Published online Jul 26, 2023. doi: 10.12998/wjcc.v11.i21.5167
Peer-review started: May 10, 2023
First decision: May 31, 2023
Revised: June 12, 2023
Accepted: July 6, 2023
Article in press: July 6, 2023
Published online: July 26, 2023
Processing time: 77 Days and 12.2 Hours
Patients with proteinase 3-antineutrophil cytoplasmic antibody associated vasculitis (AAV) experience different manifestations at the initial onset and relapse. However, such cases of different initial and relapse manifestations have not been reported in myeloperoxidase (MPO)-AAV patients.
A 52-year-old woman was admitted to our hospital because of headache. Laboratory findings indicated nephrotic range proteinuria and microscopic hematuria, serum creatinine of 243 μmol/L, anti-MPO antibody titer of > 400 RU/mL, and positive perinuclearantineutrophil cytoplasmic antibody. Renal biopsy showed pauci-immune crescentic glomerulonephritis. The cerebrospinal fluid examination and brain magnetic resonance imaging did not show any abnormality. Therefore, MPO-AAV was diagnosed. Corticosteroids, plasma
We have reported a rare case of MPO-AAV who initially presented with headache and kidney involvement. However, relapse presented with only arthralgia, which was completely different from the initial manifestations. This case suggests that AAV relapse should be highly suspected in MPO-AAV patients after remission, when clinical manifestations at relapse are different from those at onset. Prednisone and MMF may provide a good choice for refractory arthralgia during relapse in MPO-AAV patients.
Core Tip: We report a rare case of myeloperoxidase (MPO)-antineutrophil cytoplasmic antibody-associated vasculitis (AAV) who initially presented with headache and kidney involvement. However, relapse presented with only arthralgia, which was completely different from the initial manifestations. This case suggests that AAV relapse should be highly suspected in MPO-AAV patients after remission, when clinical manifestations at relapse are different from those at onset. Prednisone and mycophenolate mofetil may provide a good choice for refractory arthralgia during relapse in MPO-AAV patients.
- Citation: Zhang X, Zhao GB, Li LK, Wang WD, Lin HL, Yang N. Myeloperoxidase-antineutrophil cytoplasmic antibody-associated vasculitis with headache and kidney involvement at presentation and with arthralgia at relapse: A case report. World J Clin Cases 2023; 11(21): 5167-5172
- URL: https://www.wjgnet.com/2307-8960/full/v11/i21/5167.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i21.5167
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of disorders characterized by necrotizing vasculitis in small- and medium-sized vessels with few or no immune deposits[1]. AAV may affect one or several organ systems. Initial AAV onset is most common in the kidney (72.3%), followed by the lungs (45%) and the ear, nose, and throat (29.7%)[2,3]. However, only 4.9%-20% of AAV patients initially present with neurological symptoms, of which peripheral neuropathy is predominant[4,5]. A total of 22.3%-53.5% of AAV patients who achieved remission experienced relapses[3,6-8]. The kidney (63.1%) and lung (47.4%) are the two most common involved organs at relapse, whereas joint involvement is rare (7%-21%)[8,9]. Among AAV patients, 72.4% had kidney involvement at the initial onset and at relapse[10]. In patients with proteinase 3 (PR3)-AAV, different organs are involved at the initial onset and relapse, whereas in patients with myeloperoxidase (MPO)-AAV, the same organ is involved at both instances[8,10]. Here, we report the rare case of an MPO-AAV patient, who presented with headache and kidney involvement at initial onset and developed arthralgia at relapse. Our case showed completely different clinical manifestations at the initial onset and relapse, which has not been reported previously.
A 52-year-old female patient had headache for 2 mo.
A 52-year-old female patient was admitted to our hospital in December 2014, because of headache for 2 mo without obvious predisposing causes.
On admission, the patient’s temperature was 37.2 °C, blood pressure was 140/90 mmHg, and her neurological examination was normal.
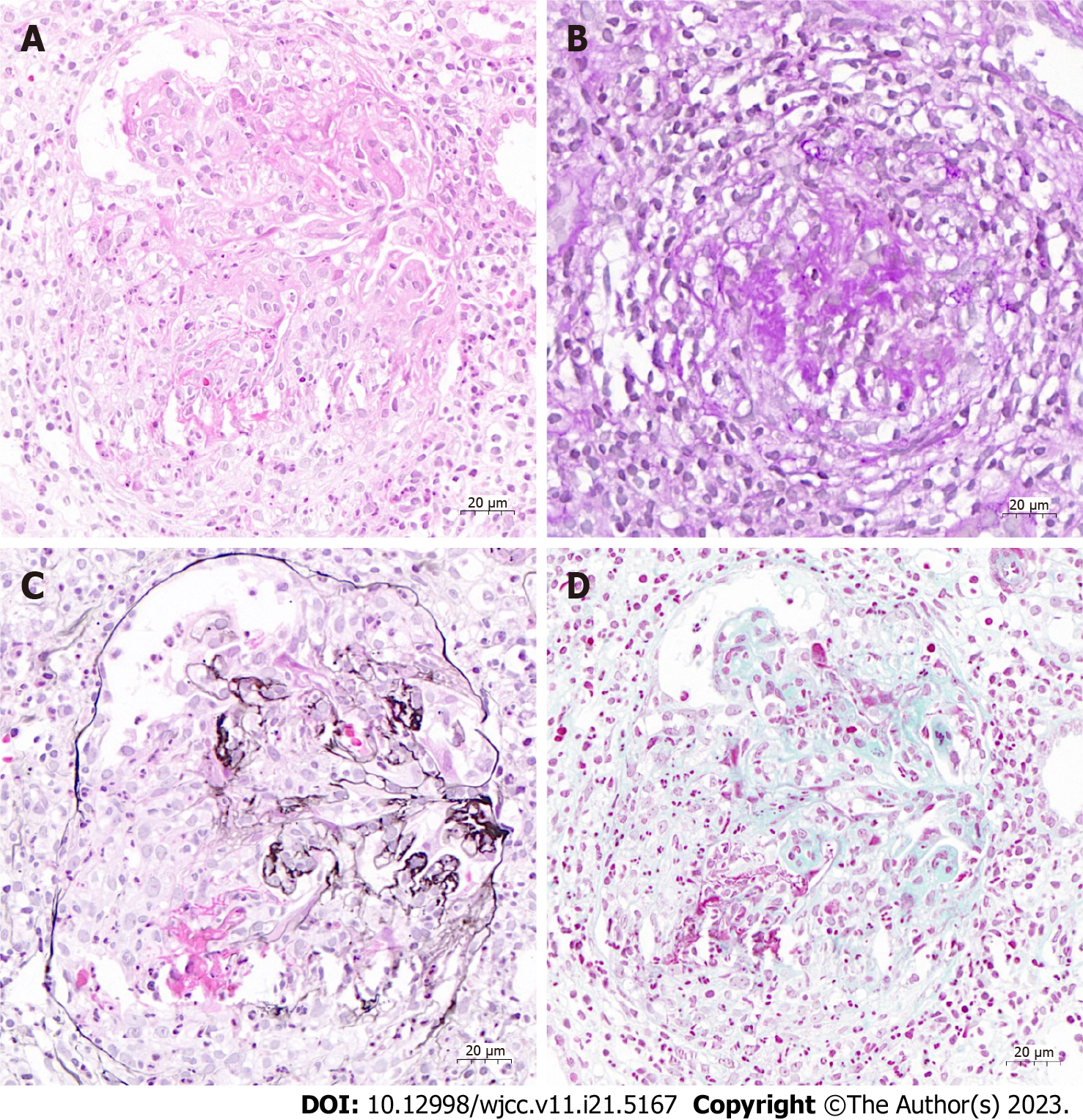
The patient’s laboratory test results were as follows: White blood cell count 5.81 × 109/L (normal range 3.5-9.5 × 109/L), hemoglobin level 74 g/L (115-150 g/L), and platelet count 204 × 109/L (125-350 × 109/L). The patient’s microscopic hematuria was 10-15 red blood cells (RBCs)/high power field (HPF); her 24-h urinary protein level was 3273 mg, and serum creatinine level was 243 μmol/L. The erythrocyte sedimentation rate was 26 mm/h (normal range 0-20 mm/h), and hypersensitive C-reactive protein level (CRP) was 61.6 mg/L (normal range 0-8 mg/L). The immunoglobulin (Ig)G, IgA, and IgM levels were normal. Complement (C)3 and C4 levels were normal. The antinuclear antibody test was negative. Rheumatoid factor level was 20.1 IU/mL (0-20 IU/mL). Serum anti-MPO antibody titer was > 400 RU/mL (normal range < 20 RU/mL), and PR3-ANCA was negative. Cerebrospinal fluid (CSF) examination revealed normal pressure, and total protein levels and cell count were within normal limits. A cytological examination for malignant cells was negative in the serum as well as in the CSF. Renal pathology revealed crescentic necrotizing glomerulonephritis on light microscopy, and no specific findings were observed on immunofluorescence microscopy and electron microscopy (Figure 1).
Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) scans of the brain showed no remarkable findings. No evidence of intracranial infection or cerebrovascular disease was observed.
The patient was diagnosed as having MPO-AAV.
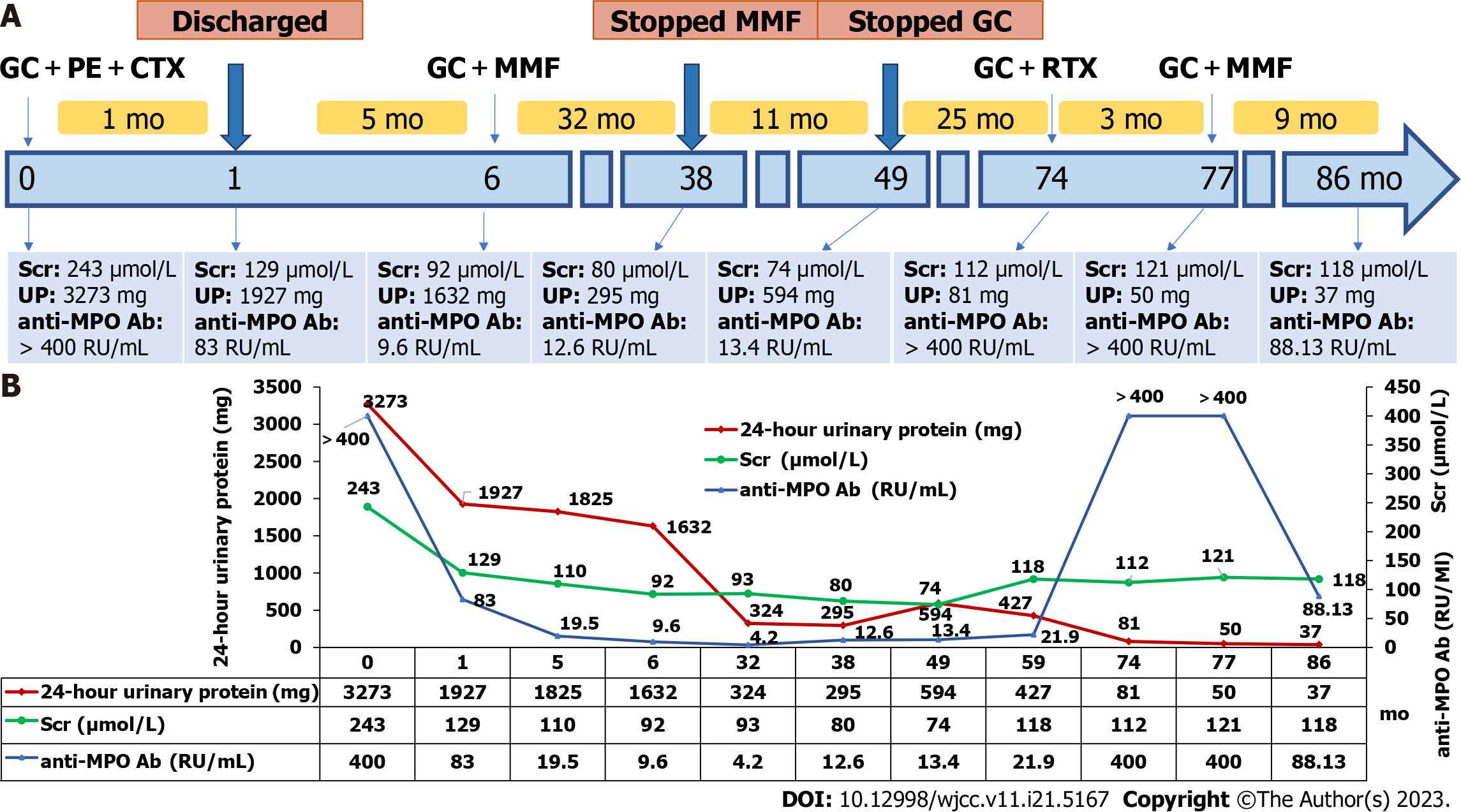
Corticosteroids in combination with cyclophosphamide and plasmapheresis as induction treatment were administered. The patient was treated with 500 mg intravenous methylprednisolone for 3 d, followed by 40 mg oral prednisone per day and 600 mg cyclophosphamide for 2 d. Ten plasma exchanges were performed every 2 d. Her symptoms gradually improved, and serum creatinine level was reduced to 129 μmol/L. The patient was discharged and was followed every month for 1 year.
After discharge, oral prednisone was slowly tapered. Intravenous cyclophosphamide was administered 600 mg every 2 wk. Four months after discharge, the patient’s headache resolved. Her serum creatinine was reduced to 110 μmol/L. Urine analysis showed 0-2 RBCs/HPF, 24-h urinary protein decreased to 1825 mg, and anti-MPO antibody titer dropped to 19.5 RU/mL. Five months after discharge, intravenous cyclophosphamide (cumulative dose of 10.8 g) was discontinued, and then, according to the KDIGO 2021 guideline, oral mycophenolate mofetil (MMF) 0.5 g was administered twice daily. In February 2019, her serum creatinine was further reduced to 74 μmol/L, 24-h urinary protein was reduced to 594 mg, microscopic hematuria disappeared, anti-MPO antibody titer decreased to 13.40 RU/mL, and the perinuclear antineutrophil cytoplasmic antibody (pANCA) test was negative. Based on these findings, oral prednisone and MMF were stopped. The patient was regularly followed every 1 to 2 mo.
In December 2019, the patient’s anti-MPO antibody titer increased to 21.9 RU/mL, and serum creatinine level increased to 118 μmol/L, but no microscopic hematuria or elevations in CRP levels were observed. The patient did not have any symptoms.
In March 2021, the patient complained of arthralgia. Laboratory test results were as follows: Serum creatinine, urinary protein, and CRP levels were 112 μmol/L, 81 mg/24 h, and 3.24 mg/L, respectively, with no microscopic hematuria. Serum anti-MPO antibody levels were > 400 RU/mL, and the patient’s pANCA test was positive. Serum anti-cyclic citrullinated peptide antibody level and hand X-ray scan were normal. High levels of anti-MPO antibody and positive pANCA indicated recurrence of MPO-AAV that presented as arthralgia, without CNS and kidney involvement. The patient was treated with 30 mg oral prednisolone per day and 600 mg of intravenous rituximab that was administered once one week for four times (375 mg/m2). Even after 3 mo, the patient’s arthralgia did not improve. The anti-MPO antibody titer was still > 400 RU/mL, and pANCA was positive. Oral prednisone was gradually tapered, and MMF (0.5 g twice a day) was administered since June 2021. With this treatment, the arthralgia gradually improved. The patient’s anti-MPO antibody titer decreased gradually. Clinical course and trends for anti-MPO antibody titer, serum creatinine level, and 24-h urinary protein level in our patient are shown in Figure 2.
In this paper, we describe a 52-year-old woman with MPO-AAV, who presented with CNS (headache) and kidney involvement at initial onset. After the initial and maintenance immunosuppressive treatment, her headache disappeared and renal function recovered. Twenty-five months after stopping immunosuppressive therapy, arthralgia was observed at relapse without nervous system and renal involvement. The patient received prednisone and MMF, with which her arthralgia completely resolved. The manifestation of MPO-AAV at relapse was completely different from that at onset, which is quite rare.
Headache in AAV patients mainly occurs with meningeal or pituitary involvement and cerebral vascular inflammation[11,12]. In our case, brain MRI and MRA scans and CSF examination showed no abnormality. Further, the patient’s headache improved after immunosuppressive therapy. No other cause of headache could be identified. Therefore, the headache was considered to be related to AAV. A previous study reported a Japanese patient with AAV who also initially presented with headache, but this case was different from ours in that the patient presented with analgesia at all four extremities but had no kidney involvement[13].
The same organ is reported to be involved at initial onset and relapse in patients with AAV[8,10], and MPO-AAV cases with involvement in different organs at initial onset and relapse have been not reported. Our MPO-AAV patient presented with headache and kidney injury at initial onset and arthralgia at relapse, making this a rare case in the literature. Therefore, AAV relapse should be highly suspected when patients with MPO-AAV who have achieved remission present with manifestations in different organ systems.
Combined treatment with prednisone and rituximab in the initial and relapse phases in AAV patients with arthralgias shows good response[14-17]. However, when no improvement is observed within 4 to 6 wk[1], an alternative treatment scheme is needed. Even after 3 mo of prednisone and rituximab therapy, our patient showed a poor response during relapse. Therefore, a combination of prednisone and MMF was administered. After 6 mo, the patient’s arthralgia resolved. Prednisone combined with MMF may be a good choice for patients with recurrent AAV with refractory arthralgia.
After the induction and maintenance therapy, the patient’s serum creatinine reached normal levels, and her serum creatinine levels increased slightly 10 mo after stopping the immunosuppressive treatment. The patient had no microscopic hematuria and proteinuria, suggesting that her renal function deterioration was not associated with AAV recurrence. Repeated renal biopsy and pathological analysis were required. However, the patient refused to undergo these analyses.
In conclusion, we present the rare case of a patient with MPO-AAV who initially presented with headache and kidney involvement and later presented with only arthralgia at relapse. This case suggests that AAV relapse should be highly suspected in MPO-AAV patients after remission, when clinical manifestations at relapse are different from those at onset. Prednisone and MMF may provide a good choice for refractory arthralgia during relapse in MPO-AAV patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Shibata Y, Japan; Ulasoglu C, Turkey S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Zhao S
| 1. | Geetha D, Jefferson JA. ANCA-Associated Vasculitis: Core Curriculum 2020. Am J Kidney Dis. 2020;75:124-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 266] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 2. | Shuai ZW, Lv YF, Zhang MM, Hu ZY. Clinical analysis of patients with myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis. Genet Mol Res. 2015;14:5296-5303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Aljuhani M, Makati D, Hoff A, Thompson J, Pellegrino B, Shawwa K, Schmidt R, Kannabhiran D. Antibody subtypes and titers predict clinical outcomes in ANCA-associated vasculitis. Rheumatol Int. 2021;41:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Zhang W, Zhou G, Shi Q, Zhang X, Zeng XF, Zhang FC. Clinical analysis of nervous system involvement in ANCA-associated systemic vasculitides. Clin Exp Rheumatol. 2009;27:S65-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Ono N, Niiro H, Ueda A, Sawabe T, Nishizaka H, Furugo I, Yoshizawa S, Tsukamoto H, Kiyohara C, Tada Y, Horiuchi T. Characteristics of MPO-ANCA-positive granulomatosis with polyangiitis: a retrospective multi-center study in Japan. Rheumatol Int. 2015;35:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Solans-Laqué R, Fraile G, Rodriguez-Carballeira M, Caminal L, Castillo MJ, Martínez-Valle F, Sáez L, Rios JJ, Solanich X, Oristrell J, Pasquau F, Fonseca E, Zamora M, Callejas JL, Frutos B, Abdilla M, Fanlo P, García-Sánchez I, López-Dupla M, Sopeña B, Pérez-Iglesias A, Bosch JA; Spanish Registry of systemic vasculitis (REVAS) from the Autoimmune Diseases Study Group (GEAS) of the Spanish Society of Internal Medicine (SEMI). Clinical characteristics and outcome of Spanish patients with ANCA-associated vasculitides: Impact of the vasculitis type, ANCA specificity, and treatment on mortality and morbidity. Medicine (Baltimore). 2017;96:e6083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Nguyen Y, Pagnoux C, Karras A, Quéméneur T, Maurier F, Hamidou M, Le Quellec A, Chiche NJ, Cohen P, Régent A, Lifermann F, Mékinian A, Khouatra C, Hachulla E, Pourrat J, Ruivard M, Godmer P, Viallard JF, Terrier B, Mouthon L, Guillevin L, Puéchal X; French Vasculitis Study Group. Microscopic polyangiitis: Clinical characteristics and long-term outcomes of 378 patients from the French Vasculitis Study Group Registry. J Autoimmun. 2020;112:102467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Outh R, Lemaire A, Mania A, Berland P, Gerbaud L, Aumaître O, André M. Relapses in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis: a retrospective study. Clin Rheumatol. 2020;39:1601-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Guillevin L, Durand-Gasselin B, Cevallos R, Gayraud M, Lhote F, Callard P, Amouroux J, Casassus P, Jarrousse B. Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum. 1999;42:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Chen M, Yu F, Zhao MH. Relapses in patients with antineutrophil cytoplasmic autoantibody-associated vasculitis: likely to begin with the same organ as initial onset. J Rheumatol. 2008;35:448-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Graf J. Central Nervous System Disease in Antineutrophil Cytoplasmic Antibodies-Associated Vasculitis. Rheum Dis Clin North Am. 2017;43:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Zheng Y, Zhang Y, Cai M, Lai N, Chen Z, Ding M. Central Nervous System Involvement in ANCA-Associated Vasculitis: What Neurologists Need to Know. Front Neurol. 2018;9:1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 13. | Ito Y, Suzuki K, Yamazaki T, Yoshizawa T, Ohkoshi N, Matsumura A. ANCA-associated vasculitis (AAV) causing bilateral cerebral infarction and subsequent intracerebral hemorrhage without renal and respiratory dysfunction. J Neurol Sci. 2006;240:99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Bottomley MJ, Gibson M, Alchi B. PR3 vasculitis presenting with symptomatic splenic and renal infarction: a case report and literature review. BMC Nephrol. 2019;20:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Wong B, Tan E, McLean-Tooke A. Pulmonary granulomas in a patient with positive ANCA and history of tuberculosis: case report. BMC Pulm Med. 2020;20:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 16. | Roccatello D, Baldovino S, Alpa M, Rossi D, Napoli F, Naretto C, Cavallo R, Giachino O. Effects of anti-CD20 monoclonal antibody as a rescue treatment for ANCA-associated idiopathic systemic vasculitis with or without overt renal involvement. Clin Exp Rheumatol. 2008;26:S67-S71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Evans R, Gueret-Wardle A, Edwards S, Salama A. ANCA-associated vasculitis and pauci-immune glomerulonephritis in HIV disease. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |