Published online Jul 16, 2023. doi: 10.12998/wjcc.v11.i20.4937
Peer-review started: April 5, 2023
First decision: April 27, 2023
Revised: May 10, 2023
Accepted: June 19, 2023
Article in press: June 19, 2023
Published online: July 16, 2023
Processing time: 91 Days and 20 Hours
Ischemic colitis (IC) is common, rising in incidence and associated with high mortality. Its presentation, disease behavior and severity vary widely, and there is significant heterogeneity in therapeutic strategies and prognosis. The common causes of IC include thromboembolism, hemodynamic insufficiency, iatrogenic factors and drug-induced. However, contrast-induced IC, especially isolated right colon ischemia is rarely reported.
A 52-year-old man was admitted to the hospital due to intermittent chest distress accompanied by palpitation. Coronary angiography was performed using 60 mL of the iodinated contrast agent iohexol (Omnipaque 300), and revealed moderate stenosis of the left anterior descending artery and right coronary artery. At 3 h post-procedure, he complained of epigastric pain without fever, diarrhea and vomiting. Vital signs remained normal. An iodixanol-enhanced abdominal computed tomography (CT) scan revealed thickening, edema of the ascending and right transverse colonic wall and inflammatory exudate, without thrombus in mesenteric arteries and veins. Following 4 days of treatment with antibiotic and supportive management, the patient had a quick and excellent recovery with disappearance of abdominal pain, normalization of leucocyte count and a significant decrease in C reactive protein. There was no recurrence of abdominal pain during the patient's two-year follow-up.
This case emphasizes that contrast-induced IC should be considered in the differential diagnosis of unexplained abdominal pain after a cardiovascular interventional procedure with the administration of contrast media. Timely imaging evaluation by CT and early diagnosis help to improve the prognosis of IC.
Core Tip: Ischemic colitis (IC) is the most frequently encountered intestinal ischemia and is associated with high mortality. Here, we describe a case of isolated right colon ischemia in a 52-year-old gentleman following a diagnostic coronary angiography that involved 60 mL of iohexol (Omnipaque 300). This case highlights that clinicans should be aware of contrast-induced IC and considered in the differential diagnosis of unexplained abdominal pain after a cardiovascular interventional procedure with the administration of contrast media. Risk stratification should be carried out as soon as possible based on clinical characteristics to ensure appropriate treatment strategies.
- Citation: Qiu H, Li WP. Contrast-induced ischemic colitis following coronary angiography: A case report. World J Clin Cases 2023; 11(20): 4937-4943
- URL: https://www.wjgnet.com/2307-8960/full/v11/i20/4937.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i20.4937
The most commonly encountered intestinal ischemia is ischemic colitis (IC), which results from diminishing blood flow to the bowel wall. In the event of ischemic injury, there can be a variety of manifestations, including superficial injury, full-thickness necrosis and perforation of the colonic wall. Any change in systemic circulation or mesenteric vascular anatomy and function can lead to IC. Common causes of IC can be categorized into hemodynamic insufficiency (often as a result of arrhythmia, heart failure, shock), thromboembolic, iatrogenic and drug-induced. Generally, IC can affect any part of the colon. However, two-thirds of all cases occur in the left colon due to its anatomical location. Isolated right colon ischemia (IRCI) is rare and occurs in around 10%. Here, we report a case of contrast-induced IC post-coronary angiography with spontaneous recovery.
A 52-year-old male patient was admitted due to 3 wk of intermittent chest distress accompanied by palpitation, but no chest pain, abdominal pain, dizzy and amaurosis.
Coronary computed tomography angiography performed using iohexol before admission showed local severe stenosis at the proximal and middle of the left anterior descending artery (LAD) and moderate stenosis at the middle of right coronary artery (RCA). The total coronary artery calcification score was 1009.9. The patient was referred to our hospital for further evaluation and treatment.
He had a prior history of cigarette smoking, arterial hypertension, old cerebral infarction, hyperlipidemia and hyperuricemia. He had been prescribed levamlodipine besylate, bisoprolol fumarate and atorvastatin for 4 years. He denied a history of alcohol consumption, type 2 diabetes mellitus, arrhythmia, chronic constipation, chronic abdominal pain, abdominal surgery and trauma, and other drug use.
He had no family history of cardiovascular and digestive system diseases.
The patient’s clinical condition was relatively good with normal blood pressure of 125/72 mmHg and heart rate of 67 bpm. There were no abnormal physical findings.
The basic laboratory values were normal with the exception of elevated plasma D-dimer of 3.14 µg/mL (normal range: 0-0.55 µg/mL). Blood leucocyte count was 8620/µL (normal range: 3500-9500/µL), hemoglobin was 151 g/dL (normal range: 130-175 g/dL) and platelet count was 311000/µL (normal range: 125000-350000/µL). Serum C-reactive protein (CRP) level was 0.52 mg/L (normal range: 0-8 mg/L).
The 12-lead electrocardiogram (ECG), echocardiography and abdominal ultrasonography were normal. On the second day of admission, coronary angiography (CAG) was performed via right radial artery access and revealed diffuse stenosis of 50%-70% from the proximal to middle segment of the LAD and localized stenosis of 50%-70% at the middle segment of the RCA (Figure 1). A total of 60 mL of iohexol (Omnipaque 300), a low-osmolar nonionic iodinated contrast agent, was administered during the procedure. The patient was also given local anesthetic (1% lignocaine) prior to CAG and standard 3000 IU heparin intra-arterially during CAG, which were completed uneventfully. We did not perform any coronary intervention according to the CAG results. Vital signs during and immediately after CAG were normal.
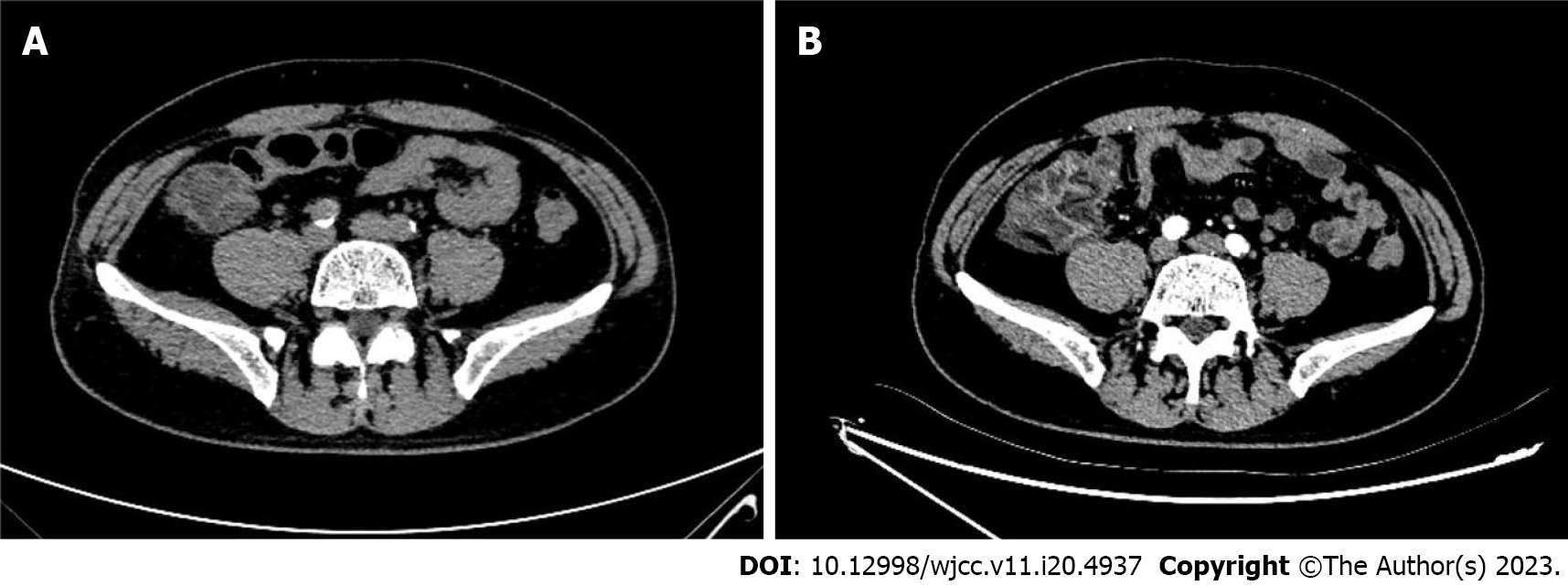
At 3 h post-CAG, the patient complained of epigastric pain and nausea without chest pain, chest tightness, vomiting, rash and pruritus. Vital signs were totally stable (temperature, 36.5℃; blood pressure, 118/62 mmHg; heart rate, 54 bpm; respiratory rate, 12 breaths/min; O2 saturation, 98% in room air). Abdominal examination showed normal bowel sounds and upper abdominal tenderness but without any peritoneal signs. ECG remained normal without any change. The patient was administered oral pantoprazole and an intramuscular injection of anisodamine in a 10-mg dose. However, his abdominal pain gradually worsened. His hemodynamic and respiratory parameters were still within the normal range without any signs of hypoperfusion. His laboratory results after CAG revealed leukocytosis (19710/µL) with neutrophilia (17580/µL, normal range: 1800-6300/µL). Hemoglobin of 14.6 g/dL, platelets of 272000/µL and CRP of 0.71 mg/L remained normal. Myocardial enzymes, amylase, lipase and lactate dehydrogenase (LDH) were also normal. Fecal occult blood test was positive. An emergency abdominal computed tomography (CT) scan (Figure 2A) revealed a thickened colonic wall corresponding to the ascending and right transverse colon segments, without obvious stenosis and without dilation of the proximal segments, accompanied by multiple exudative changes indicating inflammatory lesions. The patient was started on water deprivation, abrosia, intravenous levofloxacin 500 mg associated with rehydration by intravenously administered crystalloids. Antiplatelet drugs were stopped.
On the 2nd day after CAG, the patient complained that abdominal pain transferred to the right epigastric and subxiphoid without fever, diarrhea and vomiting. His vital signs were still stable. Physical examination showed tenderness, tension and rebound pain in the right upper abdomen and below the xiphoid. Antibiotics were replaced with latamoxef (4 g/d). On the 3rd day after CAG, an abdominal CT scan with intravenous iodixanol of 100 mL demonstrated that thickening, edema of the ascending and right transverse colonic wall and the inflammatory exudate became more serious (Figure 2B and Figure 3). There was no thrombus in the superior and inferior mesenteric arteries and veins (Figure 4). Laboratory studies revealed leukocytosis (17810/µL) with neutrophilia (14230/µL), elevated CRP (68.79 mg/dL) at 8-times the upper limit of normal and elevated D-dimer (4.3 µg/mL). Fecal occult blood test was still positive.
No arterial or venous thrombosis was detected in the colonic vascularization by CT scan, and the clinical manifestation was supportive of a local inflammatory process and diagnosis of IC. Furthermore, the 48-h dynamic electrocardiogram showed sinus rhythm, ventricular premature beats with no dynamic ST-T changes and atrial fibrillation. No abnormal shunt and thrombus were found on echocardiography and the transcranial Doppler (TCD) bubble test was negative. Color Doppler ultrasound examination of the lower extremities showed no thrombosis. Anticardiolipin antibody was negative.
On the 3rd day after CAG, the patient had a gradual reduction of abdominal pain. On the fourth day after CAG, the patient's abdominal pain changed from persistent pain to paroxysmal pain. Following 4 days of treatment with antibiotic and supportive management, the patient had a quick and excellent recovery with disappearance of abdominal pain on the fifth day after CAG, normalization of leucocyte count, a significant decrease in CRP (19.21 mg/dL) and D-dimer (2.27 µg/mL).
One week after CAG, the patient was discharged without any further instructions regarding antibiotic therapy or IC medication. The patient did not experience recurrence of abdominal pain during the 2-year follow-up period.
Ischemic bowel disease (ISBODI) is the most common vascular disorder affecting the gastrointestinal tract. Insufficient supply of oxygenated blood to the bowel wall will lead to ISBODI and there are three main clinical types, including chronic mesenteric ischemia, acute mesenteric ischemia and IC. The morbidity and mortality associated with ISBODI is high if it is not diagnosed early or timely treated. Notably, the incidence of IC was reported to be approximately 6.1 cases per 100000 person-year in 1976-1980 after adjusting for age and gender, and increased by approximately 4-fold to 22.9/100000 person-year in 2005-2009[1]. To the best of our knowledge, IC following diagnostic CAG, such as in our case, has not previously been reported.
The common causes of IC include thromboembolism, hemodynamic insufficiency, iatrogenic factors and drug-induced. Other risk factors are as follows: Atrial fibrillation, hypertension, diabetes mellitus, hemodialysis, coronary artery disease, peripheral artery disease, severe dehydration, shock, congestive heart failure, chronic obstructive lung disease, rheumatic autoimmune diseases, irritable bowel syndrome, hereditary and acquired thrombophilia, and substance abuse[2]. Moreover, enterocolonic ischemia may also be caused by systemic or infectious vasculitis, colonic obstruction, fibromuscular dysplasia, amyloidosis and radiation. Interestingly, colonoscopy itself has been described in the literature as a cause of IC. Multiple factors can lead to IC after colonoscopy. During colonoscopy, patients may experience excessive intestinal movements and spasms that can lead to vasoconstriction. Other factors include usage of hyperosmotic laxatives for bowel preparation, long procedure time and overinflation with air during the procedure[3].
IC is commonly seen in the elderly population with mesenteric vascular atherosclerosis[4]. The middle-aged male patient in this case had multiple risk factors for IC, including hypertension, hyperlipidemia, smoking history and coronary artery disease. Iatrogenic factors should be excluded firstly as abdominal pain occurred only 3 h after CAG, which was performed via right radial artery access without further coronary interventional procedure. Therefore, the embolism caused by the interventional procedure can be ruled out. D-dimer was slightly elevated at admission, suggesting a hypercoagulable state. To avoid the occurrence of thrombosis, the patient was administered oral aspirin and clopidogrel before CAG, intravenous heparin during the procedure, and encouraged to drink more water after CAG. Abdominal enhanced CT confirmed that there was no evidence of mesenteric artery and vein thrombosis.
Hypotension or shock can also cause IC and mesenteric ischemia will occur if mean arterial pressure is above 45 mmHg or the blood supply is reduced by more than 50%. However, this patient’s vital signs remained normal. Besides, patients with IC often have cardiovascular risk factors and have a potential cardiac precipitant of thromboembolic IC (e.g., valvular abnormality or arrhythmia). Atrial fibrillation is the most common cause and occurred in 20% of patients with IC[5]. The patient denied a previous history of paroxysmal atrial fibrillation and there was no atrial fibrillation detected on the 48-h Holter monitor. The patient also had no deep venous thrombosis and no abnormal right-to-left (R–L) cardiac shunt verified by a combination of echocardiography and the TCD bubble test.
The time course of events suggested that the IC had been precipitated by the CAG procedure. IC has four pathophysiological manifestations: Superior mesenteric artery (SMA) thrombosis, SMA embolism, non-occlusive mesenteric ischemia (NOMI) and mesenteric venous thrombosis. It is known that NOMI is associated with mesenteric vasoconstriction due to systemic hypotension and low flow status in the splanchnic circulation. In this case, we considered the probability of NOMI. As the common causes including iatrogenic factors, hemodynamic insufficiency and thromboembolism were excluded, we can speculate that IC here was related to administration of the contrast agent, Omnipaque, which induced mesenteric artery spasm and local diminution of blood supply.
Potential drugs that cause IC reported in previous studies are wide-ranging and include vasopressors, chemotherapeutic agents, cocaine, estrogen, non-steroid anti-inflammatory drugs, amphetamines, antipsychotics, sergotamine and others[6,7]. These agents should be specifically considered when collecting medical history from patients with suspected IC. Omnipaque, which is a second-generation nonionic contrast agent, is eliminated without any significant metabolism or transformation by renal excretion. In most cases of contrast-induced adverse reactions, the prognosis is excellent, with rapid recovery after supportive management only. In general, relevant symptoms appear within minutes to hours after contrast administration and recover spontaneously within 72 h, as the contrast agent is cleared by the kidneys. Another concept worth mentioning here is anaphylactic reaction to contrast agent. Nonionic and water-soluble agents, such as Omnipaque, although considered to be less toxic are capable of inducing anaphylactic reactions. Moreover, Omnipaque can induce changes in intravascular pressure and endothelial permeability. Recently, Omnipaque was reported to induce pituitary apoplexy[8], status epilepticus[9], immune hemolytic anemia[10], trigeminocardiac reflex[11] and encephalopathy[12] in some rare cases. In this case, Omnipaque-induced IC remains the most likely diagnosis based on the complete resolution of symptoms with antibiotic therapy and supportive management only.
Another striking feature of this case is IRCI. Blood is supplied to three watershed zones in the colon by two large arteries at their distal branches. When the blood supply is reduced, these watershed zones are prone to develop non-occlusive IC since they have the fewest vascular collaterals. Colonic ischemia can affect any part of the colon but the left colon, particularly the splenic flexure, is involved in two-thirds of patients[13]. Most often, the descending colon and sigmoid colon are involved. It is estimated that 10% of cases result in IRCI. In this case, IRCI might have been caused by local contraction of vessels that supply the right colon. Furthermore, the marginal artery is underdeveloped in the right colon in up to 50% of cases[14], which may explain why the right colon is fragile in low flow status and why some patients are particularly prone to have right-side involvement.
IC is mostly a benign and self-limiting disease in most cases. The most common symptoms are abdominal pain (87%), rectal bleeding (84%) and diarrhea (56%). Hypogastric pain is more commonly observed than hematochezia in patients with right colon ischemia[15], similar to this patient's clinical symptoms. Laboratory diagnostic criteria are not available for IC. Complete blood count, coagulation tests, serum lactate, LDH, creatinine phosphokinase and amylase, and complete metabolic profile can contribute to determining the severity of colonic ischemia. The prominent manifestation in this case was the obvious increase in white blood cell count and CRP. As a frequently misdiagnosed condition, it is often picked up as part of a work-up for ‘acute abdomen’, which usually recommends CT. CT should be performed with intravenous contrast when renal function allows, especially in cases with suspected right-sided colonic ischemia (RSCI) or when the diagnosis of acute mesenteric ischemia needs to be ruled out. Patients with IC often present imaging characteristics of colitis, for example pericolic fat stranding and intestinal wall thickening. Timely abdominal CT examination in this case contributed to the early diagnosis of IC. Colonoscopy with biopsy is the next step to confirm the diagnosis of IC. As there is evidence to show that the diagnostic yield reduces over time, early endoscopy is recommended within the first 48 h. Considering that the symptoms in this patient improved significantly 24 h after diagnosis and antiplatelet drugs were taken before CAG, further colonoscopy was not performed.
In clinical practice, IC is mostly managed with supportive care, but risk stratification is crucial to determine whether only supportive care or surgical intervention is needed. The American College of Gastroenterology in 2015 recommended that IC can be categorized into mild, moderate, and severe disease according to the presence or absence of certain risk factors associated with poor outcomes[16]. These risk factors include: (1) Male; (2) Abdominal pain without rectal bleeding; (3) Hypotension (systolic blood pressure less than 90 mmHg); (4) Tachycardia (heart rate > 100 bpm); (5) Leukocytosis (white blood cell count above 15000/µL; (6) Anemia (hemoglobin below 12 g/dL); (7) High serum LDH level (more than 350 units/L) and hyponatremia (less than 136 meq/L); and (8) Azotemia (blood urea nitrogen more than 20 mg/dL. The absence of rectal bleeding and right-sided colonic involvement are the most frequently cited factors for predicting poor prognosis of IC[17,18]. IC patients usually recover within 1 to 2 wk and the overall mortality is approximately 10%. The thrust of monitoring for response to treatment should include careful monitoring of vital signs and frequent clinical observations (including abdominal examination)[19-21]. In this study, the patient had only one risk factor (leukocytosis) but isolated right-sided colonic involvement. His rapid and excellent recovery was most likely due to the absence of persistent mesenteric artery ischemia.
In summary, we report a rare case of IC precipitated by iohexol (Omnipaque) administration following CAG. Clinicians should be aware of contrast-induced IC in the differential diagnosis of unexplained abdominal pain after contrast administration. Moreover, timely imaging evaluation with CT and early diagnosis help to improve the prognosis of IC. Risk stratification should be carried out as soon as possible based on clinical characteristics to ensure appropriate treatment strategies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Dias E, Portugal; Fusaroli P, Italy; Zhang X, United States S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | Yadav S, Dave M, Edakkanambeth Varayil J, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sweetser SR, Melton LJ 3rd, Sandborn WJ, Loftus EV Jr. A population-based study of incidence, risk factors, clinical spectrum, and outcomes of ischemic colitis. Clin Gastroenterol Hepatol. 2015;13:731-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (2)] |
| 2. | Acosta S, Alhadad A, Svensson P, Ekberg O. Epidemiology, risk and prognostic factors in mesenteric venous thrombosis. Br J Surg. 2008;95:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 3. | Mahajan A, Musunuri B, Shetty S. Colonoscopy induced ischemic colitis: An endoscopic and histological assay. Clin Res Hepatol Gastroenterol. 2022;46:101975. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Greenwald DA, Brandt LJ, Reinus JF. Ischemic bowel disease in the elderly. Gastroenterol Clin North Am. 2001;30:445-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Hourmand-Ollivier I, Bouin M, Saloux E, Morello R, Rousselot P, Piquet MA, Dao T, Verwaerde JC. Cardiac sources of embolism should be routinely screened in ischemic colitis. Am J Gastroenterol. 2003;98:1573-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Hass DJ, Kozuch P, Brandt LJ. Pharmacologically mediated colon ischemia. Am J Gastroenterol. 2007;102:1765-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Longstreth GF, Yao JF. Diseases and drugs that increase risk of acute large bowel ischemia. Clin Gastroenterol Hepatol. 2010;8:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Skljarevski V, Khoshyomn S, Fries TJ. Pituitary apoplexy in the setting of coronary angiography. J Neuroimaging. 2003;13:276-279. [PubMed] |
| 9. | Alimohammadi H, Abdalvand A, Safari S, Mazinanian A. Status epilepticus after myelography with iohexol (Omnipaque). Am J Emerg Med. 2012;30:2092.e1-2092.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Al Ghailani HH, Al Alawi AM, Al Hashim AH. Contrast Media-Induced Immune Hemolytic Anemia. Cureus. 2021;13:e14522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Tamura T, Rex DE, Marosfoi MG, Puri AS, Gounis MJ, Wakhloo AK. Trigeminocardiac reflex caused by selective angiography of the middle meningeal artery. J Neurointerv Surg. 2017;9:e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Dattani A, Au L, Tay KH, Davey P. Contrast-Induced Encephalopathy following Coronary Angiography with No Radiological Features: A Case Report and Literature Review. Cardiology. 2018;139:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Gandhi SK, Hanson MM, Vernava AM, Kaminski DL, Longo WE. Ischemic colitis. Dis Colon Rectum. 1996;39:88-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 190] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med. 2003;70:920-921, 925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Washington C, Carmichael JC. Management of ischemic colitis. Clin Colon Rectal Surg. 2012;25:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Oglat A, Quigley EM. Colonic ischemia: usual and unusual presentations and their management. Curr Opin Gastroenterol. 2017;33:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Choi SR, Jee SR, Song GA, Park SJ, Lee JH, Song CS, Park HU. Predictive Factors for Severe Outcomes in Ischemic Colitis. Gut Liver. 2015;9:761-766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Ten Heggeler LB, van Dam LJ, Bijlsma A, Visschedijk MC, Geelkerken RH, Meijssen MA, Kolkman JJ. Colon ischemia: Right-sided colon involvement has a different presentation, etiology and worse outcome. A large retrospective cohort study in histology proven patients. Best Pract Res Clin Gastroenterol. 2017;31:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Añón R, Boscá MM, Sanchiz V, Tosca J, Almela P, Amorós C, Benages A. Factors predicting poor prognosis in ischemic colitis. World J Gastroenterol. 2006;12:4875-4878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 20. | Cosme A, Montoro M, Santolaria S, Sanchez-Puertolas AB, Ponce M, Durán M, Cabriada JL, Borda N, Sarasqueta C, Bujanda L. Prognosis and follow-up of 135 patients with ischemic colitis over a five-year period. World J Gastroenterol. 2013;19:8042-8046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Hung A, Calderbank T, Samaan MA, Plumb AA, Webster G. Ischaemic colitis: practical challenges and evidence-based recommendations for management. Frontline Gastroenterol. 2021;12:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |