Published online Jul 26, 2023. doi: 10.12998/wjcc.v11.i21.5179
Peer-review started: May 24, 2023
First decision: June 15, 2023
Revised: June 24, 2023
Accepted: July 7, 2023
Article in press: July 7, 2023
Published online: July 26, 2023
Processing time: 63 Days and 20.9 Hours
Congenital lymphangiectasia is a rare disease characterized by dilated interstitial lymphatic vessels and cystic expansion of the lymphatic vessels. Congenital lym
We analysed the case of a neonate who had presented with only pleural effusion at a late gestational age and eventually died due to its inability to establish spon
Considering the presented case, obstetricians should observe unexplained foetal pleural effusion, and perform pathology analysis and whole exome sequencing for a conclusive diagnosis and prompt treatment.
Core Tip: Congenital lymphangiectasia is a rare disease characterized by an increased number of lymphatic vessels in the interstitium and cystic expansion of the lymphatic vessels. The genetic variations in proteins that control the development of lymphatic vessels are suggested as the pathophysiology of this disease. We analysed the case of a neonate who presented with only pleural effusion during late gestational age. However, the patient died after delivery because of the inability to establish spontaneous breathing. The autopsy indicated lymphangiectasia within systemic organs. Further, whole exome sequencing revealed rare heterozygous mutations in the lymphangiogenesis-controlling genes ADAMTS3 and FLT4.
- Citation: Liang ZW, Gao WL. ADAMTS3 and FLT4 gene mutations result in congenital lymphangiectasia in newborns: A case report. World J Clin Cases 2023; 11(21): 5179-5186
- URL: https://www.wjgnet.com/2307-8960/full/v11/i21/5179.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i21.5179
Congenital lymphangiectasia is a rare condition characterized by dilatated lymphatic vessels of the interstitial tissue, with cystic dilatation of the lymphatic vessels as the main pathological feature. It is a congenital developmental abnormality with a poor prognosis that has not been well studied. This condition may not exhibit obvious abnormalities during prenatal examinations. However, affected newborns quickly develop respiratory distress, which causes rapid deterioration in their condition and eventually leads to death. Without autopsy and genetic testing, it is difficult to detect this abnormality in newborns, which can potentially cause medical disputes.
Embryonic lymphatic vessel development begins at 9-16 wk gestation and starts regressing around 20 wk. Failure of normal regression can result in lymphangiectasia[1], leading to foetal oedema; Its pathogenesis includes genetic variations in proteins that regulate lymphatic vessel development. Previous studies have reported gene mutations, including CCBE1, FOXC2, FLT4, and ADAMTS3, associated with the disease[2]. However, most cases involve single-gene mutations.
In this case report, the foetus reportedly appeared normal during early and mid-pregnancy examinations; however, pleural effusion was observed during late pregnancy examinations. After birth, the newborn failed to respond to resuscitation efforts and died owing to severe respiratory distress. An autopsy revealed congenital lymphangiectasia with severe lymphatic vessel dilatation in multiple organ systems. Whole exome genetic testing identified ADAMTS3 and FLT4 heterozygous variants in the newborn. Sanger sequencing confirmed a similar genetic mutation in the mother. In summary, obstetric clinicians should be aware of the serious underlying problems in newborns presenting with severe respiratory distress shortly after birth.
At 37 wk gestation, the pregnant woman presented to the emergency department with a complaint of foetal movement cessation for 2 d and was admitted. Foetal heart monitoring and oxytocin challenge test were performed, which revealed frequent late decelerations and poor variability, indicating foetal distress. An emergency caesarean section was performed. The delivery was uneventful, with the umbilical cord wrapped around the neck of the newborn. After clearing its airway and clamping its umbilical cord, the newborn had poor muscle tone and no spontaneous breathing. The Apgar scores were 1 at 1 min (skin colour: 1) and 2 at 5 and 10 min (skin colour: 1, heart rate: 1).
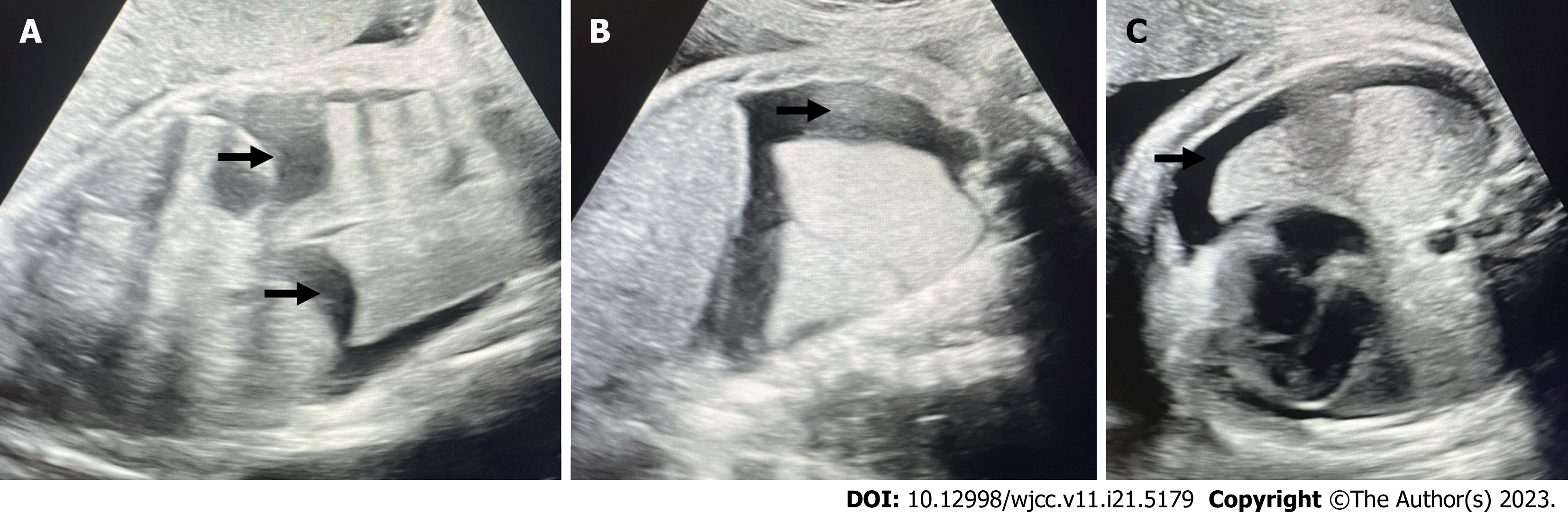
A pregnant woman aged 31 years was admitted on February 13, 2022 with the chief complaint of ‘cessation of foetal movement for 2 d at 37 wk gestation’. This pregnancy was her first, conceived naturally with gestational age confirmation at 12 wk by nuchal translucency measurement of 0.16 cm. Regular antenatal examinations were performed, with foetal movement observed at 17 wk gestation. Non-invasive prenatal testing performed at 16 wk gestation indicated a low risk of abnormality. Two ultrasound screenings at 24 and 28 wk gestation showed no significant abnormalities. At 34 wk gestation, an ultrasound revealed foetal bilateral pleural effusion with a maximum depth of approximately 0.88 cm on the left side and 2.08 cm on the right side. The patient was referred to the prenatal diagnosis centre for foetal echocardiography, which showed no abnormalities. Surgical consultation for paediatric thoracic surgery was recommended; however, the parents did not proceed with it. At 37 wk gestation (3 d before delivery), a follow-up ultrasound showed increased foetal bilateral pleural effusion, measuring approximately 1.9 cm in the vertical dimension and 1.6 cm in the horizontal dimension on the left side, and 1.8 cm in the vertical dimension and 1.3 cm in the horizontal dimension on the right side (Figure 1).
No trauma or fever was identified as a cause during the course of the disease. The patient had no history of chronic conditions, no exposure to cattle or sheep, no use of special drugs.
The parents of the deceased newborn had no significant medical history, were physically healthy, and not close blood relations.
Upon examination, swelling was observed on the right side of the neck with no abnormality on the left side and the abdomen was distended with high tension.
To determine the cause of congenital lymphatic dysplasia in the newborn, the family consented to undergo genetic testing. After signing an informed consent form, skin and subcutaneous tissue samples were obtained from the right inguinal region of the deceased newborn on February 13, 2022. Additionally, 2 mL of peripheral venous blood samples were collected from the newborn's parents on May 17, 2022 and transferred into ethylenediaminetetraacetic acid anticoagulant tubes. These samples were sent to the Shenzhen BGI Medical Laboratory for genetic analysis. Whole exome sequencing was performed on the newborn's genomic DNA, followed by variant analysis for point mutations and small insertions/deletions, and copy number variation analysis based on second-generation sequencing data. Candidate variant sites were further validated by Sanger sequencing using DNA samples from family members.
High-throughput sequencing identified three gene variants in the proband (Figure 2): (1) ADAMTS3 gene heterozygous variant [c.2667delC (p.Asn889Lys fs*22)]; (2) FLT4 gene heterozygous variant (c.2021-13C>T); and (3) AGT gene heterozygous variant (c.1184_1185delTGinsGT p.Leu395Arg). The ADAMTS3 mutation in the newborn was confirmed by Sanger sequencing and found to originate from the mother. The upper panel represented the proband's mutation, the middle panel showed the mother's mutation, and it was evident that the mutation site and type were identical. The lower panel displayed the sequencing results of the corresponding site in the father, where no relevant mutation was observed. A similar situation existed for the FLT4 gene, wherein the upper panel represented the proband's mutation, the middle panel showed the mother's mutation, and again, the mutation site and type were completely consistent. The AGT gene mutation in the proband was inherited from the father, whereas no relevant mutation was observed in the mother.
Bedside ultrasound revealed freely floating anechoic areas in the bilateral pleural cavities with a thickness of 1.6 cm on the right side and 1.2 cm on the left side, and an echogenic solid lung tissue floating within the pleural fluid. No obvious intestinal dilatation was observed in the abdominal cavity; however, free fluid measuring approximately 0.9 cm in depth with poor echogenicity was observed. The diagnosis was bilateral pleural effusion (moderate to large amount) with solid lung tissue floating within the pleural fluid.
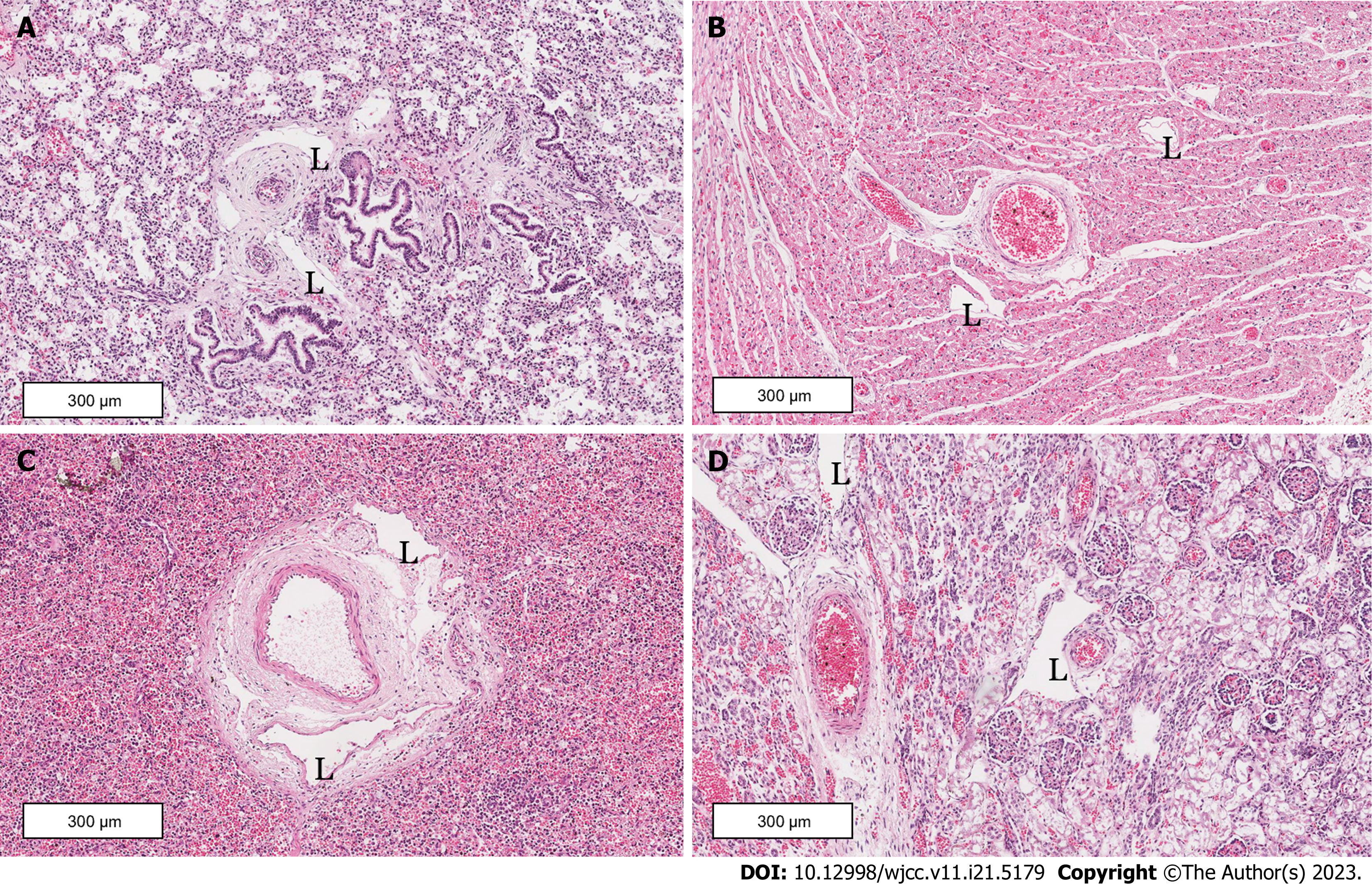
A clinical autopsy revealed significant pleural effusion in the newborn (Figure 3), measuring 70 mL on the left thoracic cavity and 80 mL on the right side. Both lungs were poorly developed, with an abnormally enlarged liver and spleen. The pathological report indicated congenital lymphatic dysplasia, with extensive lymphatic vessel dilatation in the lung, heart, liver, kidney, pancreas, and subcutaneous tissues, most prominently observed in the lung tissue (Figure 4). The fluid accumulation was predominantly in the pleural cavity, followed by the pericardial and abdominal cavities. Other findings included right ventricular wall thickening, ductus arteriosus dilatation, and lung and liver tissue congestions. No abnormality was observed in other organs.
The final diagnosis is congenital lymphangiectasia.
Immediate resuscitation measures were initiated, including endotracheal intubation, positive pressure ventilation with oxygen, and chest compressions; however, the newborn's heart rate was weak at 40-50 beats per minute, with 0%-50% blood oxygen saturation.
Despite resuscitation efforts for 2 h, the heart rate and breathing were not recovered. Subsequently, the family decided to withdraw treatment, and the newborn died.
Congenital lymphangiectasia in newborns presents with a wide range of clinical manifestations, depending on the site of lymphatic vessel dilatation. Generally, localized lymphatic vessel dilatation involving a single organ system is more common. For instance, lymphatic vessel dilatation occurs in the lungs and can lead to life-threatening pleural effusion. Literature reports often describe pulmonary lymphatic dysplasia with associated pleural effusion, with a mortality rate of 50%-98% and an incidence rate of 1 in 10000 or 1 in 15000 pregnancies[3]. Only 40% of infants with this condition can be successfully delivered. Lymphatic vessel dilatation in the limbs may result in limb enlargement and distinctive facial features. When it occurs in the intestinal tract, recurrent diarrhoea and feeding difficulties may be observed, which is typically detected during the developmental process. However, widespread lymphatic vessel dilatation involving mul
Abnormal foetal lymphatic development can occur when the various proteins involved in lymphatic vessel formation are affected by genetic mutations. Previous studies have reported over 20 gene variants, including CCBE1, FOXC2, FLT4, and ADAMTS3, associated with congenital lymphatic dysplasia, although most are single gene variants. Combined ADAMTS3 and FLT4 mutations identified in this study have not been reported previously.
ADAMTS3 is essential for lymphatic vessel development. Previous laboratory and animal studies have shown that functional defects involving this gene variant can lead to severe lymphatic development abnormalities in rats and zebrafish[6]. ADAMTS3 gene mutations disrupt the signalling process involved in lymphatic vessel formation, resulting in Hennekam-3 syndrome characterized by primary lymphedema, lymphatic vessel dilation, and distinctive facial features in newborns. The ADAMTS3 gene variant observed in this case is a less common type of ADAMTS3 gene mutation. It involves C protein deletion at position 2667 in the chromosome 4 coding region, leading to the substitution of asparagine with lysine at position 889 and a subsequent frameshift of 22 amino acids. Sanger sequencing confirmed that the mother had an identical variant at the same position without exhibiting clinical symptoms, whereas the father lacked a corresponding mutation at that site. Similar cases have been reported in previous studies on Hennekam-3 syndrome. In 2017, Brouillard et al[7] reported a family with Hennekam-3 syndrome, where a son and a daughter exhibited typical symptoms of Hennekam-3 syndrome, whereas the parents were asymptomatic. Genetic testing revealed two heterozygous ADAMTS3 mutations in the affected children, one inherited from the father and the other from the mother.
In addition, our case involves a concurrent heterozygous FLT4 gene mutation. FLT4 gene mutations are among the most common genetic variants associated with congenital lymphatic dysplasia in newborns. Connell et al[8] analysed 52 patients with primary lymphedema and found FLT4-related gene mutations in 75% and 68% of patients with a positive and negative family history of primary lymphedema, respectively. The protein encoded by FLT4 regulates the proliferation, migration, and growth of lymphatic endothelial cells. Over 50 FLT4 gene mutation sites have been reported, all of which can cause lymphatic vessel developmental abnormalities. The FLT4 mutation in this case is c.2021-13C>T, which is a recently discovered heterozygous variant with limited related reports. Considering the clinical manifestations and variant carrier among family members, this mutation is speculated to be a pathogenic variant. The mother of the affected newborn has an identical gene defect but does not exhibit symptoms, whereas the father does not have a corresponding mutation at that site.
This case is unique because of the simultaneous occurrence of the heterozygous mutations of both ADAMTS3 and FLT4 genes, which has not been observed in previous research. The consequence is likely systemic lymphatic vessel dilatation. In our case, lymphatic vessel dilatation affected vital organs, such as the heart, lungs, liver, and kidneys. Autopsy revealed significant pleural effusion with 70 mL in the left thoracic cavity and 80 mL in the right side, and consolidated lungs that failed to expand. The newborn was unable to establish respiration and blood circulation after birth, leading to unsuccessful resuscitation. This outcome is strongly associated with the complex genetic mutations observed and should be given due attention.
Diagnosing and identifying neonatal lymphatic dysplasia is extremely challenging owing to its diverse clinical presentations, making targeted examinations particularly important. Obstetricians should obtain a complete family history and maternal obstetric history. If ultrasound reveals signs of foetal oedema, such as skin thickness > 5 mm, enlarged placenta, pericardial effusion, pleural effusion, and ascites, lymphatic vessel dilation-related oedema should be considered. In addition, an ultrasound should be performed to confirm the presence of cervical lymphatic vessel dilatations if a newborn exhibits swelling in the cervical region after birth (as in our case). A study by Catalano et al[9] confirmed that the high spatial resolution of ultrasound in superficial layers can be a powerful tool for etiological discrimination. Neonatal cystic lymphangiectasis of the neck should be differentiated from thyroglossal duct cysts. Thyroglossal duct cysts is the most common congenital anomaly of the thyroglossal duct, which connects the thyroid to the tongue during the 4th and 7th wk of development. The study by Corvino et al[10] details the operational method of identification using ultrasound. For full-term newborns, if severe respiratory distress with pleural effusion, especially chylothorax, occurs after birth, clinicians should majorly suspect pulmonary lymphatic dysplasia. Imaging studies, pulmonary function tests, and pathological examinations (lung biopsy, bronchoscopy, and pleural fluid analysis) can be performed to establish a definitive diagnosis. Definitive diagnosis of neonatal lymphatic dysplasia relies on histopathological examination. Autopsy findings in neonates with lymphatic dysplasia include variable-sized transparent cysts on the lung surface with a honeycomb pattern on sectioning. Microscopically, cystic dilatation and lymphatic vessel proliferation occur in the subpleural space, in
In cases of foetal oedema and pleural effusion in newborns, if severe respiratory failure occurs after birth that contradicts the prenatal assessment results, pulmonary lymphatic dysplasia should be highly suspected, and potential causes should be systematically investigated. After obtaining a definitive pathological diagnosis, obstetricians should actively conduct whole-genome sequencing for the corresponding diseases, to ultimately uncover the underlying aetiology. This approach would facilitate accurate diagnosis, reduces misdiagnosis and missed diagnoses, enable early intervention, assess prognosis, and guide genetic counselling.
We thank Dr. Ge-Hong Dong from the pathology department of Beijing Tiantan Hospital for the pathological exami
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Al-Ani RM, Iraq; Corvino A, Italy; Oley MH, Indonesia S-Editor: Lin C L-Editor: A P-Editor: Cai YX
| 1. | Brouillard P, Witte MH, Erickson RP, Damstra RJ, Becker C, Quéré I, Vikkula M. Primary lymphoedema. Nat Rev Dis Primers. 2021;7:77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 2. | Jeltsch M, Jha SK, Tvorogov D, Anisimov A, Leppänen VM, Holopainen T, Kivelä R, Ortega S, Kärpanen T, Alitalo K. CCBE1 enhances lymphangiogenesis via A disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation. Circulation. 2014;129:1962-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 3. | Bos FL, Caunt M, Peterson-Maduro J, Planas-Paz L, Kowalski J, Karpanen T, van Impel A, Tong R, Ernst JA, Korving J, van Es JH, Lammert E, Duckers HJ, Schulte-Merker S. CCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor-C in vivo. Circ Res. 2011;109:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Ahlawat S, Fayad LM, Durand DJ, Puttgen K, Tekes A. International Society for the Study of Vascular Anomalies Classification of Soft Tissue Vascular Anomalies: Survey-Based Assessment of Musculoskeletal Radiologists' Use in Clinical Practice. Curr Probl Diagn Radiol. 2019;48:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Mettauer N, Agrawal S, Pierce C, Ashworth M, Petros A. Outcome of children with pulmonary lymphangiectasis. Pediatr Pulmonol. 2009;44:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Gupta K, Das A, Menon P, Kakkar N, Rao KL, Joshi K. Revisiting the histopathologic spectrum of congenital pulmonary developmental disorders. Fetal Pediatr Pathol. 2012;31:74-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Brouillard P, Dupont L, Helaers R, Coulie R, Tiller GE, Peeden J, Colige A, Vikkula M. Loss of ADAMTS3 activity causes Hennekam lymphangiectasia-lymphedema syndrome 3. Hum Mol Genet. 2017;26:4095-4104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Connell FC, Ostergaard P, Carver C, Brice G, Williams N, Mansour S, Mortimer PS, Jeffery S; Lymphoedema Consortium. Analysis of the coding regions of VEGFR3 and VEGFC in Milroy disease and other primary lymphoedemas. Hum Genet. 2009;124:625-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Catalano O, Varelli C, Sbordone C, Corvino A, De Rosa D, Vallone G, Wortsman X. A bump: what to do next? Ultrasound imaging of superficial soft-tissue palpable lesions. J Ultrasound. 2020;23:287-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Corvino A, Pignata S, Campanino MR, Corvino F, Giurazza F, Tafuri D, Pinto F, Catalano O. Thyroglossal duct cysts and site-specific differential diagnoses: imaging findings with emphasis on ultrasound assessment. J Ultrasound. 2020;23:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Santo S, Mansour S, Thilaganathan B, Homfray T, Papageorghiou A, Calvert S, Bhide A. Prenatal diagnosis of non-immune hydrops fetalis: what do we tell the parents? Prenat Diagn. 2011;31:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Hagmann C, Berger TM. Images in clinical medicine. Congenital pulmonary lymphangiectasia. N Engl J Med. 2003;349:e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |