Published online Jul 26, 2023. doi: 10.12998/wjcc.v11.i21.5047
Peer-review started: April 10, 2023
First decision: May 8, 2023
Revised: May 17, 2023
Accepted: July 4, 2023
Article in press: July 4, 2023
Published online: July 26, 2023
Processing time: 107 Days and 9.8 Hours
Mechanical thrombectomy is the most effective treatment for great cerebral artery embolization within a set time window. Typically, an arteriogram does not show the localization of the stent after release and whether a thrombus is captured or not. Thus, improving the visualization of a stent in interventional therapy will be helpful for clinicians.
To analyze stent imaging findings to enhance clinicians’ understanding of a special circumstance, wherein a Solitaire AB retrievable stent was visible during the imaging of a thrombus capture that improved the success rate of stent-based mechanical thrombectomy.
This was a retrospective study with four acute ischemic stroke (AIS) patients who underwent stent-based mechanical thrombectomy.
Patient 1 was a 64-year-old man admitted after 5 h of confusion; angiography revealed basilar artery occlusion. We inserted a stent into the left posterior cerebral artery-P2 segment and visualized the expanded stent that successfully captured a thrombus. Patient 2 was a 74-year-old man admitted with confusion, which lasted approximately 3 h. Angiography revealed a left middle cerebral artery (MCA)-M1 segment occlusion. A stent was deployed in the distal M2 segment, and we could visualize the stent by capturing the thrombus. Patient 3 was a 74-year-old woman admitted after experiencing left hemiplegia for 3 h. We deployed a stent at the distal right MCA-M2 segment, and the developing stent captured a large thrombus. Patient 4 was an 82-year-old man who presented with confusion for 3 h. A developing stent was placed in the distal left MCA-M1 segment, which captured a large thrombus and several fragmented thrombi.
To the best of our knowledge, this is the first report of stent imaging in patients with AIS. We demonstrated the usefulness and substantial potential of stent imaging in stent-based mechanical thrombectomy for AIS.
Core Tip: We present the case report of four patients with acute ischemic stroke (AIS) who underwent stent-based mechanical thrombectomy. In this study, we were able to visualize the developing stent, including the capture of the thrombi during mechanical thrombectomy. This study has demonstrated that visualizing stents using imaging is suitable for assessing stent deployment and procedural optimization during stent-based mechanical thrombectomy. To the best of our knowledge, this is the first report of stent imaging in patients with AIS undergoing stent-based mechanical thrombectomy. This study contributes significantly to the literature by evaluating the usefulness and enormous potential of stent imaging in stent-based mechanical thrombectomy for AIS.
- Citation: Yao QY, Fu ML, Zhao Q, Zheng XM, Tang K, Cao LM. Image-based visualization of stents in mechanical thrombectomy for acute ischemic stroke: Preliminary findings from a series of cases. World J Clin Cases 2023; 11(21): 5047-5055
- URL: https://www.wjgnet.com/2307-8960/full/v11/i21/5047.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i21.5047
Patients with large vessel occlusions, such as internal carotid artery (ICA), middle cerebral artery (MCA), or basilar artery (BA) occlusions, are potential candidates for mechanical thrombectomy, which is the most effective treatment for great cerebral artery embolization within a set time window. Cardioembolic stroke and atherothrombotic stroke are the subtypes of ischemic infarct with the highest in-hospital mortality, and their short-term prognosis is poor compared with other ischemic stroke subtypes[1]. Stents have become increasingly sophisticated, thus imposing stent imaging difficulties during intravascular procedures. Typically, an arteriogram does not show the localization of the stent after release and whether a thrombus is captured or not. Therefore, improving the visualization of a stent in interventional therapy will be helpful for clinicians.
Image enhancement techniques are used to assess stent expansion and overlap size and localize postdilation balloons, but they have been rarely performed until recently[2]. Currently, stent boost imaging is used in percutaneous coronary interventions[2-4]. The analytical software program StentBoost (Philips Medical Systems) improves the angiographic visualization of the stent and its relationship with the corresponding vessel lumen by enhancing the X-ray focus of the region in which the stent is placed[5]. However, StentBoost is rarely used during stent-based mechanical thrombectomies for acute ischemic stroke (AIS). The Solitaire AB stent is a safe and effective option for stenting MCA and BA occlusions and is extensively used in China[6]. We found that the Solitaire AB retrievable stent (without StentBoost) was visible on imaging when the thrombus was captured, thus improving the success rate of stent-based mechanical thrombectomy. These interesting findings are similar to those observed when StentBoost is used.
The clinical manifestations, treatment, and follow-up results of four AIS patients treated by mechanical thrombectomies at the 910th Hospital of the Joint Logistics Support Force of the Chinese PLA were analyzed. In this study, we used the Solitaire AB stent (Micro Therapeutics Inc., DBA ev3 Neurovascular, Irvine, California, United States) for stent-based mechanical thrombectomies. Three experienced physicians performed all the stent-based mechanical thrombectomy procedures. The study design was approved by the ethics review board of the 910th Hospital of the Joint Logistics Support Force of the Chinese PLA (No: 2021-29), and informed consent was obtained from all the patients in this study.
The 6F arterial sheath was inserted using the Seldinger technique after local anesthesia, and whole-brain digital subtraction angiography (DSA) was performed to identify the vascular lesions and understand the state of collateral circulation. Stent-based mechanical thrombectomy was performed after clear indications and informed consent were obtained. Further, a 6F guiding catheter was inserted into proximal large vessels on the diseased side under the guidance of a 0.035 inch guidewire. Subsequently, the Rebar-18 microcatheter (Micro Therapeutics Inc., DBA ev3 Neurovascular) was placed in the distal vascular occlusion over a 0.014 inch microwire (Stryker Neurovascular, Salt Lake City, Utah), and distal vascular patency was confirmed through angiography by the microcatheter. A Solitaire AB stent was deployed through the Rebar microcatheter in the vascular occlusion. The stent was then withdrawn with syringe withdrawal and a captured thrombus. If a subsequent angiogram showed good occluded artery recanalization, the surgery ended. If not, the stent-based mechanical thrombectomy would be performed again.
The indications for stent-based mechanical thrombectomy included: (1) AIS caused by large arterial occlusion confirmed by neuroimaging findings; (2) Exclusion of intracranial hemorrhage by computed tomography (CT); (3) The time window: Anterior circulation occlusion within 6 h of onset, and anterior circulation occlusion within 6-24 h of onset and after rigorous screening using imaging: Posterior circulation large vessel occlusion within 24 h of onset; and (4) The patient or legal representative provided signed informed consent[7].
The contraindications for stent-based mechanical thrombectomy included: (1) Patients with severe active bleeding or bleeding tendency; (2) Severe dysfunction of organs such as the heart, liver, and kidneys; and (3) The expected survival period was less than 90 d based on the patient's condition and results of the examinations.
The clinical requirements for thrombectomy were: (1) Less than 85 years old; (2) Obvious neurological dysfunction that persisted or worsened for more than 1 h; (3) No severe complications, such as severe liver, kidney, and heart failure; and (4) No restrictions in the confusional state at the onset or gender.
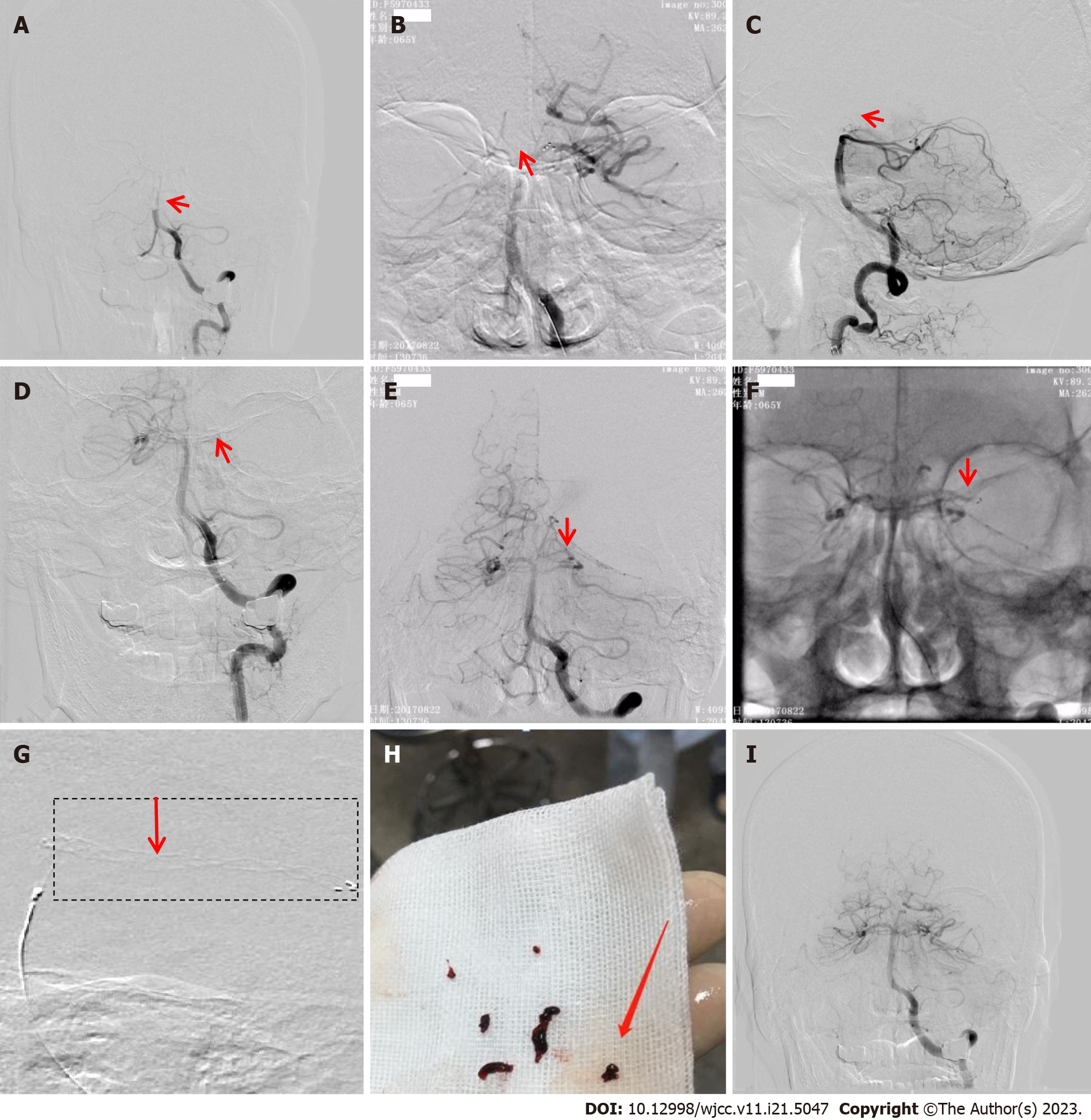
A 64-year-old man was admitted following 5 h of confusion. He had a history of smoking, and his neurological examination on admission showed mild coma, contraction of bilateral pupils, and no reaction to pain stimulation in four extremities. His National Institutes of Health Stroke Scale (NIHSS) score was 28. Brain CT, coagulation function, and blood biochemistry tests on admission revealed no evident abnormalities. The patient’s white blood cell (WBC) count (12.59 × 109/L) and neutrophil percentage (87.30%) were increased; the electrocardiogram showed a T-wave change. The patient was diagnosed with AIS, and an emergency DSA was performed, which revealed a proximal BA occlusion (Figure 1A).
A 6F guiding catheter was inserted into the distal left vertebral artery-V2 segment. The Rebar-18 microcatheter (Micro Therapeutics Inc., DBA ev3 Neurovascular) was coaxially advanced within the guiding catheter (Boston Scientific Corporation, Massachusetts, United States) over a 0.014 inch microwire (Stryker Neurovascular, Salt Lake City, Utah) placed in the left posterior cerebral artery (LPCA)-P1 segment, was then withdrawn, and an arteriogram of the distal artery was obtained. A Solitaire AB stent (6 mm × 30 mm) was deployed at the proximal LPCA-P1 segment and BA (without stent imaging, Figure 1B) through the Rebar microcatheter, which captured three fragmented thrombi. An angiogram showed occlusions at the top of the BA and bilateral posterior cerebral artery (PCA) (Figure 1C).
Furthermore, the Solitaire AB stent was deployed in the proximal right PCA (RPCA)-P1 segment and captured a fragmented thrombus (Figure 1D). A repeat angiogram showed the BA with normalization of the vessel size and appearance. The RPCA was visualized along with the proximal LPCA-P1 segment occlusion (Figure 1E). We placed the Solitaire AB stent in the distal LPCA-P1 segment for the third time (without stent imaging, Figure 1F) without capturing thrombi. We deployed the Solitaire AB stent in the LPCA-P2 segment. The stent had expanded (Figure 1G) and captured a thrombus (Figure 1H). A subsequent angiogram showed good recanalization of the occluded artery (Figure 1I). Postoperatively, the patient was in a mild coma (NIHSS score, 30). On day 2, magnetic resonance imaging revealed acute infarcts in the brainstem, left cerebellum, and left basal ganglia region, with minor brainstem hemorrhage. The patient was rehydrated and administered antibiotics, antiplatelet therapy, and statins; we also prescribed rehabilitation exercises. Afterward, his symptoms, such as mental status, feeding, dysarthria, and hemiplegia, improved. At the 3-mo follow-up, his NIHSS and modified Rankin Scale (mRS) scores were 7 and 3, respectively.
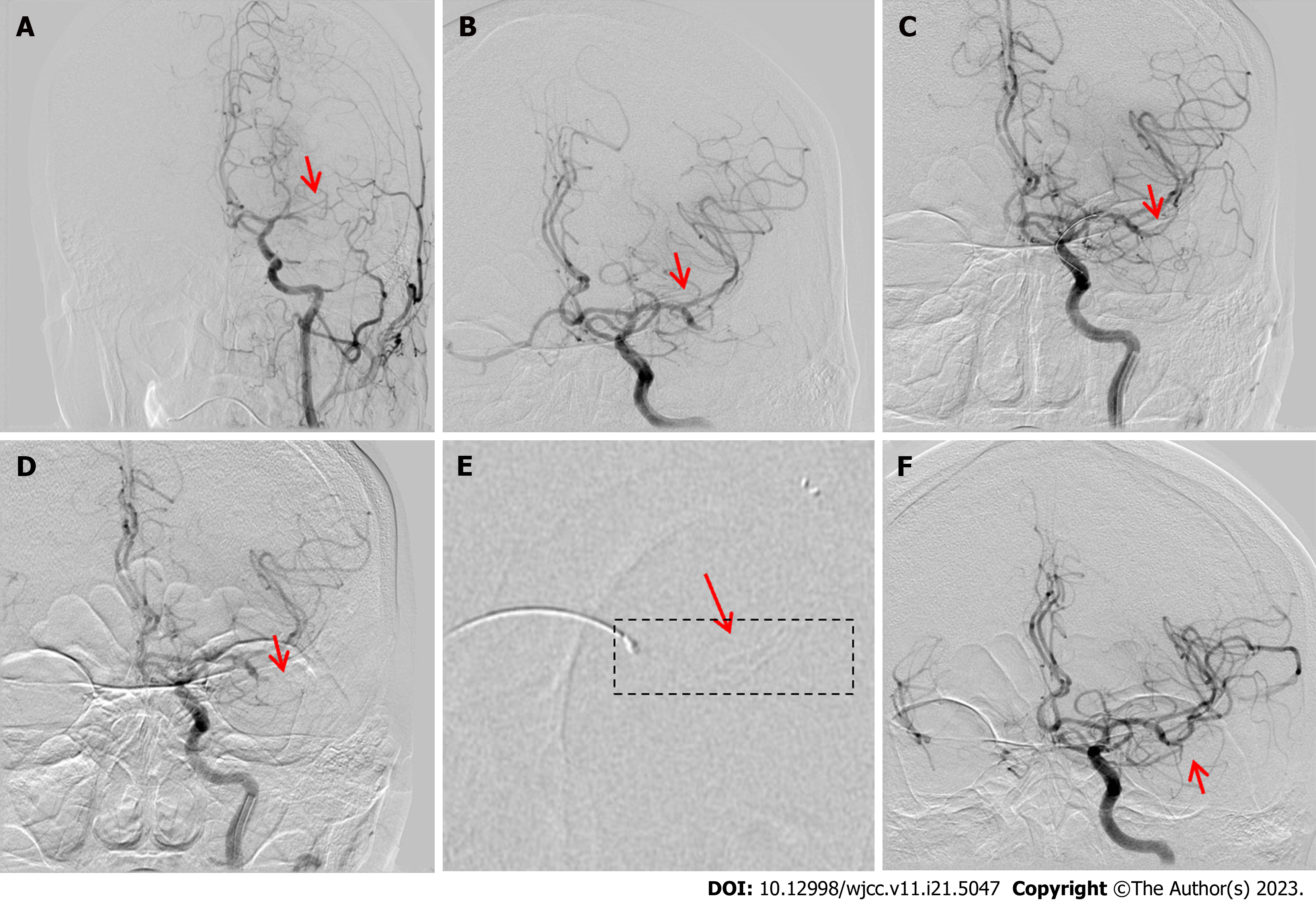
A 74-year-old man was admitted with confusion signs lasting approximately 3 h. He had a history of hypertriglyceridemia, coronary atherosclerotic heart disease, myocardial infarction, angina, left ventricular aneurysm, and frequent premature ventricular contractions. Neurological examination revealed mild coma, oculomotor nerve paralysis, shallow nasolabial sulcus, and no reaction to pain stimulation in the right extremities. An emergency CT revealed a left lenticular nucleus and an insular lobe without clearly defined boundaries. Conversely, his routine blood, blood biochemistry, coagulation function, and cardiac troponin tests, and the electrocardiography results were normal.
The patient had a massive AIS and was immediately evaluated by DSA, which revealed left MCA (LMCA)-M1 segment occlusion with poor compensatory blood flow (Figure 2A). A 6F guiding catheter was advanced into the left ICA (LICA)-C2 segment; a Rebar microcatheter was advanced over a 0.014 inch microwire across the MCA occlusion and into the M1 segment. We navigated a microcatheter past the LMCA-M1 occlusion over a microwire. An angiogram showed that some thrombi had escaped, although there was good recanalization of the MCA-M2 segment upper and lower branch occlusions (Figure 2B). A Solitaire AB stent (4 mm × 20 mm) was placed in the M2 segment via the microcatheter (without stent imaging, Figure 2C) and captured a fragmented thrombus. A second (repeat) angiogram showed no recanalization of the LMCA-M2 segment (Figure 2D). The Solitaire AB stent was redeployed through the microcatheter at the distal M2 segment. Subsequently, we were able to visualize the stent capturing the thrombus (Figure 2E). DSA after stent deployment showed good recanalization of the LMCA-M2 segment (Figure 2F). On the same postoperative day, the patient developed a moderate coma. Central respiratory failure occurred, possibly due to a massive ischemic stroke, cerebral hyperperfusion syndrome, or brain hemorrhagic transformation, and the patient was urgently transferred to the intensive care unit. He was later transferred to a local hospital, where antibiotics, antiplatelets, and statins were administered, which gradually improved his symptoms. At the 3-mo postoperative visit, the patient had a normal mental status, motor aphasia, grade 4/5 (manual muscle testing) muscle strength in the right extremities, and NIHSS and mRS scores of 3 and 1, respectively.
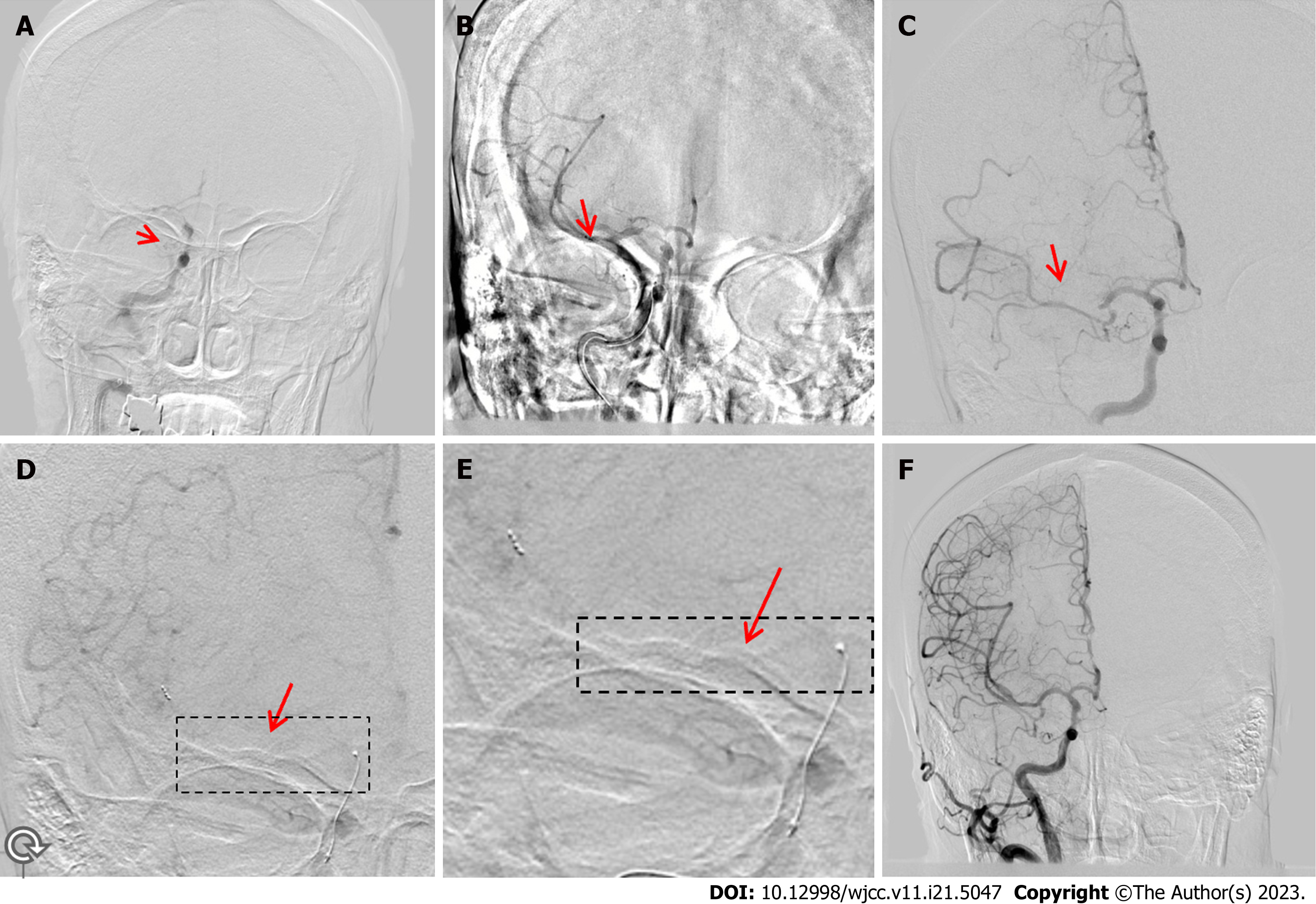
A 74-year-old woman was admitted after experiencing left hemiplegia for 3 h. On admission, her NIHSS score was 16. She had a history of hypertension for more than 10 years. Physical examination on admission showed a blood pressure (BP) of 230/113 mmHg, arrhythmia, confusion, dysphasia, oculomotor nerve paralysis, shallow nasolabial sulcus, grade 0/5 muscle strength in the left extremities, grade 4/5 muscle strength in the right extremities, and hypoesthesia in the left extremities. Her blood sodium (148.30 mmol/L), creatine kinase (233.3 U/L), lactate dehydrogenase (462.9 U/L), blood sugar (6.49 mmol/L), creatinine (109.4 μmol/L), and homocysteine (20.22 μmol/L) levels were elevated. Moreover, the patient’s WBC count (17.80 × 109/L) and neutrophil percentage (89.30%) were increased. Initial head CT showed no obvious abnormalities.
She underwent intravenous thrombolysis with tissue-type plasminogen activator (tPA, 45 mg) bridging stent-based mechanical thrombectomy. DSA revealed a distal right ICA (RICA)-C7 segment occlusion with poor collateral circulation (Figure 3A). Subsequently, a 6F guiding catheter was placed in the proximal RICA-C1 segment without stent imaging (Figure 3B). The Rebar-18 microcatheter was coaxially advanced within the guide catheter over a 0.014 inch microwire to the distal RICA. The microwire was withdrawn, and angiography was performed through the microcatheter to ensure that the catheter was in the true lumen. A Solitaire AB stent (6 mm × 30 mm) was deployed through the microcatheter and retrieved, capturing a fragmented thrombus at the proximal right MCA (RMCA)-M1 segment (Figure 3B). Repeat angiograms showed good recanalization of the RICA, right anterior cerebral artery, and RMCA-M1 and M2 segment lower branches. However, the RMCA-M2 segment upper branch remained occluded (Figure 3C). The Solitaire AB stent was deployed at the distal RMCA-M2 and retrieved without capturing a thrombus. The Solitaire AB stent was deployed again at the distal RMCA-M2 segment (Figure 3D and E) and retrieved after a large thrombus was captured. A repeat angiogram revealed good recanalization of the RMCA, and the distal artery was visualized (Figure 3F).
In this case, management included reducing the intracranial pressure, inhibiting brain edema, promoting brain metabolism, scavenging oxygen-free radicals, and administering statins and antibiotics postoperatively. On postoperative day 2, the patient’s condition worsened. Repeat CT revealed right cerebral hemisphere swelling and intraparenchymal and subarachnoid hemorrhages. After consultation with the neurosurgical team, we performed a decompressive craniectomy. At her postoperative 3-mo follow-up, she had Glasgow Coma Scale and mRS scores of 5 and 5, respectively.
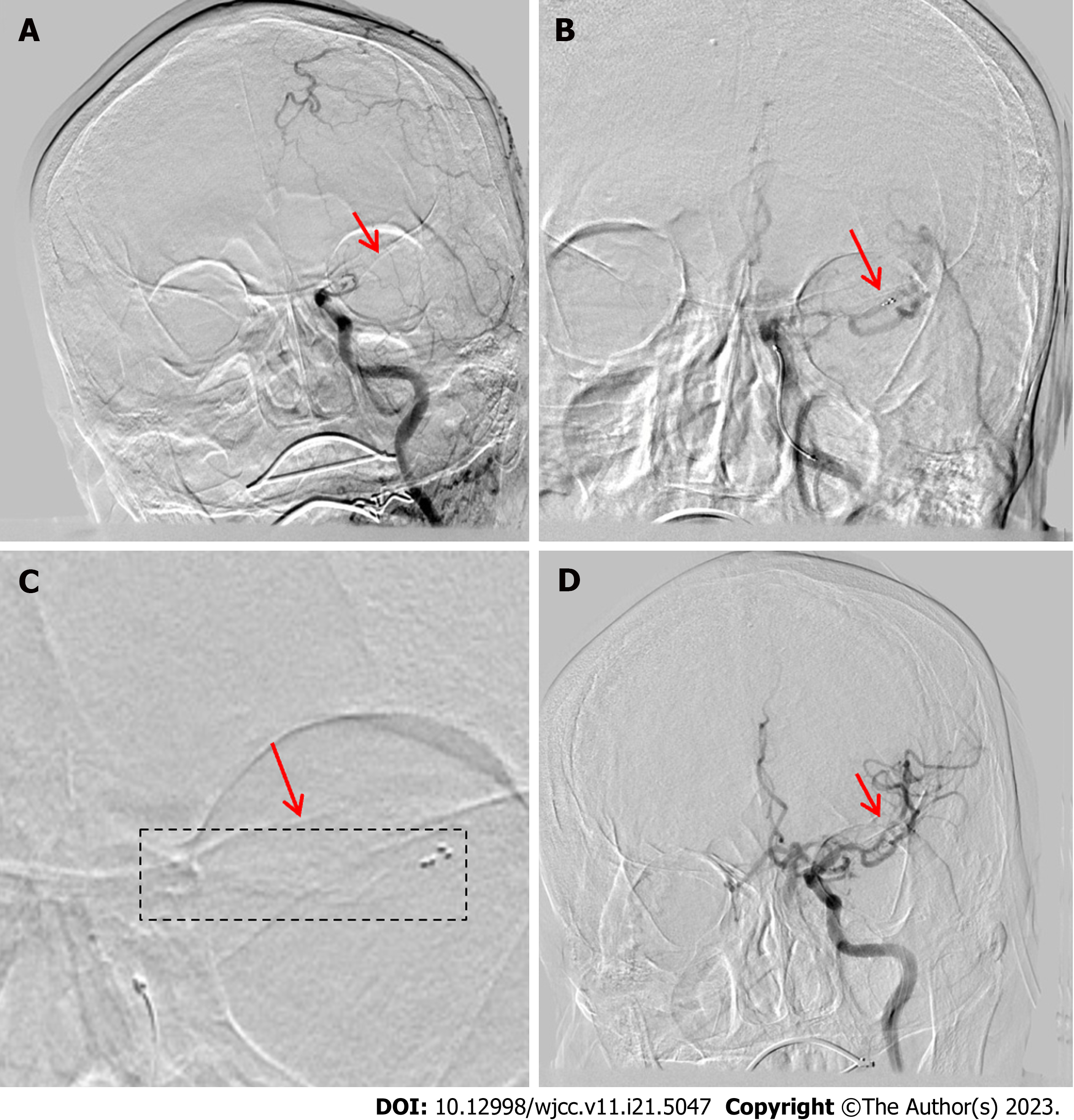
An 82-year-old man presented with confusion signs, which lasted for 3 h, and had an NIHSS score of 30. CT revealed high density in the LMC, and he was diagnosed with cardiogenic AIS. Subsequently, he underwent intravenous thrombolysis bridging mechanical thrombectomy.
On admission, his physical examination showed a BP of 135/75 mmHg, arrhythmia, mild coma, oculomotor nerve paralysis, dimpled right nasolabial sulcus, and a right Babinski sign. His plasma type B natriuretic peptide (3433.9 pg/mL), serum myoglobin (1296.7 ng/mL), cardiac troponin T (0.017 ng/mL), D-dimer (1.87 mg/L), and C-reactive protein (4.23 mg/L) levels were all high. The patient’s WBC count (12.05 × 109/L) and neutrophil percentage (88.51%) also increased. An electrocardiogram revealed fast auricular fibrillation and ST-T change, and brain CT revealed high-density areas in the LMCA.
The patient was diagnosed with an extensive cardiogenic AIS and underwent intravenous thrombolysis with tPA (50 mg) bridging mechanical thrombectomy. DSA revealed distal left LICA occlusion with poor compensatory blood flow (Figure 4A). A 6F guiding catheter was deployed in the proximal LICA-C2 segment. The microcatheter was coaxially advanced over a microwire through the guiding catheter across the LICA occlusion, and a stent was deployed in the distal M2 segment. We withdrew the microwire and confirmed its location in the true lumen. A Solitaire AB (6 mm × 30 mm) stent was deployed through the microcatheter at the distal LMCA-M1 segment (Figure 4B). The stent imaging outcomes are shown in Figure 4C; this was followed by the stent withdrawal, which captured a large thrombus and several fragmented thrombi. A repeat angiogram revealed good recanalization of the LICA, and the distal artery was visualized (Figure 4D). In this case, treatment involved the reduction of intracranial pressure and controlling BP postoperatively. CT on postoperative day 2 revealed a minor basal ganglia hemorrhage. The patient was treated by ad
We found that stent imaging can indicate the recapture of the thrombus and help to assess stent deployment and procedural optimization during a stent-based mechanical thrombectomy. Stent imaging reduces the requirement of contrast medium for localization of the stent, shortens the operative time, and requires no specialized operator training.
The Solitaire AB retrievable stent has good plasticity and is convenient for use in stent-based mechanical thrombectomies, as approved by the Federal Drug Administration. Using the Solitaire AB stent retriever for acute BA occlusion resulted in a high recanalization rate without procedural complications and good clinical outcomes[8]. Thus, Solitaire AB stent angioplasty may be safe and effective in treating MCA-occlusive AIS[9].
Stent underexpansion is a major risk factor for mechanical thrombectomy failure. Stent imaging may be used to assess stent expansion and size overlap or to localize the stent. To the best of our knowledge, stent development is rarely reported during stent-based mechanical thrombectomy for AIS. The Solitaire AB stent is made of a nickel-titanium alloy; typically, the stent is not visualized in a DSA except for three or four markers at its tip and the dielectric welding point. Our study demonstrated that stent imaging helped to capture the thrombus [success rate, 100% (4/4)]. However, mechanical thrombectomy without stent imaging does not necessarily fail [e.g., success rate, 55.6% (5/9)]. Our experience is that there is a high probability of the stent capturing a thrombus once the stent is visible, as stent imaging has important implications for thrombus capture.
We found that stent imaging can show different forms of the stent, including a completely (Figure 1G) and partially visualized stent (Figure 2E). In our study, stent imaging presented a grid-form shadow (Figure 1G) and strip-shaped distribution (Figure 3E), and the first two forms overlay each other (Figure 4C). The ability to visualize the stent with the use of imaging may be related to the changes in structure and density during thrombus capture. The exact mechanism of Solitaire AB stent imaging is unknown and is worthy of further study.
The stent imaging used in this study may differ from StentBoost. StentBoost is an angiography imaging technology based on X-ray fluoroscopy that aids stent visualization. It can be used to either assess stent expansion and size overlap or localize postdilation balloons; however, these techniques have rarely been used until recently[10,11]. One imaging mechanism is enhancing the stent's radiologic edge by digital management of regular X-ray images[12]. StentBoost enhancement was also found to improve stent visualization and identification of stent underexpansion to guide postdilatation procedures[4].
Patients 2 and 4 presented with malignant MCA infarction, a devastating type of ischemic stroke. They both had suddenly decreased consciousness and continued to show no improvement. In a recent clinical series, decreased consciousness, nausea or vomiting, and heavy smoking were prognostic factors for malignant MCA infarction[13]. Therefore, when treating stroke patients with a sudden decline in consciousness, we should pay attention to whether they have malignant MCA infarctions or not. Early recognition and effective treatment are very important for prognosis.
The limitation of this study is that although we included several representative cases, it was performed at a single center, and the results are only applicable to the selected population group. Larger multicenter studies evaluating more patients are needed to confirm our findings.
To the best of our knowledge, this is the first report of stent imaging in patients with AIS undergoing stent-based mechanical thrombectomy. The mechanism of imaging may be related to changes in the structure and density during thrombus capture. These results demonstrate the usefulness and substantial potential of stent imaging during mechanical thrombectomy for AIS. Additional studies are required to confirm our findings and elucidate the underlying mechanism involved.
Mechanical thrombectomy is the most effective treatment for great cerebral artery embolization within a set time window. Typically, an arteriogram does not show the localization of the stent after release and whether a thrombus is captured or not.
Improving the visualization of a stent will be helpful for clinicians to perform stent-based mechanical thrombectomy.
This study aimed to analyze stent imaging findings to enhance clinicians’ understanding that improved the success rate of stent-based mechanical thrombectomy.
This was a retrospective study with acute ischemic stroke (AIS) patients who underwent stent-based mechanical thrombectomy.
Four patients with acute cerebral large-vessel occlusion presented the localization of the stent after release, where a thrombus was captured in mechanical thrombosis.
To our knowledge, this is the first report of stent imaging in patients with AIS. We demonstrated the usefulness and substantial potential of stent imaging in stent-based mechanical thrombectomy.
Additional studies are required to confirm our findings and elucidate the underlying mechanism involved.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Arboix A, Spain; Ciarambino T, Italy S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Cai YX
| 1. | Arboix A, Oliveres M, Massons J, Pujades R, Garcia-Eroles L. Early differentiation of cardioembolic from atherothrombotic cerebral infarction: a multivariate analysis. Eur J Neurol. 1999;6:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Arat Ozkan A, Sinan UY, Gurmen AT. Zotarolimus-eluting stent fracture at initial implantation diagnosed with StentBoost. SAGE Open Med Case Rep. 2016;4:2050313X16645754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Wang G, Wu X. Ostial lesion of the anterior descending coronary artery treated via Szabo technique supported by stent boost imaging: a case report. J Cardiothorac Surg. 2021;16:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Laimoud M, Nassar Y, Omar W, Abdelbarry A, Elghawaby H. Stent boost enhancement compared to intravascular ultrasound in the evaluation of stent expansion in elective percutaneous coronary interventions. Egypt Heart J. 2018;70:21-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Koolen JJ, van het Veer M, Hanekamp CE. StentBoost image enhancement: first clinical experience. Medicamundi. 2005;49:1-6. [DOI] [Full Text] |
| 6. | Cao X, Wang J, Tian C, Du Z, Su H, Liu X, Lv B, Yu S, Chen X, Hui F. Solitaire AB stent-angioplasty for stenoses in perforator rich segments: A single-center experience. Interv Neuroradiol. 2020;26:608-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Chinese Society of Neurology, Chinese Stroke Society; Neurovascular Intervention Group of Chinese Society of Neurology. Chinese guidelines for the endovascular treatment of acute ischemic stroke 2022. Zhonghua Shenjingke Zazhi. 2022;55:565-580. [DOI] [Full Text] |
| 8. | Du S, Mao G, Li D, Qiu M, Nie Q, Zhu H, Yang Y, Zhang Y, Li Y, Wu Z. Mechanical thrombectomy with the Solitaire AB stent for treatment of acute basilar artery occlusion: A single-center experience. J Clin Neurosci. 2016;32:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Wang XF, Wang M, Li G, Xu XY, Shen W, Liu J, Xiao SS, Zhou JH. Efficacy of Solitaire AB stent-release angioplasty in acute middle cerebral artery atherosclerosis obliterative cerebral infarction. World J Clin Cases. 2021;9:5028-5036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Kanabar K, Prasad K, Santosh K, Kaur N, Panda P, Sharma YP. Role of StentBoost in Successful Guidewire Recrossing. J Invasive Cardiol. 2020;32:E78. [PubMed] |
| 11. | Chen Q, Zhang LW, Huang DS, Zhang CH, Wang QS, Shen D, Xiong MJ, Yang FF. Five-year Clinical Outcomes of CAD Patients Complicated with Diabetes after StentBoost-optimized Percutaneous Coronary Intervention. Chin Med Sci J. 2019;34:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Zhang J, Duan Y, Jin Z, Wei Y, Yang S, Luo J, Ma D, Jing L, Liu H. Stent boost subtract imaging for the assessment of optimal stent deployment in coronary ostial lesion intervention: comparison with intravascular ultrasound. Int Heart J. 2015;56:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Arboix A, García-Eroles L, Oliveres M, Comes E, Sánchez MJ, Massons J. Malignant middle cerebral artery infarction: a clinical study of 32 patients. Rev Invest Clin. 2015;67:64-70. [PubMed] |