Published online Jul 26, 2023. doi: 10.12998/wjcc.v11.i21.5122
Peer-review started: April 8, 2023
First decision: May 31, 2023
Revised: June 5, 2023
Accepted: June 27, 2023
Article in press: June 27, 2023
Published online: July 26, 2023
Processing time: 109 Days and 12 Hours
Angiosarcoma (AS) is a rare and highly aggressive soft tissue disease that most commonly arises in deep soft tissues. There are only a few reported cases of AS involving the ovary and even fewer reports of the underlying molecular abnor
Case 1: A 51-year-old female patient was admitted with right lower limb pain for 5 mo, and lower abdominal pain with hematuria for 1 mo. Partial removal of rectus abdominis muscle and fascia, partial hysterectomy, bilateral salpingo-oophorectomy, and inguinal and pelvic lymphadenectomy were performed. Pathology revealed primary oAS. Fluorescence in situ hybridization revealed c-MYC gene amplification. MESNA + ADM + IFO + DTIC (MAID) regimen was administered, but stable disease was achieved. The patient died 1 mo later. Case 2: A 41-year-old female patient presented with fatigue, nausea, decreased appetite, and diffuse abdominal pain. On physical examination, the abdomen was distended and a complex cystic mass was palpable in the right pelvic cavity. Pathology revealed primary oAS. MAID chemotherapy was administered and programmed death ligand 1 (PD-L1) staining was performed on the tumor samples. The patient benefited from anti-PD-1 immunotherapy and is alive without any evidence of disease 27 mo off therapy in follow-up.
Long-term survival benefit for primary oAS can be achieved by alternative thera
Core Tip: Angiosarcoma (AS) is a rare and highly aggressive type of soft tissue that most commonly arises in deep soft tissues. Alternative therapeutic strategies such as targeted therapies and anti-programmed cell death 1/programmed cell death ligand 1 therapy may be promising for primary ovarian AS.
- Citation: Zhou Y, Sun YW, Liu XY, Shen DH. Primary ovarian angiosarcoma: Two case reports and review of literature. World J Clin Cases 2023; 11(21): 5122-5128
- URL: https://www.wjgnet.com/2307-8960/full/v11/i21/5122.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i21.5122
Angiosarcoma (AS) is a rare and highly aggressive soft tissue disease that most commonly arises in deep soft tissues[1], with only 1% of cases occurring in the ovarian system[2]. AS is generally associated with exposure to chemical substances, radiation, chronic lymphedema, and trauma.
Primary ovarian AS (oAS) is a highly aggressive neoplasm with rapid disease progression and poor prognosis[3]. Adjacent structures, including the small intestine, cecum, and uterus, are frequently involved. Metastases include those in the lungs, liver, lymph nodes, adrenal glands, bones, and brain[4]. Consequently, a better understanding of the oAS characteristics is urgently required.
Here, we present two cases of primary AS of the ovary: One with amplification of the c-MYC locus 8q21.24 and the other with positive programmed cell death protein 1 (PD-1, 22C3) immunohistochemical analysis. The clinical features, diagnosis, differential diagnosis, new treatment approaches, and prognosis of the disease are discussed based on a literature review.
Case 1: A 51-year-old female patient was admitted to our hospital on February 25, 2020, because of aggravated abdominal pain.
Case 2: A 41-year-old female patient presented with fatigue, nausea, decreased appetite, and diffuse abdominal pain.
Case 1: The patient had right lower limb pain for five months and lower abdominal pain with hematuria for one month.
Case 2: Since June 2018, the patient experienced intermittent lower abdominal distension and low back pain.
Cases 1 and 2: The patients had no history of illness. They had no medical or radiation history.
Cases 1 and 2: The patient denied a family history of malignant tumors.
Case 1: Gynecological examination revealed an enlarged uterus and a hard, palpable mass in the right adnexal area.
Case 2: The abdomen was distended, and a complex cystic mass (major diameter: 10 cm) was palpable in the right pelvic cavity with poor mobility.
Cases 1 and 2: Only serum carbohydrate antigen 125 (CA-125) tumor marker was elevated, 71.5 kU/L and 119.3 kU/L, respectively. Other markers were normal.
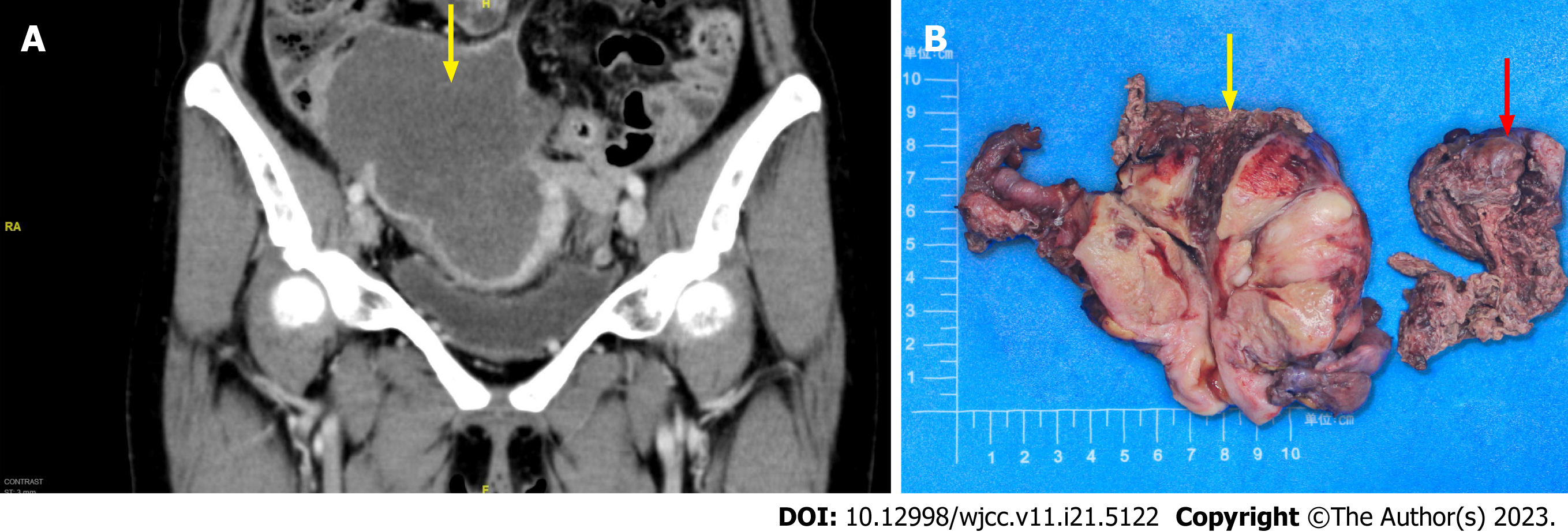
Case 1: A subsequent computed tomography scan of the abdomen and pelvis revealed a large solid mass (12.8 cm × 11.6 cm × 7.8 cm) in the right adnexal region, which invaded the right lower segment of the ureter, leading to obstruction and dilatation of the middle and upper segments of the ureter, and invaded the adjacent small intestine and cecum (Figure 1A).
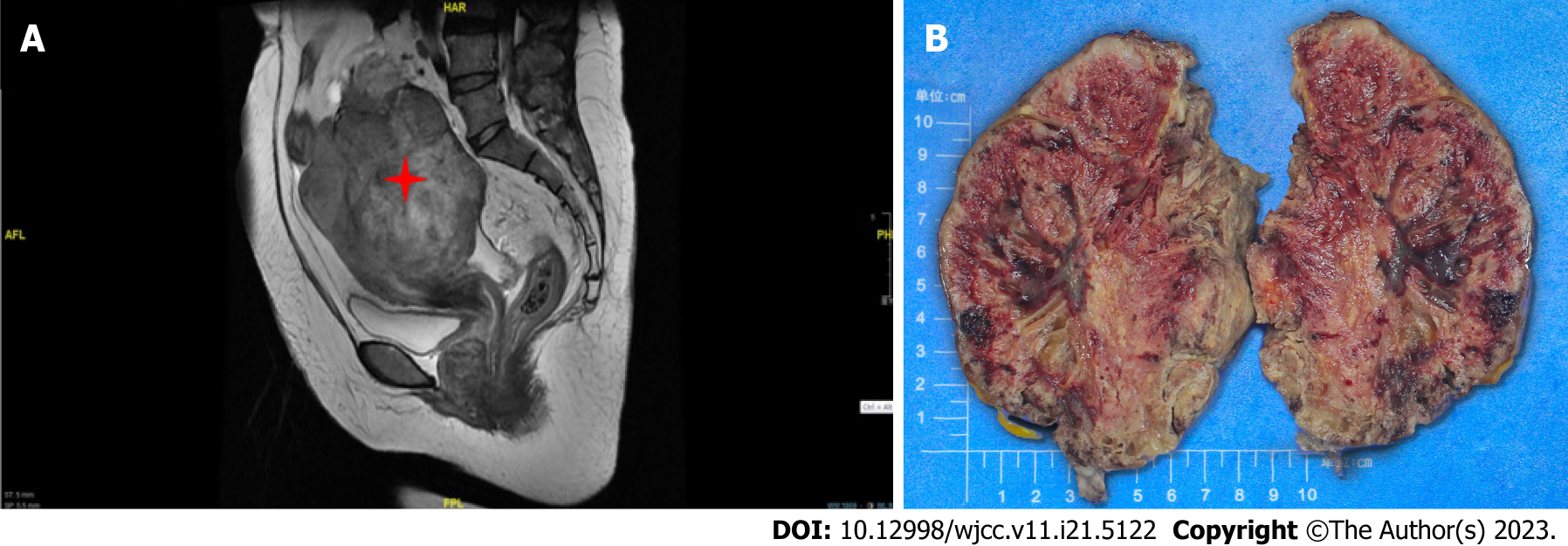
Case 2: Magnetic resonance imaging documented a solid mass 10.3 cm × 9.2 cm in dimensions, uniform density, irregular margin, and unclear boundary in the right adnexal area. Malignant ovarian tumors were also considered. (Figure 2A).
The postoperative pathological findings of the present two cases were as follows.
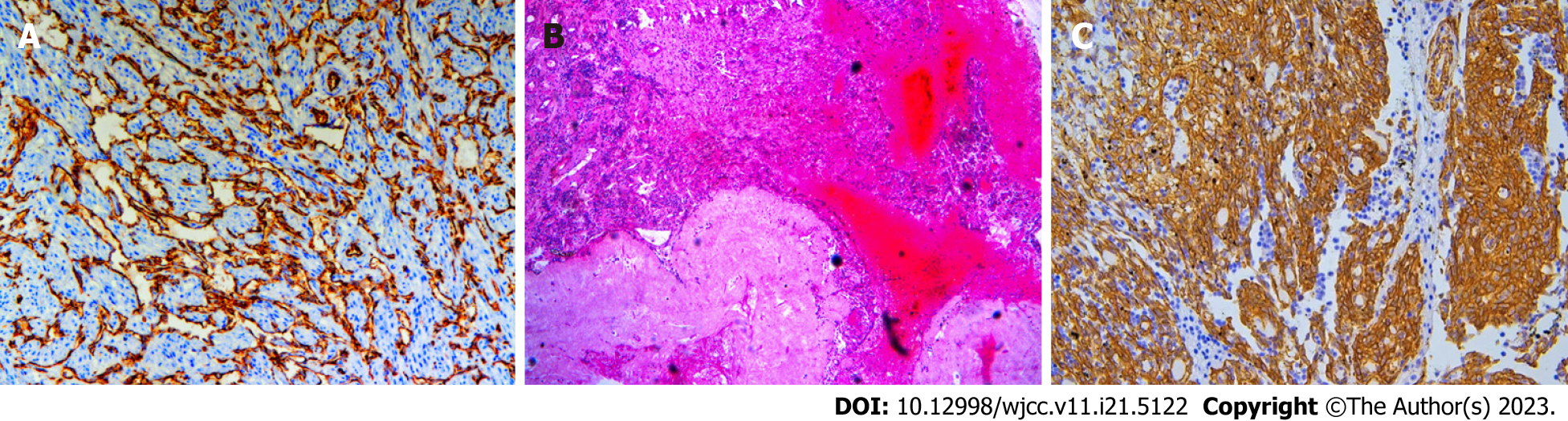
Case 1: On gross examination, the left ovary appeared unremarkable, and the right ovary was greatly enlarged and nodular, with cystic and solid, red-white cut surfaces. The small intestinal segment was densely adhered to the left colonic segment, with an intervening serosal mass. Multiple serosal deposits and lymph nodes were also isolated (Figure 1B). Upon microscopic examination, the tumor cells showed spindle-shaped, intercellular, slit-like structures, and neoplastic proliferation of atypical cells with enlarged pleomorphic nuclei and prominent nucleoli. Mitotic activity was high (12/10 high power fields). Extensive hemorrhage and necrosis were observed. The tumor cells showed positive immunohistochemical staining for caldesmon, CD31, and CD34. Approximately 50% of the cases were positive for cell proliferation-associated nuclear antigen (Ki-67) protein and negative for cytokeratin (CK), epithelial membrane antigen (EMA), CK5/6, desmin, CD10, p16, estrogen receptor alpha (ER), progesterone receptor (PR), programmed death ligand 1 (PD-L1), and placental prolactin protein. Due to the poor differentiation of the tumor cells and a Ki-67 high index, fluorescence in situ hybridization was performed, and c-MYC amplification was detected (Figures 3A-C).
Case 2: On gross examination, the right ovary was markedly swollen and completely displaced by a solid mass. The cut surface was red and the spongy areas occupied a substantial portion (Figure 2B). Microscopically, the tumor cells were composed of nail-like endothelial cells and a small number of scattered large cells arranged in a solid, fascicular, and tubular staggered arrangement. Vascular lacunae were formed in the area with an obvious lumen. Immunohistochemistry performed on a representative section showed strong and diffuse expression of vimentin, CD31, CD34, FLi-1, as well as a Ki-67 proliferative index that was focally increased up to 20%, while immunoreactions for calretinin, Pan cytokeratin monoclonal antibody, steroidogenic factor-1, EMA, PAX-8, WT-1, a-inhibin, ER, PR were all negative (Figures 4A-C).
Case 1 and 2: The final pathology returned as primary oAS.
Case 1: Given the clinical presentation and significantly elevated serum CA-125 levels, an ovarian malignant tumor was suspected before surgery. The right ureter was wrapped by another mass measuring approximately 6 cm × 4 cm, and removal of the mass together with the involved ureter might cause a ureteral defect. Partial removal of the rectus abdominis muscle and fascia, partial hysterectomy, bilateral salpingo-oophorectomy, and inguinal and pelvic lymphadenectomy were performed first. The patient was administered neoadjuvant chemotherapy with six cycles of MESNA + ADM + IFO + DTIC (MAID) chemotherapy (means 1500 mg/m2 on days 1-4, doxorubicin 20 mg/m2 on days 1-3, ifosfamide 2500 mg/m2 on days 1-3, and dacarbazine 300 mg/m2 on days 1-3). Responses were evaluated after two cycles of MAID; however, stable disease was achieved. The oncology department was consulted, and upon discussion of risks and benefits, the patient decided to participate in a clinical trial to take apatinib 500 mg orally once daily; however, she developed severe dermatitis and pleural effusion after undergoing a series of treatments. The dose was then halted. Her condition worsened clinically because of accumulation of ascitic fluid and shortness of breath. Palliative chemotherapy was administered as the disease was in its terminal stage.
Case 2: The patient underwent hysterectomy and bilateral salpingo-oophorectomy. Postoperatively, the patient received six cycles of MAID chemotherapy (mesna, adriamycin/doxorubicin, ifosfamide, and dacarbazine). Three months later, she experienced tumor recurrence with pelvic seeding; hence, she received a series of adjuvant chemotherapies again. In addition, PD-L1 (PD-L1 22C3 pharmDx antibody, Dako, Inc.) was performed on the tumor samples, which indicated PD-L1 focally up to 90% (Figure 2). As PD-1 checkpoint inhibition has seldom been investigated in AS, the oncology department was consulted upon discussion of treatment strategies for risks and benefits. The patient was treated with anti-PD-1 immunotherapy (nivolumab).
Case 1: The death of the patient disease occurred 1 mo after treatment.
Case 2: The patient has survived without evidence of disease for 27 mo off therapy.
Primary oAS is very rare, with an incidence of one in one million malignant ovarian tumors. The clinical manifestations of primary oAS lack specificity, most of which are abdominal pain and masses, and some of which are long-term unexplained gastrointestinal or urinary symptoms. Among the tumor markers, serum CA-125 level is increased (49.2-189.1 kU/L), while the serum CA-199, α-fetoprotein, and carcinoembryonic antigen levels are mostly normal[5].
The differential diagnosis of oAS mainly focuses on pathological diagnosis. Primary oAS has complex and diverse histomorphology, consisting of endothelial cells with varying degrees of atypia. When well-differentiated, they form lumps, and when poorly-differentiated, only single-cell lacunae can be observed. The diagnosis of primary oAS should be combined with the results of routine pathological examinations and immunohistochemistry panels, especially positive endothelial cell markers (mainly CD31, CD34, FV, and vimentin)[6]. Therefore, oAS may present a challenge for intraoperative frozen section diagnosis of an ovarian mass[7].
Primary oAS should be differentiated from benign vascular hyperplasia, ovarian hemangiomas, juvenile hemangioendotheliomas, and other benign lesions. Immunohistochemical detection of vascular endothelial cell markers in these lesions was positive; however, the tumor cells lacked atypia. Primary oAS should be differentiated from malignant melanoma, leiomyosarcoma, and other malignant tumors. Malignant melanoma is positive for the S100 protein, anti-melanoma-specific antibody, and melanoma differentiation antigen. Leiomyosarcoma is positive for myogenic markers, such as desmin, smooth muscle actin, and caldesmon[8]. Tumor size at the time of diagnosis and the presence or absence of metastases are the most important prognostic factors for primary oAS. The prognosis of tumors < 5 cm in diameter is significantly better than that of tumors > 5 cm[9].
Presently, treatment strategies vary, but none have produced substantial long-term success[10]. The commonly used chemotherapy regimens for primary oAS include MAID[11], ifosfamide + doxorubicin, and gemcitabine + cisplatin[12]. Primary oAS with c-MYC amplification or high PD-L1 expression is rare. Understanding the progress in molecular pathological research on AS and searching for possible effective targets for targeted therapy is the hope of prolonging the survival of patients[13-17].
In many cases, c-MYC amplification is associated with prognosis by mediating acquired resistance to anticancer therapies. Although c-MYC has been extensively investigated as a therapeutic target for various cancers, including radiation-induced and secondary AS[18], there are few studies have examined the clinical significance of c-MYC gene amplification in primary oAS[19].
The treatment of AS with c-MYC gene amplification has been discussed in a few case reports[20-22]. In a previous study, patients with radiation-induced abdominal AS exhibited a dramatic response to apatinib[23]. Due to the rarity of incidents and lack of clinical treatment experience, we referred to suggestions for experimental drug use, but our patient showed apatinib resistance and little effect was achieved. It remains unclear whether apatinib resistance is related to radiation-induced genesis, location of the primary tumor, or potential molecular factors. Further clinical studies are warranted to corroborate these findings, and treatment with c-MYC inhibitors may be suggested as an alternative thera
Recently, several monoclonal antibodies targeting PD-1 or its ligand PD-L1 have been associated with favorable outcomes in some solid tumors in large clinical trials[24-26]. A strong predictive association has been validated between PD-L1 expression and clinical outcomes for PD-1/PD-L1 inhibitors, and PD-1 and PD-L1 were evaluated in certain tumor specimens using specialized immunohistochemical techniques to determine PD-L1 immunoreactivity in tumor cells[27]. PD-1 checkpoint inhibition has seldom been investigated in AS and may have therapeutic utility[28,29]. In our second case, because of metastasis and it was unclear what additional treatment was performed at that time, patients’ tumors were evaluated for PD-L1 expression with PD-L1 22C3 pharmDx antibody (Dako, Inc.) and showed strong PD-L1 expression (average of 50%, hotpot with 99%). The patient benefited from anti-PD1/PD-L1 therapy treated with nivolumab. Although it is not possible to draw conclusions about the prognosis or potential therapies in this case, an evaluation of the effectiveness of immune checkpoint inhibitors for oAS is awaited[30].
Primary oAS is very rare; surgical resection combined with postoperative adjuvant chemotherapy is the first-line treatment but has not shown long-term survival benefits. The diagnosis of this disease depends on the pathological diagnosis, and the detection of certain pathological indicators also provides more evidence for clinical treatment. Alternative therapeutic strategies, such as targeted therapies and anti-PD1/PD-L1 therapy treatment be promising for primary oAS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bredt LC, Brazil; Ghazy A, Egypt S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Deb PQ, Weiss RE, Heller DS. Angiosarcoma of the Uterus: A Systematic Review. Int J Gynecol Pathol. 2022;41:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 2. | Bradford L, Swartz K, Rose S. Primary angiosarcoma of the ovary complicated by hemoperitoneum: a case report and review of the literature. Arch Gynecol Obstet. 2010;281:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 3. | Furihata M, Takeuchi T, Iwata J, Sonobe H, Ohtsuki Y, Wakatsuki A, Morioka N, Sagara Y. Primary ovarian angiosarcoma: a case report and literature review. Pathol Int. 1998;48:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Vavilis D, Papadopoulos N, Agorastos T, Efstratiou I, Kommoss F, Bontis IN. Primary ovarian angiosarcoma--review of the literature and report of a case with coexisting chylothorax. Eur J Gynaecol Oncol. 2007;28:287-289. [PubMed] |
| 5. | Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. 2010;11:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 663] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 6. | May Lee M, Pierobon E, Riva G, Germi L, Feliciani C, Naldi L. Angiosarcoma and Vascular Surgery: A Case Report and Review of Literature. Vasc Endovascular Surg. 2022;56:762-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Khunamornpong S, Settakorn J, Sukpan K, Pongsuvareeyakul T, Siriaunkgul S. Angiosarcoma Arising in Ovarian Mucinous Tumor: A Challenge in Intraoperative Frozen Section Diagnosis. Case Rep Pathol. 2016;2016:8508624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Glazebrook KN, Magut MJ, Reynolds C. Angiosarcoma of the breast. AJR Am J Roentgenol. 2008;190:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Quesenberry CD, Li C, Chen AH, Zweizig SL, Ball HG 3rd. Primary angiosarcoma of the ovary: a case report of Stage I disease. Gynecol Oncol. 2005;99:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Messiou C, Moskovic E, Vanel D, Morosi C, Benchimol R, Strauss D, Miah A, Douis H, van Houdt W, Bonvalot S. Primary retroperitoneal soft tissue sarcoma: Imaging appearances, pitfalls and diagnostic algorithm. Eur J Surg Oncol. 2017;43:1191-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Li EX, Zhang YT, Shang JT, Xu Z, Geng Y, Li SM, Shi F, Wu YY. [Effect of modified MAID regimen for patients with advanced soft tissue sarcoma]. Ai Zheng. 2006;25:1048-1051. [PubMed] |
| 12. | Liu R, Chen D, Dong F, Zheng MH. [Advances in molecular pathology of primary cardiac angiosarcoma]. Zhonghua Bing Li Xue Za Zhi. 2023;52:87-90. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Yang L, Liu L, Han B, Han W, Zhao M. Apatinib treatment for KIT- and KDR-amplified angiosarcoma: a case report. BMC Cancer. 2018;18:618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Jones RL, Ravi V, Brohl AS, Chawla S, Ganjoo KN, Italiano A, Attia S, Burgess MA, Thornton K, Cranmer LD, Cheang MCU, Liu L, Robertson L, Adams B, Theuer C, Maki RG. Efficacy and Safety of TRC105 Plus Pazopanib vs Pazopanib Alone for Treatment of Patients With Advanced Angiosarcoma: A Randomized Clinical Trial. JAMA Oncol. 2022;8:740-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 15. | Panda SP, Panigrahy UP, Prasanth D, Gorla US, Guntupalli C, Panda DP, Jena BR. A trimethoxy flavonoid isolated from stem extract of Tabebuia chrysantha suppresses angiogenesis in angiosarcoma. J Pharm Pharmacol. 2020;72:990-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Pink D, Andreou D, Bauer S, Brodowicz T, Kasper B, Reichardt P, Richter S, Lindner LH, Szkandera J, Grünwald V, Kebenko M, Kirchner M, Hohenberger P. Treatment of Angiosarcoma with Pazopanib and Paclitaxel: Results of the EVA (Evaluation of Votrient(®) in Angiosarcoma) Phase II Trial of the German Interdisciplinary Sarcoma Group (GISG-06). Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Wagner MJ, Lyons YA, Siedel JH, Dood R, Nagaraja AS, Haemmerle M, Mangala LS, Chanana P, Lazar AJ, Wang WL, Ravi V, Holland EC, Sood AK. Combined VEGFR and MAPK pathway inhibition in angiosarcoma. Sci Rep. 2021;11:9362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Yan M, Gilmore H, Bomeisl P, Harbhajanka A. Clinicopathologic and immunohistochemical study of breast angiosarcoma. Ann Diagn Pathol. 2021;54:151795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Abada E, Jang H, Kim S, Ali-Fehmi R, Bandyopadhyay S. A clinicopathologic and immunohistochemical study of primary and secondary breast angiosarcoma. J Pathol Transl Med. 2022;56:342-353. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Webb C, Partain N, Koduru P, Hwang H, Sarode VR. Secondary Angiosarcoma With C-MYC Amplification Following Prophylactic Bilateral Mastectomy and Autologous Breast Reconstruction: Report of a Case and Review of the Literature. Int J Surg Pathol. 2021;29:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Fraga-Guedes C, André S, Mastropasqua MG, Botteri E, Toesca A, Rocha RM, Peradze N, Rotmensz N, Viale G, Veronesi P, Gobbi H. Angiosarcoma and atypical vascular lesions of the breast: diagnostic and prognostic role of MYC gene amplification and protein expression. Breast Cancer Res Treat. 2015;151:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Yonezawa I, Waki M, Tamura Y, Onoda R, Narushima M, Ishizuka T, Tajima S. Gemcitabine-based regimen for primary ovarian angiosarcoma with MYC amplification. Curr Oncol. 2014;21:e782-e789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Guan J, Luo Z, Xiao Z, Xie Y, Lin L. Treatment of consistent BRAF/HRAS gene mutation and MYC amplification radiation-induced abdominal wall angiosarcoma with low-dose apatinib: a case report. BMC Cancer. 2019;19:1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR; KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5948] [Cited by in RCA: 7470] [Article Influence: 830.0] [Reference Citation Analysis (0)] |
| 25. | Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L, Pluzanski A, Reckamp KL, Burgio MA, Kohlhäeufl M, Waterhouse D, Barlesi F, Antonia S, Arrieta O, Fayette J, Crinò L, Rizvi N, Reck M, Hellmann MD, Geese WJ, Li A, Blackwood-Chirchir A, Healey D, Brahmer J, Eberhardt WEE. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35:3924-3933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 685] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 26. | Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2868] [Cited by in RCA: 3682] [Article Influence: 460.3] [Reference Citation Analysis (0)] |
| 27. | Filiukova OB, Snastina TI, Belotskiĭ SM, Marchuk AI, Timin EN. [Stimulation of the respiratory burst of the neutrophils from healthy subjects by different Staphylococcus aureus concentrations]. Zh Mikrobiol Epidemiol Immunobiol. 1987;58-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 28. | Sindhu S, Gimber LH, Cranmer L, McBride A, Kraft AS. Angiosarcoma treated successfully with anti-PD-1 therapy - a case report. J Immunother Cancer. 2017;5:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 29. | Boichard A, Wagner MJ, Kurzrock R. Angiosarcoma heterogeneity and potential therapeutic vulnerability to immune checkpoint blockade: insights from genomic sequencing. Genome Med. 2020;12:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Ye H, Lin M, Li R, Qin S, Hou G, Chen H, Li X. Primary ovarian angiosarcoma: a rare and recognizable ovarian tumor. J Ovarian Res. 2021;14:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |