Published online Jul 26, 2023. doi: 10.12998/wjcc.v11.i21.5136
Peer-review started: April 18, 2023
First decision: May 19, 2023
Revised: June 14, 2023
Accepted: July 6, 2023
Article in press: July 6, 2023
Published online: July 26, 2023
Processing time: 92 Days and 17.7 Hours
Fibroblastic rheumatism (FR) is a rare fibroproliferative disease with an unknown etiology. The absence of typical symptoms makes early diagnosis challenging. This study aims to systematically review FR cases and present a case from our center to provide a comprehensive description of the clinical manifestations, diag
FR is a rare fibroproliferative disease with an unknown etiology. It is characterized by rapidly progressive and destructive symmetrical inflammatory multi
A comprehensive review of the available literature emphasizes the importance of early and accurate diagnosis coupled with appropriate treatment for achieving favorable outcomes and preventing joint destruction and limb contractures.
Core Tip: Fibroblastic rheumatism is a rare fibroproliferative disease with unknown etiology characterized by onset of rapidly progressive and destructive symmetrical inflammatory multiple arthritis. We report a rare case of a 50-year-old female presented with symmetric inflammatory polyarthriti. We found that a complete medical history, histopathology, and immunohistochemistry combined with clinical manifestations of skin nodules, arthralgia, and arthritis are the keys to a successful diagnosis of this disease. In terms of treatment, our patient was similarly treated with non-steroidal anti-inflammatory drugs, corticosteroids, methotrexate, and tacrolimus, but the symptoms did not resolve and the joint destruction continued to develop. All in all, early diagnosis and aggressive and correct use of steroids and immunosuppressants are essential to prevent potentially harmful joint destruction and limb contracture. Meanwhile, further research is needed to determine effective treatment strategies.
- Citation: Guo H, Liang Q, Dong C, Zhang Q, Gu ZF. Systematic review of fibroblastic rheumatism: A case report. World J Clin Cases 2023; 11(21): 5136-5146
- URL: https://www.wjgnet.com/2307-8960/full/v11/i21/5136.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i21.5136
Fibroblastic rheumatism (FR) is a rare fibroproliferative disease with an unknown etiology. It was first reported by Chaouat et al[1] in 1980. FR is characterized by rapidly progressive and destructive symmetrical inflammatory multiple arthritis. The key features of FR include the following: Firstly, clinical manifestations typically involve the presence of multiple flesh-colored to pink nodules on the fingers, along with multiple arthritis and finger flexion contracture[2]. Histopathological evaluation of cutaneous nodules reveals diffuse dermal fibrosis with prominent spindle cells, proliferation in the dermis, and a decrease in elastic fibers[3]. Immunohistochemistry demonstrates positive staining for α-smooth muscle actin (α-SMA) in spindle cells, indicating myoblast fibroblast proliferation[4]. The most common pre
A 50-year-old female, previously treated with steroid therapy, presented with symmetric inflammatory polyarthritis persisting for 17 years. The patient initially developed cutaneous nodules in 2003, which were distributed symmetrically across the joints of the hands, ankles, shoulders, and elbows. Over the following 6 mo, stiffness, joint deformities, contractures, and restricted mobility affected multiple joints, including bilateral elbows, wrists, knees, ankles, feet, MCP joints, bilateral PIP joints, and MTP and DIP joints.
The patient had a positive medical history of fever, decreased appetite, cough, breathlessness.
A previous subdiaphragmatic abscess resection and ovarian cyst removal.
No special notes.
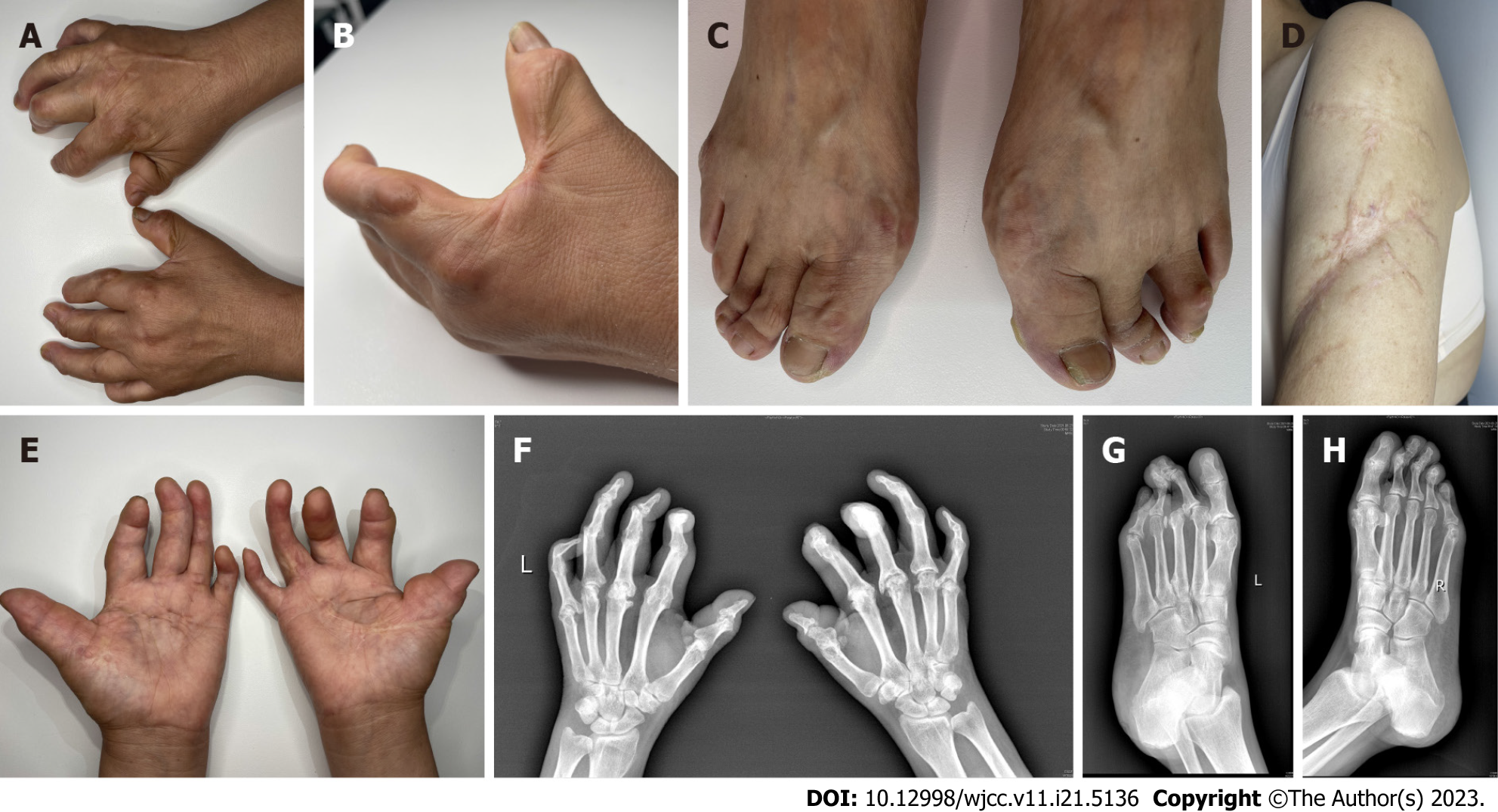
Physical examination revealed multiple smooth nodules, ranging in diameter from approximately 5-20 mm, on the dorsal surfaces of the patient's palms, upper arms, MCP joints, MTP joints, and PIP and DIP joints. These nodules exhibited symmetric distribution, firm texture, free mobility, and were non-tender to touch (Figure 1A-C). Furthermore, a large scar connective tissue was observed along the lateral margin of both upper arms (Figure 1D). Joint flexion contractures were prominent, particularly affecting the thumb, ring, and little fingers, resulting in a +ACI-main en griffe+ACI- (claw hand) appearance (Figure 1E). No signs of Raynaud phenomenon, calcinosis, or palmoplantar thickening were noted.
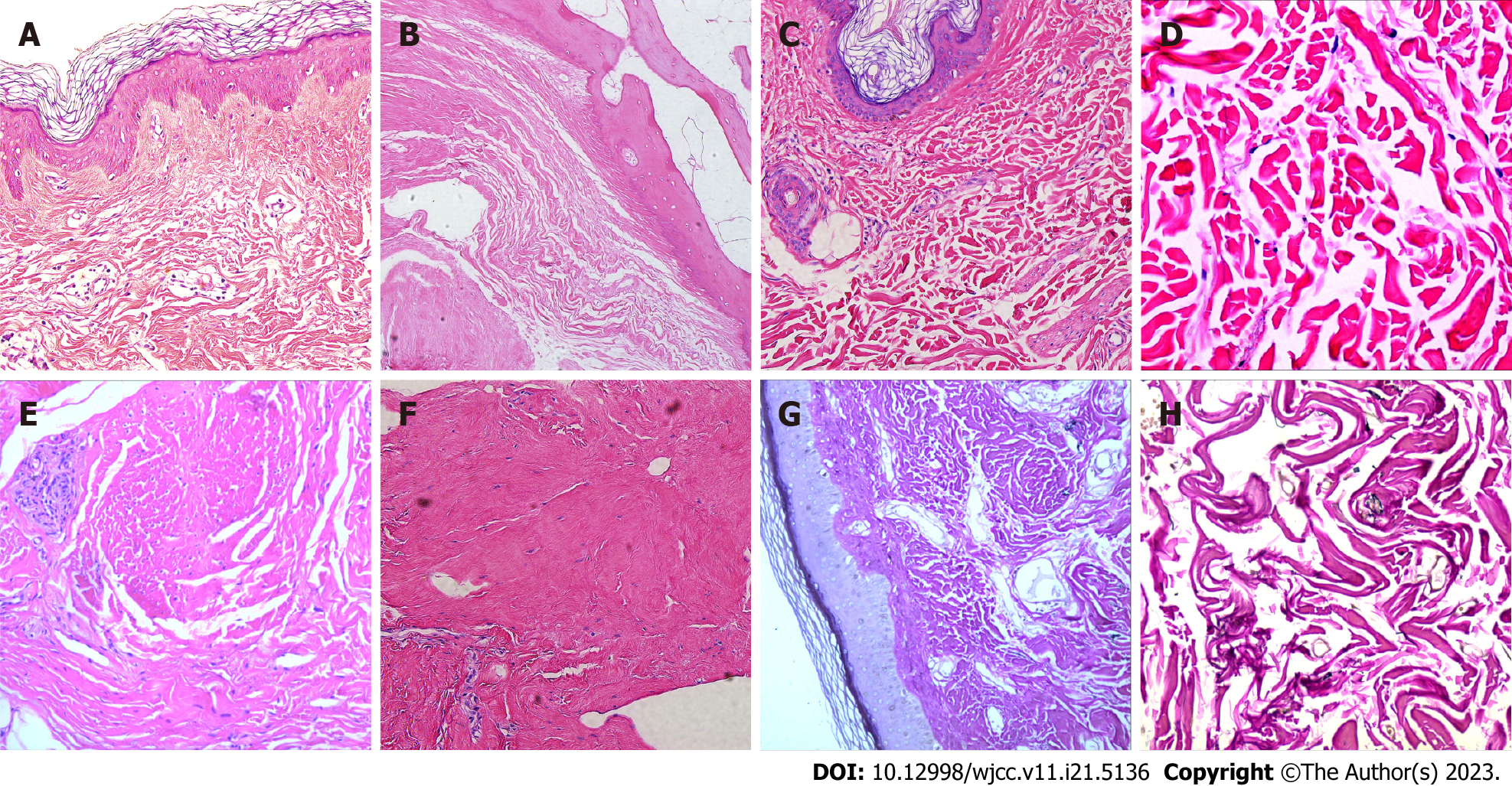
Biopsy samples were obtained for pathological examination from right arch palmar aponeurosis, left foot articular cartilage and right upper arm scar tissue. The results showed dermal fibrous hyperplasia, elastic fiber disorder, excessive skin coking, irregular thickening of the spinous layer, dermal collagen fiber hyperplasia, synovial fibrous tissue hyperplasia, thickened collagen fibers, and central degeneration and necrosis of cartilage tissue with calcification (Figure 2A-F). Elastica van Gieson staining showed the reduction of elastic fibers in the dermis (Figure 2G and H). In immunohistochemical examination, the spindle cells were positive for vimentin, and α-SMA, but CD68, CD163, CD34, and S100 were negative (Figure 3A-F).
Laboratory tests indicated positive results for both rheumatoid factor (RF) and carcinoembryonic antigen (CEA), while all other routine and immunological tests, including anti-DNA antibodies, antineutrophil cytoplasmic antibodies, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), antinuclear antibodies (ANAs), anti-extractable nuclear antigen scl-70, anti-cyclic citrullinated peptide antibodies, anti-Jo-1, anti-SSA, and anti-SSB antibodies, were negative or within normal ranges.
Fibroblastic Rheumatism is a rare disease first described by Chaouat et al[1] and is characterized by a combination of rheumatologic and dermatological manifestations. Rheumatologic features are symmetrical polyarthralgias with joint stiffness, associated with cutaneous nodules and sclerodactyly[11]. Histology shows an increased number of fibroblasts and a marked dermal fibrosis.
Upon diagnosing the patient with FR, we initially recommended surgical intervention to correct joint deformities and restore finger movement. Subsequently, we initiated immunosuppressive therapy, starting with oral methylprednisolone (32 mg/d) for a duration of 3 mo. This was followed by a course of MTX (20 mg/wk) for 8 mo until improvement of the skin lesions.
As of the 12-mo follow-up period, there has been no further progression of the FR condition.
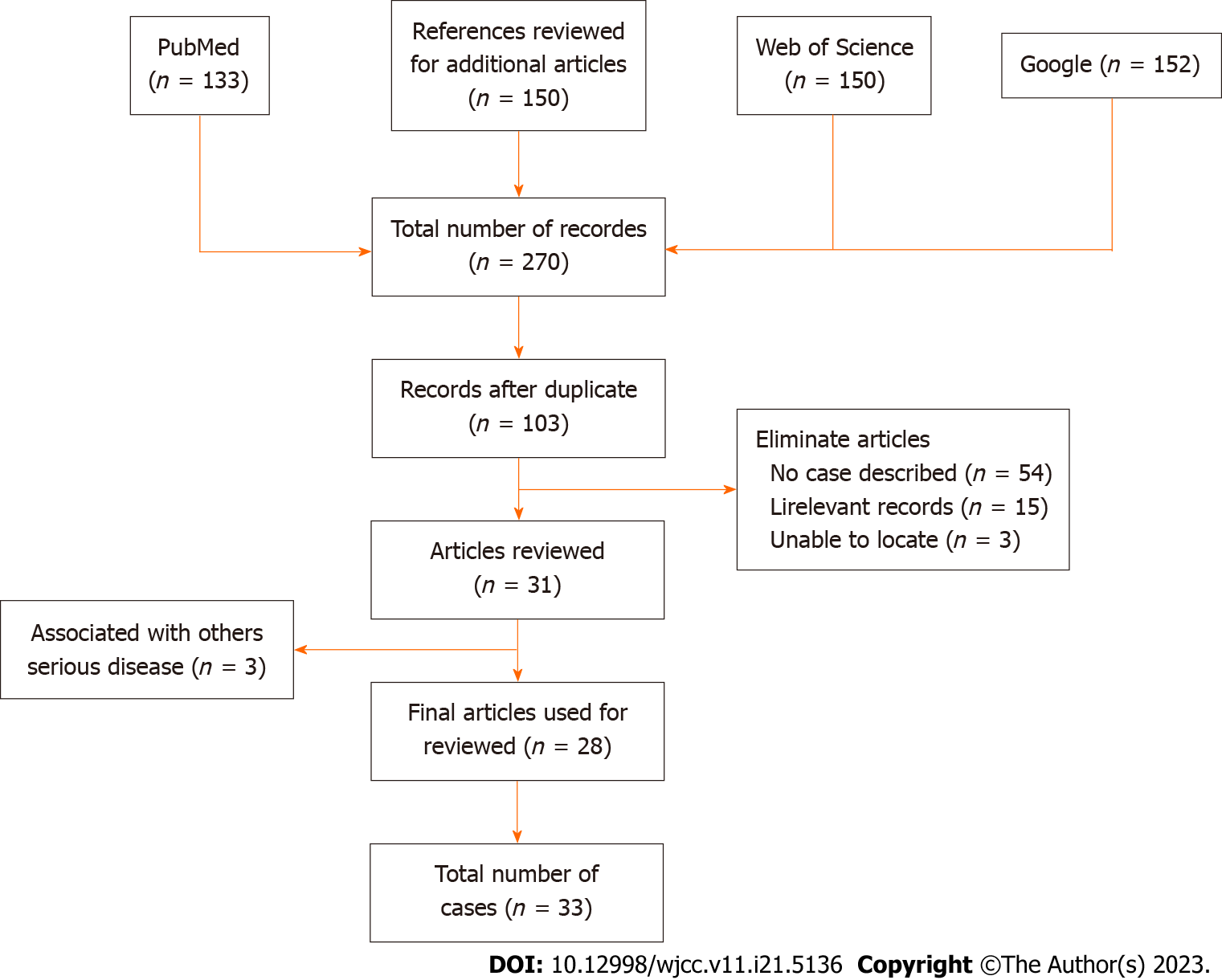
Using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) model[12], we conducted a systematic review aiming to provide a comprehensive analysis of recent publications on FR. Our search was not limited by language or region, and we utilized public data to retrieve case reports and series reports on FR. The search terms included "arthralgia, arthritis, arthropathy, cutaneous, disease, fibroblast, fibroblastic, rheumatism, rheumatology, rheumatic, and case report". The articles included in our review were sourced from PubMed, Google, and Web of Science and were published between 1980 and 2021.
During the article screening process, we adhered strictly to predefined eligibility criteria. Two authors independently evaluated the articles for inclusion, and any discrepancies were resolved by a third party. Articles that were duplicates, descriptive cases, lacked initial diagnostic test data, or focused on reviews, commentaries, or editorials were excluded. Ultimately, all included articles received unanimous agreement from the reviewers.
For each included article, we extracted the following information: Author, year of publication, geographic distribution or country of cases, imaging results, histologic features, immunohistochemical findings, cutaneous manifestations, rheumatologic manifestations, other symptoms/diseases (such as upper respiratory tract infection, autoimmune disease, and trauma), and strategies of systemic treatment. We employed Microsoft Excel to calculate the simple frequency, percentage, and ratio of case reports.
Our search yielded a total of 263 articles from PubMed and Web of Science. Additionally, we identified five more articles from the references of the initially searched articles. After excluding articles that did not meet the eligibility criteria, we identified 28 relevant articles for our research. The PRISMA guide was followed to present the search strategy and flow chart (Figure 4). Supplementary Table 1 provides a summary of the 28 papers, including laboratory examination results, immunohistochemistry findings, histopathology, imaging data, clinical symptoms (such as kin nodules and arthritis symptoms), complications, and treatment strategies. These papers, published between 1980 and 2020, reported on 33 patients with FR.
Table 1 presents detailed demographic information and FR characteristics of the patients .Of the total cases, 66.67% (22/33) were males, 33.33% (11/33) were females, and the median age was 40 years (ranging from 6 to 61 years). Notably, individuals aged 0-19 years (n = 12) and 40-59 years (n = 13) accounted for 75.76% of the total patient population. The most prevalent clinical symptoms observed were cutaneous periarticular nodules on the hands (n = 28; 84.85%), polyarthritis affecting distal joints (n = 19; 57.58%), arthralgias (n = 19; 57.58%), sclerodactyly (n = 19; 57.58%), and cutaneous periarticular nodules on the feet (n = 13; 39.39%).
| Variables | Types | No. (n = 33) | % |
| Gender | Man | 22 | 66.67 |
| Female | 11 | 33.33 | |
| Age | 0-19 | 12 | 36.36 |
| 20-39 | 3 | 9.09 | |
| 40-59 | 13 | 39.39 | |
| ≥ 60 | 5 | 15.16 | |
| Presenting symptoms | Cutaneous periarticular nodules on the hands | 28 | 84.85 |
| Cutaneous periarticular nodules on the feet | 13 | 39.39 | |
| Cutaneous periarticular nodules on others parts | 9 | 27.27 | |
| Sclerodactyly | 19 | 57.58 | |
| Raynaud’s phenomenon | 9 | 27.27 | |
| Redness and swelling | 13 | 39.39 | |
| Polyarthritis (classically affecting distal joints) | 19 | 57.58 | |
| Arthralgias | 19 | 57.58 | |
| Tendonitis | 10 | 30.3 | |
| Thickened palmar/plantar fascia | 10 | 30.3 | |
| Joint contractures | 12 | 36.36 | |
| Diagnostic imaging tests | Acroosteolysis | 16 | 48.48 |
| Erosive arthropathy | 13 | 39.39 | |
| Osteoproliferation | 3 | 9.09 | |
| Joint space stenosis | 12 | 36.36 | |
| Histologic features | Absence or decrease of elastic fibers | 9 | 27.27 |
| Increased proliferation of dermal fibroblasts | 24 | 72.72 |
Sixteen patients had available imaging data in the systematic review. X-ray was the most commonly employed imaging modality, accounting for 48.48% (16/33) of all cases. X-ray findings primarily included acroosteolysis in 48.48% (16/33) of cases, erosive arthropathy in 39.39% (13/33) of cases, and joint space stenosis in 36.36% (12/33) of cases. The use of MRI was relatively low, with only 15.15% (5/33) of cases reporting MRI findings.
Histological changes were reported in 33 cases (Supplementary Table 1). Among them, 51.52% (17/33) exhibited full thickness dermal involvement with increased collagen fibers, some of which were arranged in a whirlpool pattern. Furthermore, 75.76% (25/33) of cases with reported histological changes demonstrated local spindle cell proliferation, and 45.46% (15/33) of cases exhibited significant reduction of elastic fibers in the dermis. Immunohistochemical staining (Table 2) revealed elevated levels of α-SMA in 87.5% (25/28) of cases and 100% (18/18) of cases tested positive for Vimentin. Five cases showed positive results for CD34 staining, while four cases reported positive results for S100. Notably, EMA and CD68 staining yielded mainly negative results.
| Immunohistochemical markers | Reactivity | Number of patients with available data (n/N, %) |
| CD34 | + | 5/20 (62.5) |
| - | 15/20 (62.5) | |
| S100 | + | 4/23 (71.88) |
| - | 19/23 (71.88) | |
| EMA | + | 1/10 (31.25) |
| - | 9/10 (31.25) | |
| α-SMA | + | 25/28 (87.5) |
| - | 3/28 (87.5) | |
| Vimentin | + | 18/18 (56.25) |
| - | 0/18 (56.25) | |
| CD68 | + | 2/12 (37.5) |
| - | 10/12 (37.5) |
Current treatment approaches for FR encompass non-steroidal anti-inflammatory drugs, steroid anti-inflammatory drugs, immunosuppression, physical therapy, and surgical intervention. However, surgical treatment is primarily focused on relieving stiffness and restoring joint movement. In our analysis, patients received various treatment options, including methylprednisolone (20 cases), methotrexate (14 cases), interferon (3 cases), physical therapy (2 cases), and surgical treatment (1 case). Three patients did not have specific treatment details provided. Of the 33 patients included in our systematic analysis, 20 received steroid treatment, 12 were treated with a combination of steroids and immunosuppressants, and only two patients underwent physical therapy alone.
FR is a rare fibroproliferative disease that can affect individuals of all ages. The cases analyzed in this study (Table 1) demonstrated an onset age range of range of 7 to 64 years, with a higher frequency observed in individuals aged 0-19 years and 40-59 years. The average age of onset, 32.47 years, aligns with previous studies[13,14]. Interestingly, a higher proportion of males (66.67%) was observed in our cases, which contrasts with recent data[5]. This suggests a potential gender difference in the incidence of FR, although the underlying reason for this trend remains unclear. The etiology of FR is not well understood, although recent literature suggests a proliferative response of fibroblasts and myofibroblasts to unidentified stimuli[15]. Clinically, FR often manifests with a sudden onset, and some patients may have a history of upper respiratory tract infection prior to the onset[5,16]. Initially, patients experience symmetrical polyarthritis, morning stiffness, joint swelling, and pain. The disease progresses rapidly, leading to flexion contractures in bilateral PIP joints, which significantly impacts daily life and may require surgical intervention. Polyarthritis predominantly affects distal small joints such as fingers and toes, although it can also involve larger joints like the knees, hips, shoulders, and elbows. Cutaneous nodules are commonly found around the joints of the hands, appearing as smooth, firm, skin-colored, or red nodules with diameters ranging from 2 to 20 mm. Nodules may also occur on the fingers, as well as on the extension sides of the elbow and knee joints, nose, ears, neck, and back. Raynaud's phenomenon is often associated with FR, but internal organ involvement is rare, while a small number of patients may develop malignant tumors[17,18]. Our patients presented with arthritis, joint pain, destructive joint changes, and multiple joint erosions, particularly affecting small joints such as fingers and toes, which is consistent with previously reported cases. Notably, even in the advanced stage of FR, joint space preservation is typically observed, distinguishing it from rheumatoid arthritis. The exact mechanism of joint destruction in FR remains unclear, but these characteristic joint symptoms and the presence of skin nodules are key factors in FR diagnosis.
Laboratory examinations, including blood tests, liver and renal function tests, ESR, and electrolyte levels, were generally within the normal range in both our case report and previous cases[19]. Additionally, as shown in Table 3, tests for RF, anti-cyclic citrullinated peptide antibody, ANAs, anti-neutrophil cytoplasmic antibodies, anti-SmD1, anti-U1-snRNP, anti-SSA, anti-SSB, anti-Scl-70, anti-Jo-1, C3, CRP, and anti-DNA were negative[20]. Similarly, our case report demonstrated normal laboratory test results throughout the disease progression, without any significant changes as the disease worsened. Therefore, assessing disease activity based on laboratory data may be challenging.
| Laboratory data | Expressed levels | Number of patients with available data (n/N, %) |
| ANAs | + | 3/31 (96.88) |
| - | 28/31 (96.88) | |
| ds-DNA | + | 0/18 (56.25) |
| - | 18/18 (56.25) | |
| ANCA | + | 3/23 (71.88) |
| - | 20/23 (71.88) | |
| Anti-SSB | + | 1/15 (46.88) |
| - | 14/15 (46.88) | |
| Anti-Scl-70 | + | 0/17 (53.13) |
| - | 17/17 (53.13) | |
| Anti-Jo-1 | + | 0/21 (65.63) |
| - | 21/21 (65.63) | |
| C3 | + | 0/13 (40.63) |
| - | 13/13 (40.63) | |
| RF | + | 0/33 (100) |
| - | 33/33 (100) | |
| CRP | + | 4/25 (78.13) |
| - | 21/25 (78.13) |
Early diagnosis and treatment are crucial in FR to prevent progressive joint involvement and the development of permanent joint deformities and disabilities[21]. Given that several diseases can present with similar fibrosis, accurate identification of other conditions associated with skin nodules and rheumatic symptoms, such as rheumatoid arthritis, multicentric reticulohistiocytosis (MCRH), and nodular scleroderma, is essential. Experimental laboratory and histopathological examinations can be performed to rule out these differential diagnoses. Table 4 summarizes the differences between FR and other conditions like psoriatic arthritis, MCRH, rheumatoid arthritis, and nodular scleroderma. Additionally, immunostaining for CD34, CD68, EMA, α-SMA, S100, and vimentin can aid in narrowing down the differential diagnosis (Table 2).
| RA | PsA | MCRH | NS | FR | |
| Clinical features | |||||
| Skin | |||||
| Sclerodactyly | +/- | - | - | + | + |
| Subcutaneous nodules | + | - | + | - | + |
| Raynaud phenomenon | + | - | - | - | + |
| Musculoskeletal | |||||
| Favorite sites | |||||
| DIP | - | + | + | + | + |
| PIP | + | + | + | + | + |
| MCP | + | +/- | + | +/- | + |
| Histopathologic features | |||||
| Proliferation of fibroblasts | - | - | - | + | + |
| Proliferation of collagen fibers | - | - | - | + | + |
| Multinucleated giant cells | - | + | + | + | +/- |
| Synovitis | + | + | + | +/- | + |
| Loss of elastin | - | - | + | - | + |
| Immunohistochemistry | |||||
| CD34 | +/- | + | +/- | + | +/- |
| SMA | - | +/- | +/- | +/- | + |
| Vimentin | - | - | - | - | + |
| C3 | + | + | +/- | +/- | - |
| Other features | |||||
| Elevation of CRP and/or ESR | + | + | +/- | + | - |
| Rheumatoid factor | + | - | - | +/- | - |
| Redness and swelling | + | +/- | - | +/- | + |
In our case report, the clinical features primarily included multiple skin-colored nodules and polyarthritis with finger flexion contractures. Histopathological examination revealed spindle cell proliferation in the dermis, increased collagen fibers and fibrosis, and a significant decrease in elastic fibers. Immunohistochemical analysis demonstrated positive staining for α-SMA and vimentin in proliferating spindle cells, confirming the diagnosis of FR. Similarly, among the 33 cases reviewed in our systematic analysis, 56% of FR patients were diagnosed based on clinical manifestations, histopathological changes, and positive immunohistochemical results. While imaging was performed in all 23 cases, the findings were nonspecific. X-ray results indicated mostly negative findings in the early stages, with signs of joint damage becoming apparent only in the advanced stages. MRI has been extensively studied for other inflammatory arthropathies, particularly rheumatoid arthritis, exhibiting higher sensitivity for early erosions compared to standard radiography. Moreover, detailed assessment of soft tissue changes, including synovitis, has shown prognostic value, and routine imaging tests can be used to monitor disease activity and progression. However, these imaging findings are not specific to FR and are shared with other inflammatory arthropathies, such as rheumatoid arthritis. Therefore, imaging examinations serve as auxiliary diagnostic tools and aids in assessing disease progression.
Various treatment options have been attempted in FR, including aspirin, non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, colchicine, hydroxychloroquine, MTX, alpha-interferon, and physical therapy, with varying degrees of success[5,22]. However, satisfactory treatments for FR are currently lacking. Some reports suggest that corticosteroid and/or methotrexate treatment can significantly improve symptoms[11,23]. Although there are no prospective studies or controlled trials, moderate to high doses of systemic steroids have been shown to be beneficial in inhibiting fibrogenic cytokine production and subsequent fibroblast activation during the early active phase of FR, thereby improving arthritis symptoms[6,24]. However, complete treatment resolution is rare in FR. In our case, our patient received NSAIDs, corticosteroids, methotrexate, and tacrolimus, but symptoms persisted, and joint destruction continued to progress.
Subsequently, in our systematic review of 32 cases, four cases showed good response to MTX, with one case achieving complete remission, suggesting a potential dose-dependent effect. Additionally, 62.5% of patients initially received NSAIDs and steroid drugs (methylprednisolone) to reduce abnormal fibroblast proliferation and alleviate symptoms. However, most patients experienced disease progression and subsequently received immunosuppressants such as MTX, tacrolimus, and interferon. While the majority of patients achieved complete resolution of skin nodules and arthritis after a few years, permanent joint damage remained. Aggressive treatment with moderate to high doses of glucocorticoids in the early stages of the disease has been reported to effectively improve arthritis symptom and reduce the incidence of permanent joint damage by inhibiting cytokine-induced fibrogenesis and preventing fibroblast activation[5,11]. Furthermore, the development of biologics has opened up new therapeutic possibilities. Animal experiments have shown that interferon-gamma (IFN-γ) can inhibit IL-1β-induced matrix metalloproteinase production in synovial fibroblasts and protect articular cartilage in early arthritis[5,19]. Antagonists of transforming growth factor-beta inhibit fibroblast-to-myofibroblast transformation, reducing extracellular matrix deposition and scarring[15].
In summary, FR is a rare disorder affecting the skin and joints, with limited reported cases. Although the presence of skin nodules on the extremities, ears, or neck in patients with a history of rheumatism is a characteristic feature, it is not diagnostically specific. However, histological and immunohistochemical findings, such as fibroblast hyperplasia, collagen fiber thickening, dermal fibrosis, and reduced elastic fibers, strongly support the definitive diagnosis of FR. Therefore, a comprehensive evaluation, including detailed medical history, histopathology, immunohistochemistry, and consideration of clinical manifestations such as skin nodules, arthralgia, and arthritis, is crucial for successful disease diagnosis. Although current pharmacological treatments for FR remain unsatisfactory, early diagnosis and the aggressive and appropriate use of steroids and immunosuppressants are vital to prevent progressive joint destruction and limb contractures. Furthermore, further research is needed to identify effective treatment strategies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Rezus E, Romania; Rothschild B, United States; Salimi M, Iran; Sultana N, Bangladesh S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Chaouat Y, Binet O, Faures-Quenet B, Aubart D, Crouzet J, Amouroux J, Hamalgrand N, Todesco S, Schiavon F, Punzi L. Fibroblastic rheumatism. A clinical and histological entity. Rev Rhum Mal Osteoartic. 1988;55:59-62. [PubMed] |
| 2. | Jurado SA, Alvin GK, Selim MA, Pipkin CA, Kress D, Jamora MJ, Billings SD. Fibroblastic rheumatism: a report of 4 cases with potential therapeutic implications. J Am Acad Dermatol. 2012;66:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Marconi IM, Rivitti-Machado MC, Sotto MN, Nico MM. Fibroblastic rheumatism. Clin Exp Dermatol. 2009;34:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Tan J, Bardi M, Lacaille D. A Case of Fibroblastic Rheumatism. Journal of Rheumatology. 2020;47:1137-1140. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Cai SCS, Tee SI, Lee JSS, Tan LS. Fibroblastic Rheumatism Versus Variant Disease of Multinucleate Cell Angiohistiocytoma. Am J Dermatopathol. 2020;42:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Motegi SI, Yamazaki S, Fujiwara C, Sekiguchi A, Ishikawa O. Fibroblastic rheumatism: A case of multiple nodules of fingers and hands, contractures of fingers and polyarthritis. J Dermatol. 2018;45:e142-e143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Bandino JP, Elston DM. Volar Papulonodules in a Patient With Seronegative Rheumatoid Arthritis: Challenge. Am J Dermatopathol. 2018;40:e95. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Zou XW, Huo J, Wang JM, Yuan JY, Ma YY, Wang QY. Fibroblastic rheumatism: the first case with infiltration of multinuclear giant cells and raised blood lead level. Clin Exp Dermatol. 2015;40:309-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Cabral R, Brinca A, Cardoso JC, Reis JP, Tellechea O, Figueiredo A. Fibroblastic rheumatism - case report. Acta Reumatologica Portuguesa. 38:128-130. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Parida JR, Agarwal V, Krishnani N. An unusual case of polyarthritis, skin nodules and patchy skin thickening: fibroblastic rheumatism. Int J Rheum Dis. 2012;15:e12-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Suri R, Azzam MJ, Heaphy MR Jr. Firm Cutaneous Nodule on the Dorsal Hand: A Case Report on Fibroblastic Rheumatism. Cureus. 2020;12:e10312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Marconi GD, Fonticoli L, Rajan TS, Lanuti P, Della Rocca Y, Pierdomenico SD, Trubiani O, Pizzicannella J, Diomede F. Transforming Growth Factor-Beta1 and Human Gingival Fibroblast-to-Myofibroblast Differentiation: Molecular and Morphological Modifications. Front Physiol. 2021;12:676512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Hemmati I, Wade J, Kelsall J. Risedronate-associated scleritis: a case report and review of the literature. Clin Rheumatol. 2012;31:1403-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Courties A, Guégan S, Miquel A, Duriez P, Berenbaum F, Sellam J. Fibroblastic rheumatism: immunosuppressive therapy is not always required. Joint Bone Spine. 2014;81:178-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Watanabe S, Kamada K, Imai H, Takeda H, Nose M, Murata N, Hasegawa H, Yamamoto H. An Asian case of fibroblastic rheumatism: clinical, radiological, and histological features. Mod Rheumatol. 2010;20:423-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Lacour JP, Maquart FX, Bellon G, Gillery P, Lepeytre P, Ziegler G, Ortonne JP. Fibroblastic rheumatism: clinical, histological, immunohistological, ultrastructural and biochemical study of a case. Br J Dermatol. 1993;128:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Romas E, Finlay M, Woodruff T. The arthropathy of fibroblastic rheumatism. Arthritis Rheum. 1997;40:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Romiti R, Levy Neto M, Menta Simonsen Nico M. Response of fibroblastic rheumatism to infliximab. Dermatol Res Pract. 2009;2009:715729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Kluger N, Dumas-Tesici A, Hamel D, Brousse N, Fraitag S. Fibroblastic rheumatism: fibromatosis rather than non-Langerhans cell histiocytosis. J Cutan Pathol. 2010;37:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Trotta F, Colina M. Multicentric reticulohistiocytosis and fibroblastic rheumatism. Best Pract Res Clin Rheumatol. 2012;26:543-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Ji L, Geng Y, Hao Y, Zhang Z. Fibroblastic rheumatism: an addition to fibromatosis. Joint Bone Spine. 2011;78:519-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Réty F, Tayebjee OA, Servettaz A, Godmer P, Le Van An JC, Laredo JD, Martin A, Guillevin L, Mouthon L. Radiological bone lesions in fibroblastic rheumatism; case report. Presse Med. 2007;36:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Toit R, Schneider JW, Whitelaw DA. Fibroblastic rheumatism. Jcr-Journal of Clinical Rheumatology. 2006;12:201-203. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Pedersen JK, Poulsen T, Hørslev-Petersen K. Fibroblastic rheumatism: a Scandinavian case report. Ann Rheum Dis. 2005;64:156-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |