 g/L to 28.22 g/L, and serum creatinine decreased to 112.7 μmol/L (Figure 2). A test for anti-PLA2R antibody titer was postponed due to the coronavirus disease 2019 epidemic, and no adverse events, such as respiratory infections or diarrhea, were observed during the treatment.
Throughout treatment, the patient's HIV remained in a state of low transcription. The latest cell count showed CD3+CD4+T cell count of 352/µL and a CD3+CD8+T cell count of 375/µL. Continued treatment and follow-up are ongoing.
Individuals positive for HIV demonstrated an elevated risk for kidney disease, including HIV-associated nephropathy, non-collapsing focal segmental glomerulosclerosis, immune-complex kidney disease, comorbid kidney disease, and kidney injury associated with prolonged exposure to antiretroviral therapy or opportunistic infection[2]. These conditions are the main contributors to end-stage renal disease in HIV-positive patients[3]. Literature reviews of HIV-associated MN cases frequently report identification of MN in the context of undetectable viral loads. In approximately 50% of cases, tissue reactivity with the PLA2R antigen can be identified, even in the absence of corresponding anti-PLA2R serum antibodies[1].
A proposed mechanism for the development of anti-PLA2R autoantibodies in HIV patients is "bystander activation", where tissue damage arises from an over-reactive antiviral immune response, followed by the release of self-antigens that perpetuate autoimmune-mediated harm via spreading epitopes[4]. Antiretroviral therapy is an important strategy to minimize the incidence of acute kidney injury and HIV-related kidney diseases[5]. However, the nephrotoxicity of antiretroviral drugs should also be taken into account. Therefore, chronic kidney disease (CKD) screening is reco
In this case, we encountered a refractory case of anti-PLA2R-MN associated with HIV infection. It is critical to identify the primary cause of progressive renal disease in HIV-positive patients. Differing treatments are needed depending on whether interstitial kidney injury resulting from prolonged exposure to antiretroviral therapy or from opportunistic infections. Other causes should also be ruled out. Kidney biopsy is the most definitive method to establish a diagnosis. Our patient’s kidney biopsy is the most definitive method to establish a diagnosis. Our patient's kidney biopsy showed immune complex deposition in the subepithelial area and a thickened glomerular basement membrane, indicative of stage I-II membranous nephropathy. Immunofluorescence showed granular deposition of PLA2R (3+) along the capillary loops, and the test of anti-PLA2R antibody was strongly positive (1:320); these findings confirmed a diagnosis of anti-PLA2R-MN. About 50%-90% of anti-PLA2R-MN patients present with nephrotic syndrome and the degree of proteinuria remission is associated with the prognosis of renal function[6]. In our case, the main complaints were proteinuria, hypoalbuminemia, and fatigue.
The primary challenge in clinically treating anti-PLA2R-MN co-existing with HIV infection is the selection of immunosuppressants. In our case, the patient was in a status of immunodeficiency with a history of tuberculosis. Instead of utilizing a small dose of glucocorticoids or cyclophosphamide, we chose losartan potassium and tacrolimus to control the progression of kidney injury. Tacrolimus, a calcineurin inhibitor, could combine with immune avidin and suppress cytoplasm calcineurin activity, subsequently blocking the T cell proliferation and avoiding bone marrow suppression[7]. Studies have shown that calcineurin inhibitors have effects on the podocyte cytoskeleton, resulting in a non-specific reduction of proteinuria[8]. Furthermore, tacrolimus could induce a rapid decrease in anti-PLA2R levels[9,10]. A meta-analysis[11] found that tacrolimus therapy was associated with high total remission and low proteinuria level compared to cyclophosphamide for patients with MN. However, in our case, after three months of tacrolimus treatment, the 24-h urine protein continued to rise without remission. Due to the elevated blood glucose level, a side effects, tacrolimus was discontinued. According to the “Expert consensus on the application of rituximab in the treatment of membranous nephropathy”[12], rituximab has been applied to treat membranous nephropathy in recent years with favorable results. As a murine/human chimeric anti-CD20 monoclonal antibody that depletes B cells, rituximab has been reported to be effective in the treatment of MN by clearing B cells, inhibiting B cell-T cell interaction, or depleting CD20dimT cells, contributing to decreased T-cell activation, or decreased effector T-cell generation, which, as a consequence, would result in rising Treg cell percentages among CD4 T cells[13] with the expert consensus’ recommendation on rituximab in treating anti-PLA2R-MN and refractory MN. Unfortunately, our patient showed an allergic reaction when we changed to rituximab therapy.
Telitacicept, a novel fusion protein comprising a recombinant transmembrane activator and calcium modulator and cyclophilin ligand interactor receptor fused to the fragment crystallizable domain of human immunoglobulin G (IgG)[14]. It’s a dual inhibitor of B-lymphocyte stimulator and a proliferation-inducing ligand (APRIL). BlyS (B cell activator, BAFF) and APRIL are crucial in maintaining the B cell pool and humoral immunity. For example, BlyS regulates differentiation and maturation of immature B cells, while APRIL oversees the function and survival of long-lived plasma cells. Both play prominent roles in the pathogenesis of autoimmune diseases. By binding to and neutralizing the activity of these two cell-signaling molecules, BlyS and APRIL, Telitacicept suppresses the development and survival of plasma cells and mature B cells. Telitacicept has shown promising clinical outcomes in the treatment of B cell-mediated autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, and multiple sclerosis, with good clinical outcomes[15].
A recent study demonstrated telitacicept’s efficacy on lupus nephritis, with all 8 pediatric patients showing varying degrees of urine protein reduction, gradual normalization of albumin, and some improvement in renal function without further deterioration[16]. However, treating anti-PLA2R-MN with HIV infection with telitacicept has not been reported before. Our case has shown a surprising therapeutic effect with no obvious side effects till now. According to the 2021 KDIGO GN Guidelines[17], our patient’s condition was reduced from high risk (proteinuria > 3.5 g/d, no decrease > 50% after six months of conservative therapy with ACEi, serum albumin < 25 g/L, and PLA2Rab > 50 RU/mL) to partial remission (proteinuria < 3.5 g/d and serum albumin > 25 g/L).
We report a case of anti-PLA2R-MN associated with HIV infection in which telitacicept was effective in reducing proteinuria. More comprehensive clinical and laboratory studies are required to assess the efficacy and safety of telitacicept in treating patients with anti-PLA2R-MN and HIV infection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koubaa M, Tunisia; Thongon N, Thailand S-Editor: Liu JH L-Editor: A P-Editor: Ju JL
| 1. | Charu V, Andeen N, Walavalkar V, Lapasia J, Kim JY, Lin A, Sibley R, Higgins J, Troxell M, Kambham N. Membranous nephropathy in patients with HIV: a report of 11 cases. BMC Nephrol. 2020;21:401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Swanepoel CR, Atta MG, D'Agati VD, Estrella MM, Fogo AB, Naicker S, Post FA, Wearne N, Winkler CA, Cheung M, Wheeler DC, Winkelmayer WC, Wyatt CM; Conference Participants. Kidney disease in the setting of HIV infection: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2018;93:545-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 3. | Prudhvi K, Schwartz D, Fisher M. Rituximab for Lupus-Like Membranous Nephropathy in the Setting of Well-Controlled HIV Infection. Am J Ther. 2020;28:e732-e734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM. Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 350] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 5. | Booth JW, Hamzah L, Jose S, Horsfield C, O'Donnell P, McAdoo S, Kumar EA, Turner-Stokes T, Khatib N, Das P, Naftalin C, Mackie N, Kingdon E, Williams D, Hendry BM, Sabin C, Jones R, Levy J, Hilton R, Connolly J, Post FA; HIV/CKD Study and the UK CHIC Study. Clinical characteristics and outcomes of HIV-associated immune complex kidney disease. Nephrol Dial Transplant. 2016;31:2099-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Di J, Qian Q, Yang M, Jiang Y, Zhou H, Li M, Zou Y. Efficacy and safety of long-course tacrolimus treatment for idiopathic membranous nephropathy. Exp Ther Med. 2018;16:979-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Huang H, Liang Z, Zheng X, Qing Q, Du X, Tang Z, Wei M, Wang C, Zhong Q, Lin X. Tacrolimus versus cyclophosphamide for patients with idiopathic membranous nephropathy and treated with steroids: a systematic review and meta-analysis of randomized controlled trials. Ren Fail. 2021;43:840-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 763] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 9. | Ramachandran R, Yadav AK, Kumar V, Siva Tez Pinnamaneni V, Nada R, Ghosh R, Rathi M, Kohli HS, Gupta KL, Sakhuja V, Jha V. Two-Year Follow-up Study of Membranous Nephropathy Treated With Tacrolimus and Corticosteroids Versus Cyclical Corticosteroids and Cyclophosphamide. Kidney Int Rep. 2017;2:610-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Fernández-Juárez G, Rojas-Rivera J, Logt AV, Justino J, Sevillano A, Caravaca-Fontán F, Ávila A, Rabasco C, Cabello V, Varela A, Díez M, Martín-Reyes G, Diezhandino MG, Quintana LF, Agraz I, Gómez-Martino JR, Cao M, Rodríguez-Moreno A, Rivas B, Galeano C, Bonet J, Romera A, Shabaka A, Plaisier E, Espinosa M, Egido J, Segarra A, Lambeau G, Ronco P, Wetzels J, Praga M; STARMEN Investigators. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int. 2021;99:986-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 11. | Lin W, Li HY, Lin S, Zhou T. Efficacy and safety of tacrolimus vs cyclophosphamide in the therapy of patients with idiopathic membranous nephropathy: a meta-analysis. Drug Des Devel Ther. 2019;13:2179-2186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Nephrology Expert Panel of the Peking University Health Science Center. [Expert consensus on the application of rituximab in the treatment of membranous nephropathy]. Zhonghua Nei Ke Za Zhi. 2022;61:282-290. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Rosenzwajg M, Languille E, Debiec H, Hygino J, Dahan K, Simon T, Klatzmann D, Ronco P. B- and T-cell subpopulations in patients with severe idiopathic membranous nephropathy may predict an early response to rituximab. Kidney Int. 2017;92:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 14. | Shi F, Xue R, Zhou X, Shen P, Wang S, Yang Y. Telitacicept as a BLyS/APRIL dual inhibitor for autoimmune disease. Immunopharmacol Immunotoxicol. 2021;43:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 15. | Dhillon S. Telitacicept: First Approval. Drugs. 2021;81:1671-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 16. | Sun L, Shen Q, Gong Y, Li Y, Lv Q, Liu H, Zhao F, Yu H, Qiu L, Li X, He X, Chen Y, Xu Z, Xu H. Safety and efficacy of telitacicept in refractory childhood-onset systemic lupus erythematosus: A self-controlled before-after trial. Lupus. 2022;31:998-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 17. | Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, Cook HT, Fervenza FC, Gibson KL, Glassock RJ, Jayne DRW, Jha V, Liew A, Liu ZH, Mejía-Vilet JM, Nester CM, Radhakrishnan J, Rave EM, Reich HN, Ronco P, Sanders JF, Sethi S, Suzuki Y, Tang SCW, Tesar V, Vivarelli M, Wetzels JFM, Lytvyn L, Craig JC, Tunnicliffe DJ, Howell M, Tonelli MA, Cheung M, Earley A, Floege J. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:753-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 459] [Article Influence: 114.8] [Reference Citation Analysis (0)] |