Published online Jan 16, 2023. doi: 10.12998/wjcc.v11.i2.464
Peer-review started: November 6, 2022
First decision: November 16, 2022
Revised: November 18, 2022
Accepted: January 3, 2023
Article in press: January 3, 2023
Published online: January 16, 2023
Processing time: 66 Days and 16.5 Hours
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccinations have been administered worldwide, with occasional reports of associated neurological complications. Specifically, the impact of vaccinations on individuals with X-linked Charcot-Marie-Tooth disease type 1 (CMTX1) is unclear. Patients with CMTX1 can have stroke-like episodes with posterior reversible encephalopathy syndrome on magnetic resonance imaging (MRI), although this is rare.
A 39-year-old man was admitted with episodic aphasia and dysphagia for 2 d. He received SARS-CoV-2 vaccination 39 d before admission. Physical examination showed pes cavus and reduced tendon reflexes. Brain MRI showed bilateral, symmetrical, restricted diffusion with T2 hyperintensities in the cerebral hemispheres. Nerve conduction studies revealed peripheral nerve damage. He was diagnosed with Charcot-Marie-Tooth disease, and a hemizygous mutation in the GJB1 gene on the X chromosome, known to be pathogenic for CMTX1, was identified. Initially, we suspected transient ischemic attack or demyelinating leukoencephalopathy. We initiated treatment with antithrombotic therapy and immunotherapy. At 1.5 mo after discharge, brain MRI showed complete resolution of lesions, with no recurrence.
SARS-CoV-2 vaccination could be a predisposing factor for CMTX1 and trigger a sudden presentation.
Core Tip: We present a case report of a young man who presented with episodic aphasia and dysphagia after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination. After a complete neurological evaluation, nerve conduction study, and DNA analysis, we diagnosed the patient with Charcot-Marie-Tooth disease type 1 (CMTX1). CMTX1 can occur after SARS-CoV-2 vaccination, and thus SARS-CoV-2 vaccination should be considered a potential predisposing factor for CMTX1. There is paucity of information on the neurological consequences of SARS-CoV-2 vaccination, even though billions of vaccines have been administered worldwide. We believe that our study makes a significant contribution to the literature based on the continued urgency of the coronavirus disease 2019 pandemic.
- Citation: Zhang Q, Wang Y, Bai RT, Lian BR, Zhang Y, Cao LM. X-linked Charcot-Marie-Tooth disease after SARS-CoV-2 vaccination mimicked stroke-like episodes: A case report. World J Clin Cases 2023; 11(2): 464-471
- URL: https://www.wjgnet.com/2307-8960/full/v11/i2/464.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i2.464
Charcot-Marie-Tooth disease (CMT) is an inherited neuropathy that mainly affects the motor and sensory fibers of the peripheral nervous system. The prevalence of CMT is 1/2500[1,2]. X-linked CMT type 1 (CMTX1) results from mutations in the GJB1 gene on chromosome Xq13.1[3,4] and is the second most common form of CMT[5,6]. A small number of patients with CMTX1 present with episodic neurological dysfunction and reversible white matter lesions, which have not been adequately reported.
Billions of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccinations have been administered worldwide, and there have been occasional reports of central nervous system (CNS) complications caused by the vaccination[7]. In a study of 704300 subjects receiving the first dose of an mRNA SARS-CoV-2 vaccination, 65% had at least one neurologic manifestation, including headache (62.2%), transient paresthesia (3.5%), and weakness (1%). There were also reports of serious adverse events, including seizures, Guillain-Barré syndrome, and transverse myelitis[7]. Some patients experienced severe neurological complications after vaccination, including cerebral venous sinus thrombosis, CNS demyelinating diseases, inflammatory peripheral neuropathies, and limbic encephalitis[8]. The impact of vaccination on individuals with CMTX1 is unclear at present.
In this report, we present the case of a patient with CMTX1 and stroke-like episodes after SARS-CoV-2 vaccination with reversible splenial lesion syndrome (RESLES). We have reviewed the relevant literature to raise attention to the impact of SARS-CoV-2 vaccination on CMTX1 expression.
A 39-year-old man presented with three episodes of acute-onset aphasia and dysphagia over 2 d in June 2021.
The patient was vaccinated with an inactivated SARS-CoV-2 vaccine (Beijing Institute of Biological Products Co., Ltd., Beijing, China)[9]. He received the first dose of the vaccine 2 mo before admission and the second dose 39 d before admission. Two days before admission, the patient reported aphasia and dysphagia for 1 h. Later that night, he had a second episode with similar symptoms as well as dyspnea, which lasted for approximately 1 h. Asyndesis, dysphagia, and dyspnea developed over 3 h on the morning of admission, which prompted his visit to our emergency department.
He had eczema and renal calculus for many years, and his eczema recurred after the second vaccine. He denied any history of major trauma or toxin exposure.
He has three brothers, one with pes cavus and another with pes cavus and CMT. His parents denied any history of hereditary diseases. His mother had died of myocardial infarction.
Physical examination showed that the patient was alert and had demonstrated weakness in chewing and swallowing, difficulty in tongue thrusting, difficulty in lifting the soft palate, diminished tendon reflexes of the limbs, mild atrophy of the muscles of the hands and distal leg, and bilateral pes cavus. There were no other positive neurological signs.
Blood analysis after admission showed that erythrocyte sedimentation rate, serum creatinine, electrolyte, glycosylated hemoglobin, and D-dimer levels were within normal limits. Laboratory tests showed slightly increased white blood cell count (11.75 × 109/L; reference range: 3.5–9.5 × 109/L), hepatitis B surface antigen levels (73.05 IU/mL; reference range: 0–0.03 IU/mL), antistreptolysin-O titers (218.00 IU/mL; reference range: 0–200 IU/mL), and total cholesterol levels (5.64 mmol/L; reference range: < 5.2 mmol/L).
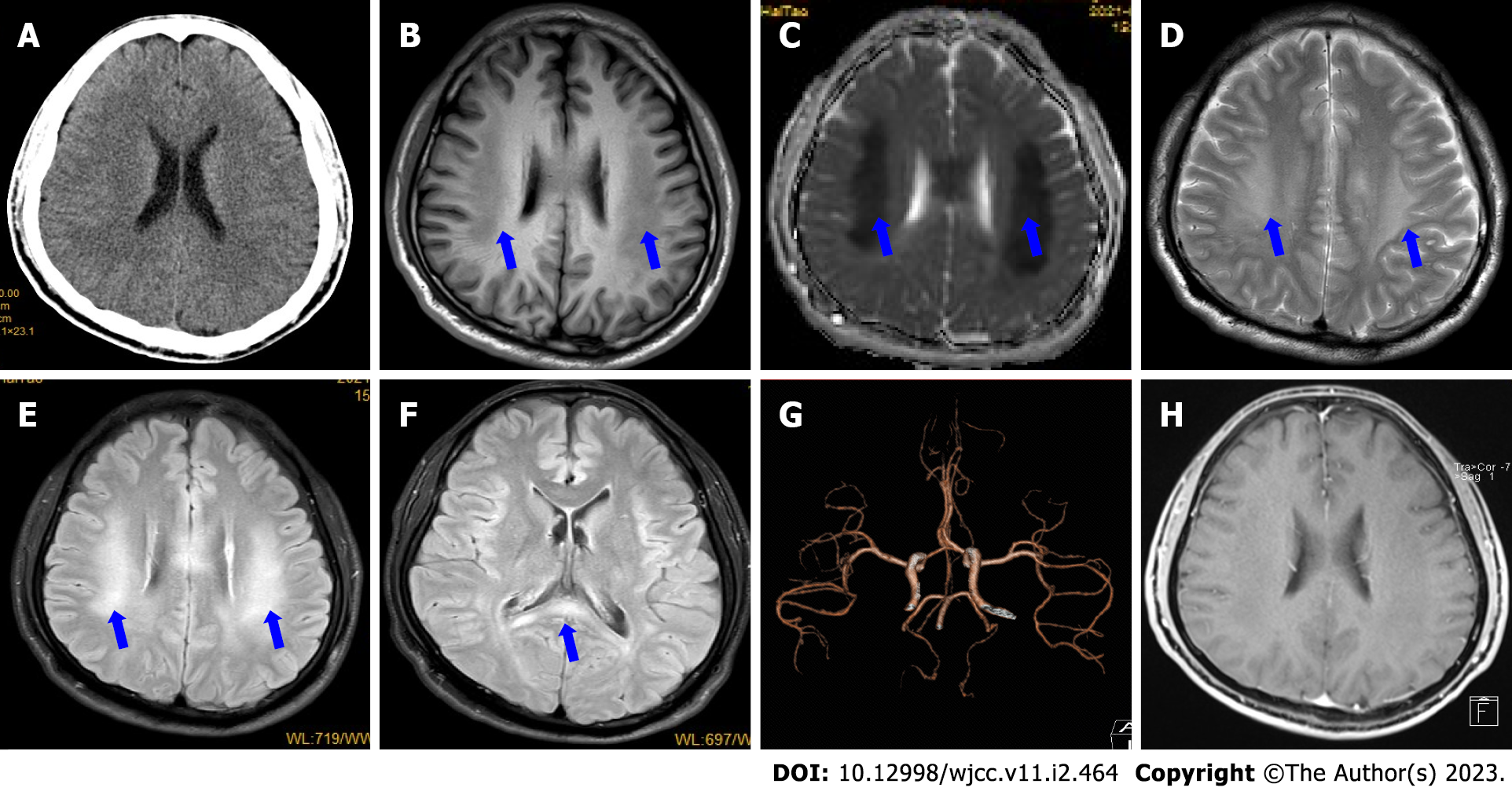
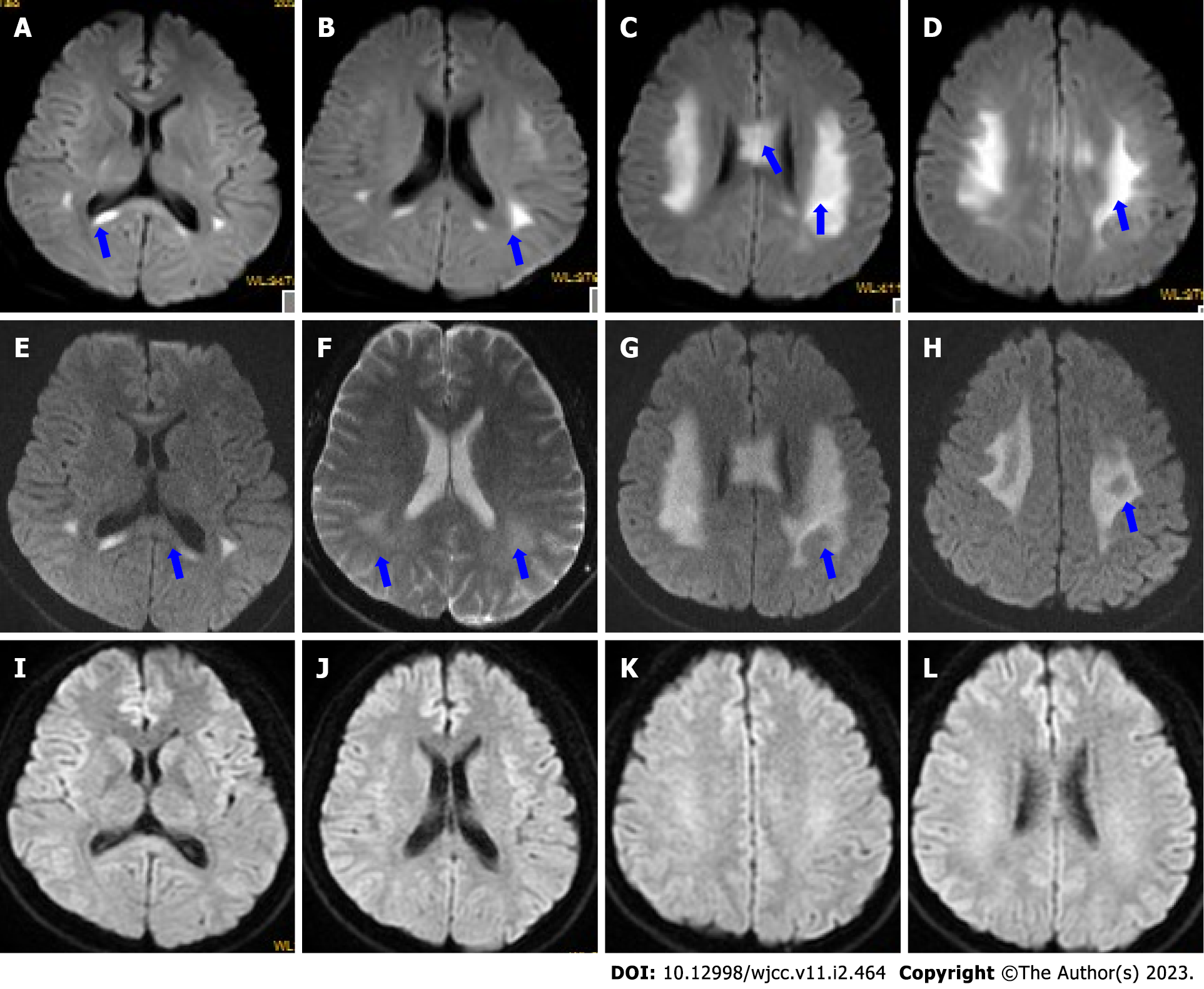
Initial head computed tomography (CT) showed no obvious abnormalities (Figure 1A). Brain magnetic resonance imaging (MRI) (Figure 1B-F) on day 2 showed bilateral, symmetrical, and restricted diffusion (Figure 2A-D), with T2 hyperintensities in the corpus callosum and supratentorial white matter. The neostigmine test, repetitive nerve stimulation, chest CT, carotid artery Doppler ultrasound, echocardiography, transcranial Doppler, CT angiography (Figure 1G), and electroencephalography showed no obvious abnormalities. Contrast-enhanced transcranial Doppler revealed a small, natural, or consecutive right-to-left shunt. Transesophageal echocardiography also showed a small intracardiac right-to-left shunt. A 24-h dynamic electrocardiogram showed accidental atrial premature beats, paroxysmal ventricular tachycardia, and paroxysmal ST-segment change.
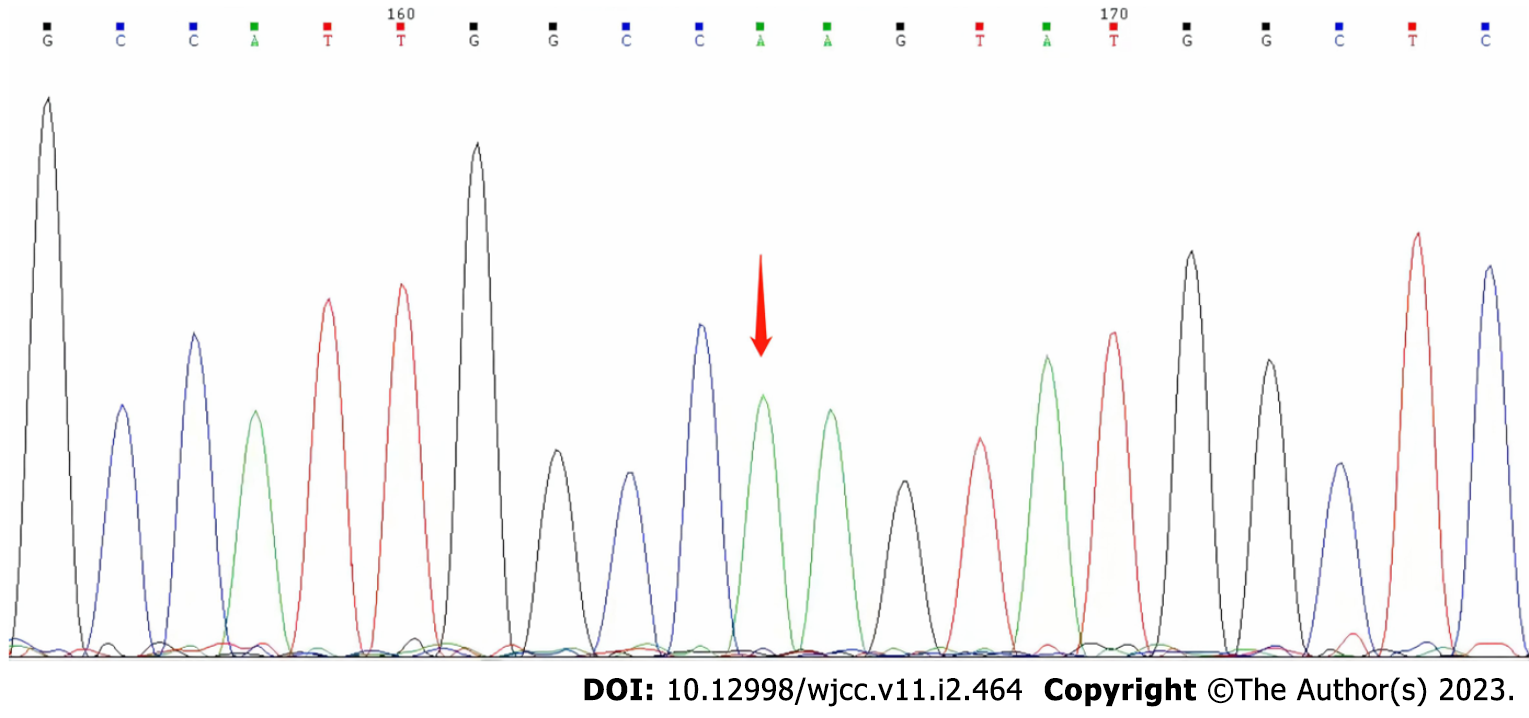
On day 25 after discharge, genetic sequencing (Beijing Golden Standard Medical Laboratory, Beijing, China) results showed a hemizygous mutation (c.65 G>A p.R22Q) in the GJB1 gene (Figure 3).
CMTX1.
Initially, we suspected that he was experiencing transient ischemic attack or demyelinating leukoencephalopathy at admission. We began immunotherapy [intravenous (IV) immunoglobulin 27.5 g/day and IV methylprednisolone 500 mg/day, followed by IV dexamethasone 10 mg/day for 5 d]; antiplatelets (aspirin and clopidogrel), improving cerebral metabolism (IV deproteinized calf serum extract[10,11]) and improving cerebral circulation (IV Erigeron breviscapus[12]); hypolipidemic agent (atorvastatin); and symptomatic therapy. His symptoms improved on the afternoon of admission. Brain MRI on day 4 showed that the lesions were not enhanced (Figure 1H), and the areas of restricted diffusion were obvious but not reduced (Figure 2E-H). Nerve conduction studies revealed prolonged compound muscle action potential, reduced amplitude, and slowed motor velocity in both the median (right 35.3 m/s; left 39.2 m/s) and tibial (right 31.1 m/s; left 33.4 m/s) nerves, and slowed sensory conduction velocity in both sural nerves. The results suggested both myelin dysfunction and axonal damage, especially in the distal limbs. Based on the clinical and laboratory examinations, we diagnosed him with CMT. He was discharged on day 7 and felt no discomfort with reduced tendon reflexes at discharge. He was satisfied with the treatment received, as well as his recovery.
At 1.5 mo after discharge, a follow-up appointment showed that he had no discomfort, and a repeat MRI showed complete resolution of the lesions (Figure 2I-L).
In this report, we presented the case of an older patient with CMTX1 with the primary symptom of repeated posterior circulation stroke-like episodes accompanied by RESLES, after a SARS-CoV-2 vaccination. We speculate that SARS-CoV-2 vaccination may be a predisposing factor for CMTX1, resulting from a hemizygous mutation in the GJB1 gene on chromosome Xq13.1. However, there are few research reports on this topic.
The most common predisposing factors for CMTX1 are infection or fever, high-altitude travel, and intense exercise[13]. However, this patient did not have any of these predisposing factors before the onset. We must consider the possibility that the SARS-CoV-2 vaccine may be associated with developing CMTX1, even though this potential relationship is not clear. However, we are highlighting a clinical research question to bring this possibility to broader attention.
Our patient had a relatively late onset of the disease after vaccination. Most patients have symptom onset within a short period after vaccination. Most neurological symptoms in patients (71.8%) with CNS demyelination occurred approximately 9 d after vaccination[14]. All patients who developed Guillain-Barré syndrome after the first dose of the vaccine (Dose 1) had a latency of 3−22 d[15]. Twenty-one cases with severe neurological complications were diagnosed within a median of 11 (range 3–23) d after SARS-CoV-2 vaccinations[8]. However, there are also some reports of later-onset cases after vaccination. A female patient with post-vaccination acute disseminated encephalomyelitis presented with a first seizure 1 mo after the second SARS-CoV-2 vaccine (Dose 2)[16]. There have been three cases of Bell’s palsy after SARS-CoV-2 vaccination that occurred on day 37 after Dose 1, day 32 after Dose 2, and day 48 after Dose 2, respectively[17]. Although most neurological symptoms appeared after Dose 1, some patients had onset after Dose 2. In another study, approximately 73% of patients (n = 8) developed a rash after SARS-CoV-2 vaccine Dose 1, and 27% of patients (n = 3) after the second dose[18]. Our patient had neurological side effects after Dose 2.
The mechanism of neurological events possibly caused by SARS-CoV-2 vaccination is not well-understood, but we speculate that it may be related to inflammation. The activation or reactivation of the immune system is thought to be the most likely cause[19]. Some experts believe cellular mechanisms may involve breaking gap junctions between oligodendrocytes and astrocytes, causing the inability of these cells to regulate fluid exchange[20]. All types of vaccines inevitably evoke immune responses. Consequently, some immune diseases recur or worsen after vaccination. In our patient, eczema recurred after the second dose of the vaccine. Additional reports of immune-related exacerbations include a report of a man with renal-limited microscopic polyangiitis, which recurred 5 wk following the first dose[21]. Fourteen patients with psoriasis experienced an exacerbation soon after the second vaccine[22]. There are reports of patients with varicella-zoster virus reactivation induced by Dose 2[23], as well as aggravation of cutaneous lupus erythematosus following the mRNA vaccine[24]. An intense immune response may cause aggravation of a pre-existing autoimmune disease and may even trigger de novo autoimmune diseases[24]. Immune disorders are closely related to neuropathies or neurological complications, especially immune-mediated neuropathies[25].
CMTX1 is highly heterogeneous, presenting initially with CNS symptoms, and can be misdiagnosed. The most common CNS manifestations of patients with CMTX1 include periodic dysarthria, ataxia, hemiparesis[26], dysphagia, facial or lingual weakness, and numbness, in addition to cranial nerve deficits, aphasia, chorea, dizziness, and lethargy. CNS symptoms manifest as facial-lingual paresis or limb weakness in 93.6% of patients, difficulty speaking or swallowing in 83.0%, hypoesthesia in 31.9%, and ataxia in 21.3%[13]. The presence of foot deformity or a characteristic consistent with X-linked inheritance increases the possibility of CMT.
The patient in this report, who was initially suspected of having demyelinating encephalopathy, began his treatment with immunotherapy. Interestingly, a report of 27 patients with CMTX1 showed that 14 (51.9%) received corticosteroid and/or IV immunoglobulin therapy after presenting CNS symptoms[13]. This suggests that we should not only distinguish CMTX1 from stroke but also from acute disseminated encephalomyelitis and adrenoleukodystrophy in clinical practice. It is important to perform a detailed family history evaluation and neurological examination. This patient had a strong family history, which is important for validating this diagnosis. A positive family history, weakened tendon reflexes, and pes cavus support a diagnosis of CMTX1.
While our patient was older, CMTX1 with transient CNS manifestations is typically a disorder that mainly affects children and adolescents[26]. This patient had reversible, bilateral, non-enhancing leukoencephalopathy and restricted diffusion on MRI, which presented with RESLES; these are characteristics of the CNS imaging phenotype of CMTX1[26]. Abnormal signals were most often found in deep brain white matter (88.9%) and the corpus callosum (80.0%) on MRI of patients with CMTX1 with episodic CNS deficits[13]. Mutations in connexin 32, produced from GJB1, are responsible for most CMTX1 cases[2].
We found that brain MRI abnormalities are one of the key features of patients with CNS involvement. After an attack, the MRI usually shows increased T2-weighted, fluid-attenuated inversion recovery (FLAIR), diffusion-weighted hyperintense signals in bilateral deep white matter, and the corpus callosum with sparing of the subcortical U fibers. We observed that the abnormal MRI signals reversed after an attack. CMTX1 should be considered another rare cause of RESLES. We observed the abatement of the clinical symptoms followed by the resolution of the T2/FLAIR abnormal signals. A repeated brain MRI showed that the lesions did not disappear but became even more obvious. Nerve conduction studies in this patient were consistent with the characteristics of CMT-related peripheral nerve damage, including prolonged latency, reduced amplitude, and slowed conduction velocity. Forearm nerve conduction velocities (NCV) are typically in the “intermediate” range of 30–40 m/s for males. An important signal is the median nerve, which has a motor NCV of 25−45 m/s and can be easily detected[13]. In male patients with CMT, it is often no more than 38 m/s in the median nerv[27].
There is currently no specific treatment for CMT, and treatment is mainly symptom-based, in addition to the avoidance and elimination of predisposing factors. The patient’s symptoms were rapidly relieved after immunotherapy, and there has been no recurrence to date. Therefore, immunotherapy may be an effective treatment for vaccine-related CMTX1 onset. The CNS phenotype of CMTX1 has a good prognosis for the patient’s CNS function[28], and recognition will avoid unnecessary tests and potentially harmful therapy.
CMT disease is a rare entity and thus its flare up from vaccination can happen in extremely few cases. It is not yet possible to draw conclusions about any significant association between SARS-CoV-2 vaccination and CMTX1. Similar cases and population cohorts should be scrutinized to ensure the constant evaluation of such risks.
CMTX1 can occur without warning after SARS-CoV-2 vaccination, and the vaccination should be considered a potential predisposing factor. This relationship requires further attention and research. CMTX1 can mimic stroke-like episodes, and RESLES is a feature of the MRI phenotype of CMTX1.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dragonieri S, Italy; Roy S, United States S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Saporta AS, Sottile SL, Miller LJ, Feely SM, Siskind CE, Shy ME. Charcot-Marie-Tooth disease subtypes and genetic testing strategies. Ann Neurol. 2011;69:22-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 397] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 2. | Lee MJ, Nelson I, Houlden H, Sweeney MG, Hilton-Jones D, Blake J, Wood NW, Reilly MM. Six novel connexin32 (GJB1) mutations in X-linked Charcot-Marie-Tooth disease. J Neurol Neurosurg Psychiatry. 2002;73:304-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Lu YY, Lyu H, Jin SQ, Zuo YH, Liu J, Wang ZX, Zhang W, Yuan Y. Clinical and Genetic Features of Chinese X-linked Charcot-Marie-Tooth Type 1 Disease. Chin Med J (Engl). 2017;130:1049-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Karadima G, Koutsis G, Raftopoulou M, Floroskufi P, Karletidi KM, Panas M. Four novel connexin 32 mutations in X-linked Charcot-Marie-Tooth disease. Phenotypic variability and central nervous system involvement. J Neurol Sci. 2014;341:158-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Wang Y, Yin F. A Review of X-linked Charcot-Marie-Tooth Disease. J Child Neurol. 2016;31:761-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Fridman V, Bundy B, Reilly MM, Pareyson D, Bacon C, Burns J, Day J, Feely S, Finkel RS, Grider T, Kirk CA, Herrmann DN, Laurá M, Li J, Lloyd T, Sumner CJ, Muntoni F, Piscosquito G, Ramchandren S, Shy R, Siskind CE, Yum SW, Moroni I, Pagliano E, Zuchner S, Scherer SS, Shy ME; Inherited Neuropathies Consortium. CMT subtypes and disease burden in patients enrolled in the Inherited Neuropathies Consortium natural history study: a cross-sectional analysis. J Neurol Neurosurg Psychiatry. 2015;86:873-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 263] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 7. | Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43:3-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 151] [Article Influence: 50.3] [Reference Citation Analysis (1)] |
| 8. | Kaulen LD, Doubrovinskaia S, Mooshage C, Jordan B, Purrucker J, Haubner C, Seliger C, Lorenz HM, Nagel S, Wildemann B, Bendszus M, Wick W, Schönenberger S. Neurological autoimmune diseases following vaccinations against SARS-CoV-2: a case series. Eur J Neurol. 2022;29:555-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 9. | Deng T, Nian XX, Zhang JY, Huang SH, Yang XM. Development and application of novel inactivated SARS-CoV-2 vaccines. Zhongguo Shengwuzhipinxue Zazhi. 2021;34:761-769. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 10. | Li W, Guo A, Sun M, Wang J, Wang Q. Neuroprotective Effects of Deproteinized Calf Serum in Ischemic Stroke. Front Neurol. 2021;12:636494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Peng Y. Effects of Deproteinized Calf Serum injection on neurological function and activities of daily living in patients with cerebral embolism caused by atrial fibrillation. Zhongguo Xunzheng Yixue Zazhi. 2018;10:425-427. |
| 12. | Li JG, Wang LQ, Yang XY, Chen Z, Lai LYW, Xu H, Liu JP. Chinese herbal medicine Dengzhan Xixin injection for acute ischemic stroke: A systematic review and meta-analysis of randomised controlled trials. Complement Ther Med. 2017;34:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Tian D, Zhao Y, Zhu R, Li Q, Liu X. Systematic review of CMTX1 patients with episodic neurological dysfunction. Ann Clin Transl Neurol. 2021;8:213-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Ismail II, Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J Neuroimmunol. 2022;362:577765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 15. | Min YG, Ju W, Ha YE, Ban JJ, Lee SA, Sung JJ, Shin JY. Sensory Guillain-Barre syndrome following the ChAdOx1 nCov-19 vaccine: Report of two cases and review of literature. J Neuroimmunol. 2021;359:577691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Lopez-Vidriero MT, Jacobs M, Clarke SW. The effect of isoprenaline on the ciliary activity of an in vitro preparation of rat trachea. Eur J Pharmacol. 1985;112:429-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Colella G, Orlandi M, Cirillo N. Bell's palsy following COVID-19 vaccination. J Neurol. 2021;268:3589-3591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 18. | Hoff NP, Freise NF, Schmidt AG, Firouzi-Memarpuri P, Reifenberger J, Luedde T, Bölke E, Meller S, Homey B, Feldt T, Jensen BEO, Keitel V, Schmidt L, Maas K, Haussmann J, Tamaskovics B, Budach W, Fischer JC, Buhren BA, Knoefel WT, Schneider M, Gerber PA, Pedoto A, Häussinger D, Grebe O, van Griensven M, Braun SA, Salzmann S, Rezazadeh A, Matuschek C. Delayed skin reaction after mRNA-1273 vaccine against SARS-CoV-2: a rare clinical reaction. Eur J Med Res. 2021;26:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Oonk NGM, Ettema AR, van Berghem H, de Klerk JJ, van der Vegt JPM, van der Meulen M. SARS-CoV-2 vaccine-related neurological complications. Neurol Sci. 2022;43:2295-2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 20. | Taylor RA, Simon EM, Marks HG, Scherer SS. The CNS phenotype of X-linked Charcot-Marie-Tooth disease: more than a peripheral problem. Neurology. 2003;61:1475-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | David R, Hanna P, Lee K, Ritchie A. Relapsed ANCA associated vasculitis following Oxford AstraZeneca ChAdOx1-S COVID-19 vaccination: A case series of two patients. Nephrology (Carlton). 2022;27:109-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Sotiriou E, Tsentemeidou A, Bakirtzi K, Lallas A, Ioannides D, Vakirlis E. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021;35:e857-e859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 23. | Santovito LS, Pinna G. A case of reactivation of varicella-zoster virus after BNT162b2 vaccine second dose? Inflamm Res. 2021;70:935-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Niebel D, Ralser-Isselstein V, Jaschke K, Braegelmann C, Bieber T, Wenzel J. Exacerbation of subacute cutaneous lupus erythematosus following vaccination with BNT162b2 mRNA vaccine. Dermatol Ther. 2021;34:e15017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Kieseier BC, Mathey EK, Sommer C, Hartung HP. Immune-mediated neuropathies. Nat Rev Dis Primers. 2018;4:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Al-Mateen M, Craig AK, Chance PF. The central nervous system phenotype of X-linked Charcot-Marie-Tooth disease: a transient disorder of children and young adults. J Child Neurol. 2014;29:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Zhang J, Huang S, Zhao H, Li X, Zhang R. The electrophysiological characteristics of the four most common genotypes of Charcot-Marie-Tooth disease. Zhonghua Shenjingke Zazhi. 2019;26-33. [DOI] [Full Text] |
| 28. | Santoro JD, Chitnis T. Strokelike Episodes in a Patient With Chronic Gait Abnormalities. JAMA Neurol. 2019;76:621-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |