Published online Jan 16, 2023. doi: 10.12998/wjcc.v11.i2.456
Peer-review started: October 17, 2022
First decision: November 25, 2022
Revised: December 8, 2022
Accepted: December 23, 2022
Article in press: December 23, 2022
Published online: January 16, 2023
Processing time: 86 Days and 16.9 Hours
Multicentric reticulohistiocytosis (MRH)/systemic lupus erythematosus (SLE) overlap syndrome is an uncommon disease in the clinic and is diagnosed through characteristic clinical manifestations, histopathology, and immunopathology. Here, we report the case of a 30-year-old woman with SLE who developed MRH.
A 30-year-old woman with a history of polyarthritis for the past 12 years had multiple skin nodules on her body for 10 years, including the sacrococcygeal area, dorsum of the hands, interphalangeal joint of the feet and sternoclavicular joint. The histopathology of a biopsy of the distal interphalangeal joint of the hands revealed granulomatous inflammation, fibrous hyperplasia with ground-glass degeneration, inflammatory cell exudation and focal necrosis. The immunohistochemical stains showed positive staining for CD68 and negative staining for S100 and acid-fast staining. The patient was diagnosed with SLE with MRH. Her symptoms were improved after a combined treatment of prednisone, hydroxychloroquine and cyclophosphamide.
MRH/SLE overlap syndrome is difficult to diagnose and treat. Cyclophosphamide may be an alternative choice for the treatment of MRH.
Core Tip: We present a case of systemic lupus erythematosus with multicentric reticulohistiocytosis that was reported for the second time. Multicentric reticulohistiocytosis/systemic lupus erythematosus overlap syndrome is an uncommon disease that is hard to diagnose and treat. This case illustrates how to diagnose and treat the comorbidities and the connection that exists between them. For treatment, cyclophosphamide may be an alternative choice for multicentric reticulohistiocytosis.
- Citation: Liu PP, Shuai ZW, Lian L, Wang K. Systemic lupus erythematosus with multicentric reticulohistiocytosis: A case report. World J Clin Cases 2023; 11(2): 456-463
- URL: https://www.wjgnet.com/2307-8960/full/v11/i2/456.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i2.456
Multicentric reticulohistiocytosis (MRH) is a rare, multisystem non-Langerhans cell disorder of unknown etiology that is characterized by erosive polyarthritis and skin papulonodular lesions. MRH is associated with underlying malignancy and autoimmune disease, including breast cancer, ovarian adenocarcinoma, melanoma, rheumatoid arthritis (RA), Sjogren’s syndrome, systemic lupus erythematosus (SLE) and multiple sclerosis[1,2]. Many cases are misdiagnosed as RA due to the manifestation of arthritis mutilans, including symmetric articular involvement, marginal erosion in bone and severe arthritis destruction. Herein, we report a case of SLE with MRH.
SLE is an autoimmune disease involving multiple organs and is associated with substantial morbidity. Active SLE may develop into a rare, difficult-to-diagnose macrophage activation syndrome (MAS) in both adults and pediatric patients[3,4]. MAS is a very severe, acute and potentially life-threatening condition that is considered a secondary form of hemophagocytic lymphohistiocytosis. It is also a serious complication of many rheumatic diseases and is associated with potential malignancy[5]. In addition to destruction of joints and skin, persistent high-grade fever, hepatosplenomegaly, lymphadenopathy and central nervous dysfunction are the main clinical manifestations, which should be used to differentiate from MRH[3,4]. There are no established treatment guidelines for the management of MRH because of its rarity and infrequency[6]. Our case of SLE with MRH is surpri
A 30-year-old woman with a history of polyarthritis for the past 12 years was accompanied by multiple skin nodules on her body for 10 years.
A 30-year-old woman with a history of polyarthritis for the past 12 years was accompanied by multiple skin nodules on her body for 10 years. Relevant tests observed leukopenia, hemolytic anemia, high titer of antinuclear antibody (ANA), positive anti-Sm antibody, RNP, SSA, SSB and lupus band test, and a low level of complement C3. The patient was diagnosed with SLE according to the 2019 European League Against Rheumatism and the American College of Rheumatology criteria[7]. The symptoms of polyarthritis improved after treatment with 60 mg prednisone and 10 mg leflunomide once a day. However, two years after the diagnosis, a peanut-sized nodule was noticed on the sacrococcygeal area of the patient. The nodule gradually increased in size and was accompanied by an ulceration and a purulence. The biopsy showed fibrous hyperplasia with infiltration of multinuclear giant cells and necrosis, which was in line with the secondary changes of epidermal cysts. Several new papulonodular lesions appeared involving the dorsum of the hands, interphalangeal joint of the feet, and sternoclavicular joint, occasionally with an ulceration. Hyperplasia of fibrous tissue with multinucleated giant cell infiltration and foam cell formation was observed in biopsy. However, the diagnosis of the nodule remains unknown.
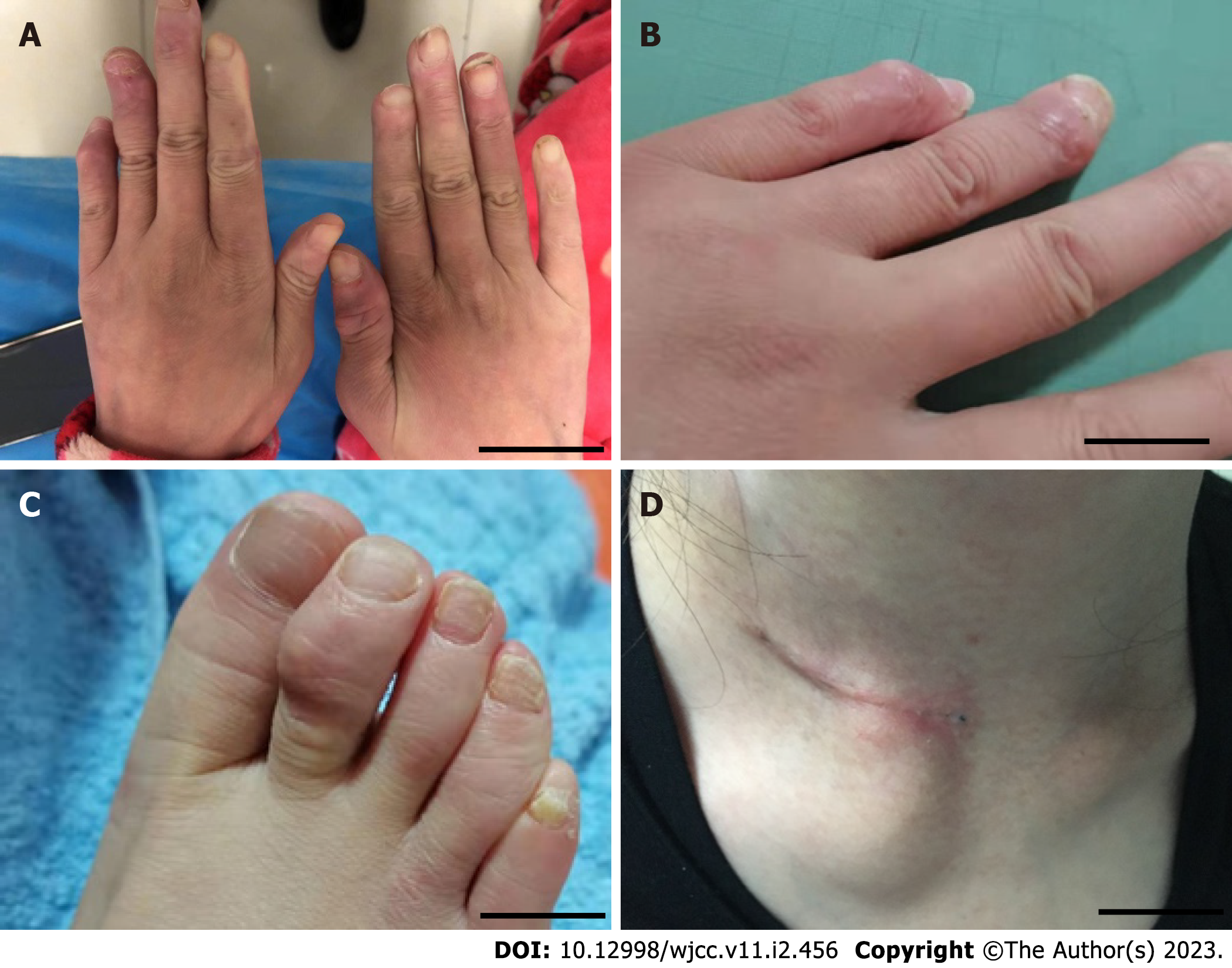
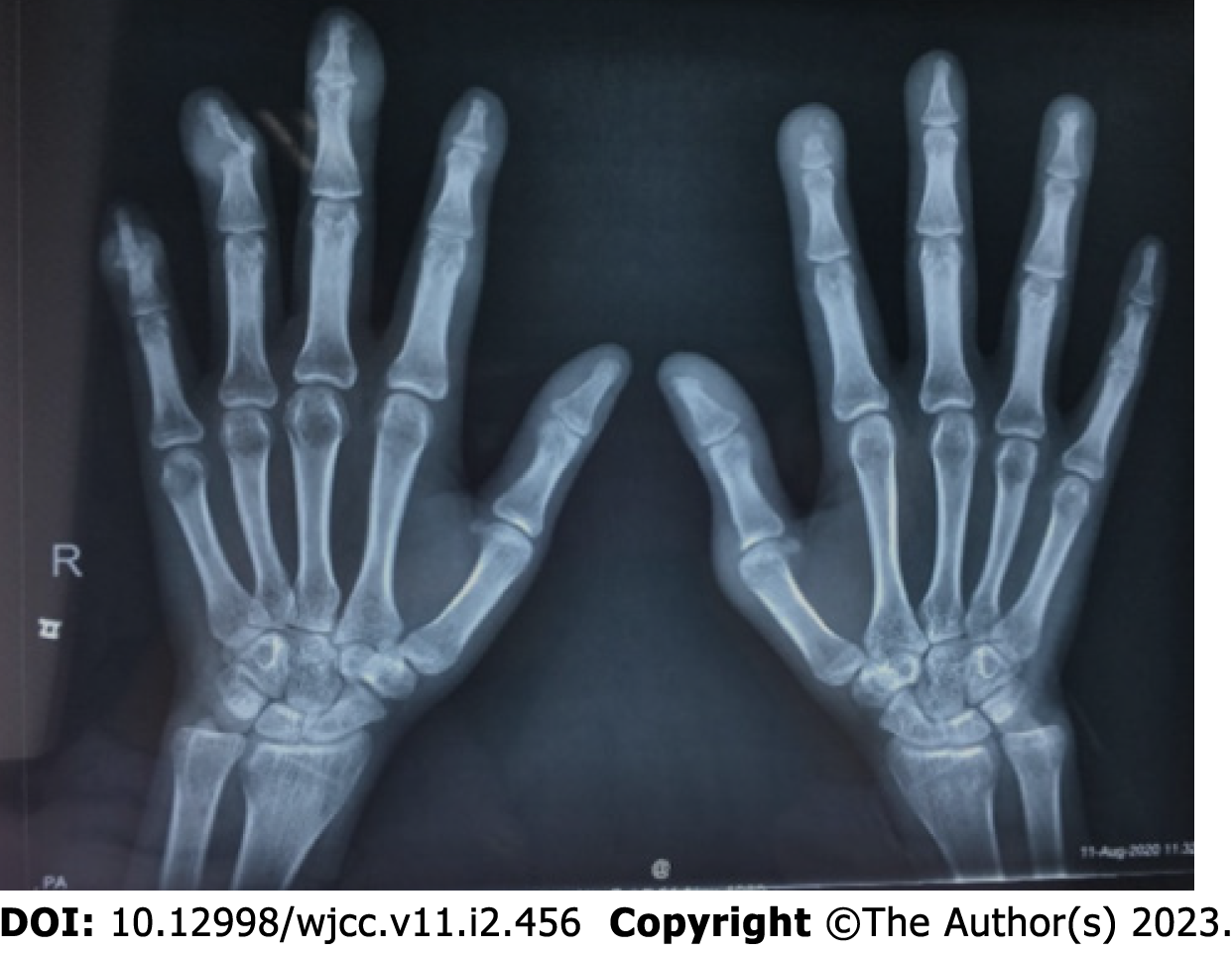
The patient was admitted to the hospital with new skin nodules on the distal interphalangeal of the hands and feet and a mass over the right sternoclavicular joint (Figure 1). The lesion is firm and has no tenderness. Laboratory tests showed hypersensitivity C-reactive protein (hs-CRP), and the erythrocyte sedimentation rate (ESR) was increased. The anti-cyclic citrullinated peptide (CCP) antibody titer was 10 RU/mL; furthermore, the tuberculosis infection T-lymphocyte spot test (T-SPOT) result was positive. ANA was strongly positive with a titer of 1:1000. A positive result was found for anti-centromere B antibody, anti-La/SS-B antibody, anti-SSA (52) and anti-SSA (60) antibody. X-ray of the hands showed bone erosion at the distal interphalangeal joints with the appearance of a pencil-in-cup deformity (Figure 2). Magnetic resonance imaging (MRI) of the hip joint revealed ischemic necrosis and synovitis in the left femoral head.
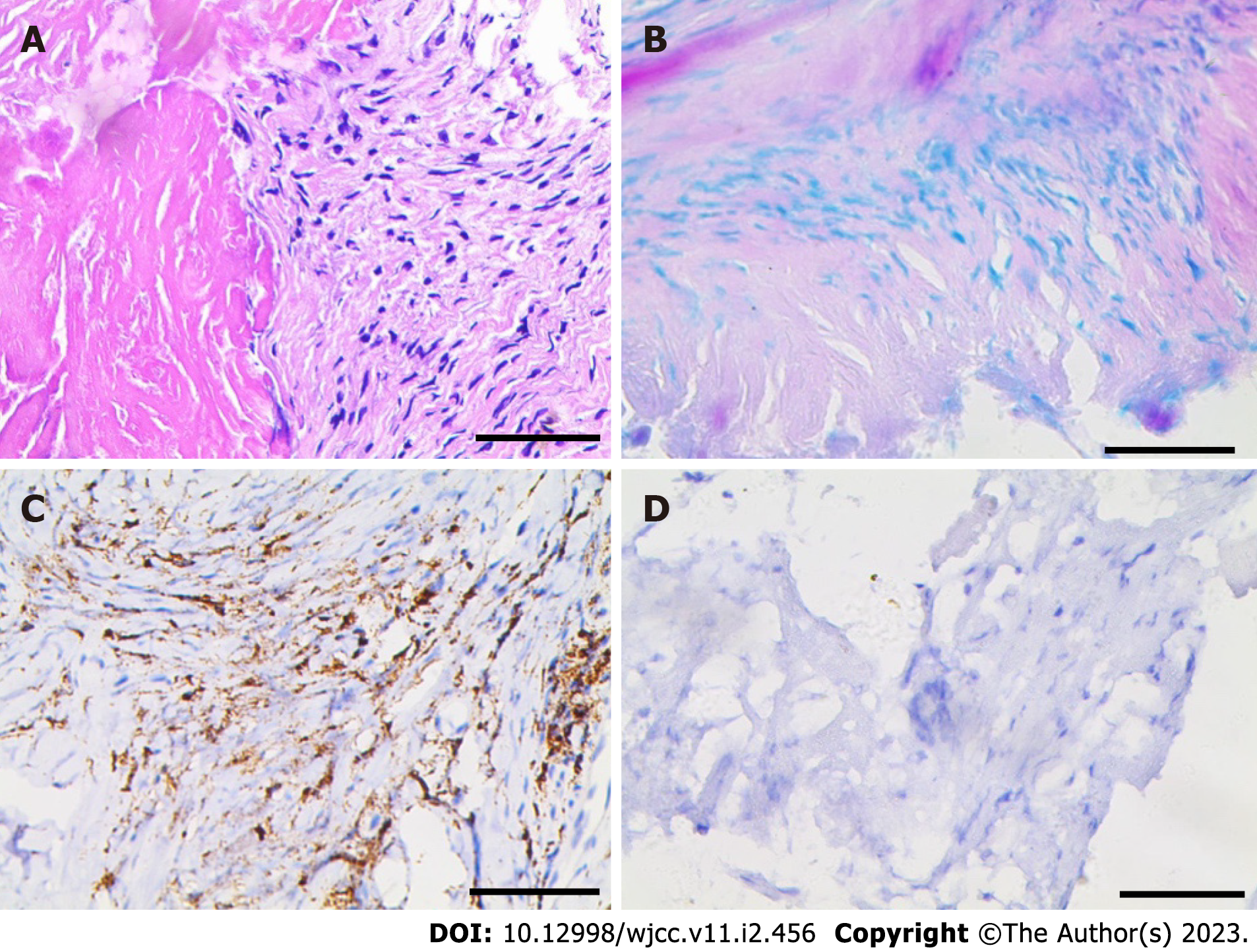
The biopsy of the nodules on the distal interphalangeal joint of the right hand revealed fibrous hyperplasia with ground-glass degeneration, inflammatory cell exudation and focal necrosis. Urate deposition was observed within the fibrous area. The immunohistochemical stains showed positive staining for CD68 but negative staining for both S-100 and acid-fast stain (Figure 3). These findings, along with clinical manifestations, were suggestive of MRH. Therefore, the patient was diagnosed with SLE with MRH on the basis of her history and relevant auxiliary examination.
She had a history of SLE for 12 years.
There was no clinically significant family history, the patient had no smoking or drinking habits, and she did not have a history of using illicit drugs or exposure to toxic substances.
A local round bulge could be seen near the clavicle in the IV region of the right neck, approximately 3 cm × 2 cm in size, soft in texture, and no tenderness. Multiple round masses around the upper part of the right humerus, bilateral fingers and toes were observed.
The anti-CCP antibody titer was 10 RU/mL; and the T-SPOT result was positive. ANA was strongly positive with a titer of 1:1000. A positive result was found for anti-centromere B antibody, anti-La/SS-B antibody, anti-SSA (52) and anti-SSA (60) antibody.
The white blood cells, red bloods cells, platelets, hemoglobin, erythrocyte sedimentation rate and hypersensitivity C-reactive protein levels are summarized in Table 1.
| Timeline | WBC (× 109/L) | RBC (× 1012/L) | PLT (× 109/L) | HGB (g/L) | ESR (mm/h) | hs-CRP (mg/L) | |
| August 2020 | 3.52 | 3.80 | 169 | 111 | 23↑ | 17↑ | |
| September 2020 | 4.79 | 2.89↓ | 270 | 82↓ | 28 | 79.38↑ | |
| October 2020 | 4.78 | 4.12 | 209 | 109↓ | 19 | 1.92 | |
| February 2021 | 2.53↓ | 3.79↓ | 110↓ | 111↓ | 21↑ | 10.79↑ | |
| May 2021 | 7.10 | 4.21 | 219 | 124 | 12 | 4.49 | |
| May 2022 | 4.56 | 3.83 | 209 | 125 | 10 | 3.50 | |
An X-ray of the hands showed bone erosion at the distal interphalangeal joints with the appearance of a pencil-in-cup deformity (Figure 2). MRI of the hip joint revealed ischemic necrosis and synovitis in the left femoral head.
The biopsy of the nodules on the distal interphalangeal joint of the right hand revealed fibrous hyperplasia with ground-glass degeneration, inflammatory cell exudation and focal necrosis. Urate deposition was observed within the fibrous area. The immunohistochemical stains showed positive staining for CD68 but negative staining for both S-100 and acid-fast stain (Figure 3).
The patient was diagnosed with SLE with MRH.
There is no specific treatment for this disease. Her treatment regimen consisted of 7.5 mg prednisone, 200 mg hydroxychloroquine and 10 mg leflunomide, all once a day orally to control the primary disease. After consultation with the Department of Bone Disease Orthopedics Oncology, a biopsy of the lump at the 4/5th distal interphalangeal joint of the right hand was performed under anesthesia, and the postoperative pathology was in line with MRH. Once a week, 10 mg methotrexate (MTX) was administered for treatment. However, poor response to MTX after 6 mo of treatment was recorded for the patient, and new nodules were detected on her hands and feet. Since the regimen was replaced with prednisone and 0.4 g CTX intravenous infusion once every two weeks accordingly, the patient has not complained about the new nodules anymore.
The current follow-up is one year, with no new systemic manifestations and few outbreaks of limited cutaneous nodular lesions.
MRH is a rare multisystem disease of unknown etiology. It can be divided into two types: reticular histiocytoma localized to the skin and erosive polyarthritis and nodular skin lesions[8]. The onset of the disease is insidious, mostly in middle-aged women approximately 40 years old[2]. Many patients are misdiagnosed with RA, and the following clues can help to understand the disease. Initially, polyarthritis is usually symmetric, and distal interphalangeal joints are affected in 75% of patients[9]. The skin is nonpruritic, and reddish-brown papules and nodules are seen on the forearms, forehead, neck and upper trunk, varying from a few millimeters to 2 cm in size[8]. Histopathological examination often reports multinucleated giant cell infiltration and foam cell formation, which need to be differential diagnoses with tuberculosis infection and lupus panniculitis. The histopathological examination of the patient reported foam cell formation. Considering the previous diagnosis of SLE, it is easy to misdiagnose it as lupus panniculitis that occurred predominantly on the fat-rich area, which was hardly suggestive of erosive arthritis. Immunohistochemical staining is positive for CD45 and CD68 but negative for S100 and CD1a, which is characteristic of Langerhans cells in MRH[9,10]. In addition, eosinophilic cytoplasm with a ground-glass appearance was also observed. All of these factors lead to the possibility of other proliferative and destructive diseases.
MRH has been reported to be associated with a variety of autoimmune diseases, which are often accompanied by RA, Sjogren’s syndrome, primary biliary cirrhosis, systemic vasculitis and SLE[2,9]. Other concomitant conditions include tuberculosis infection, tumor and gout, which were also recorded in our patient. A positive T spot result was reported in our case, consistent with a literature report that some patients have a positive skin PPD test[11]. Our patient was also diagnosed with a left fallopian tube cyst and underwent surgery one month later, consistent with the report that up to 25% of patients have reported malignancies, such as breast, cervical, ovarian, and stomach tumors[9,12]. Last, the pathological biopsy of our patient reported urate deposition, which may be because urate is prone to be deposited under acidic conditions, consistent with the report that the MRH patient has thyroid disease and gout[13,14].
MRH may be associated with various autoimmune diseases in approximately 29% of cases[6]. In our case report, laboratory findings, including high ANA titers (1:1000), elevated ESR, leukopenia and hypocomplementemia, were all suggestive of active SLE, and this patient consistently had small lumps on different parts of the body. When SLE was in a stable state, the progression of MRH also appeared to be in remission. This is consistent with the relationship between MRH and SLE activity reported by Saito et al[15]. The mechanism of association between MRH and SLE remains unknown, which may reflect a coincidence or a common pathogenesis[6]. However, it is interesting to show trends in the expression of class II human leukocyte antigen DR (HLA DR) and proinflammatory cytokine elevation, including tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and IL-12, which are secreted by macrophages and lymphocytes in both disorders. In SLE, the class II HLA DR genes play a role in conferring disease susceptibility, clinical and immunological expression, and levels of TNF-α, IL-6 and IL-12 are high in the serum of lupus patients compared to heathy controls[16,17]. Meanwhile, in MRH, the macrophage-specific marker of class II HLA DR is expressed in synovial fluid of mononuclear cells from patients with arthritis. Furthermore, quantitative amounts of the proinflammatory cytokines TNF-α, IL-6 and IL-12 are elevated in synovial fluid and serum[6]. Bennàssar et al[10] detected a decrease in proinflammatory cytokines after treatment in MRH. According to the above data, it is noteworthy that progression and resolution of MRH may correlate with SLE disease activity by the upregulation of soluble factors, such as cytokines, and hyperactivity of macrophages and lymphocytes, suggesting that immunological disorders might participate in the pathogenesis of MRH.
Given the rare and infrequent presentation of this disease, there are no established treatment guidelines for the management of MRH. Considering the high risk of progressive joint destruction and potentially disfiguring skin lesions, effective and timely therapy is crucial to patient outcomes. Limited evidence supports the use of nonsteroidal anti-inflammatory drugs and disease-modifying antirheumatic drugs commonly used in rheumatoid arthritis, including MTX, leflunomide, hydroxychloroquine, azathioprine, CTX and cyclosporin A, and in some cases combined with biologic treatment[6]. As MRH is associated with elevated TNF levels, anti-TNF agents are able to block TNF, and adalimumab and infliximab were proven to be valid for MRH treatment. Sarilumab and tocilizumab have been proposed as specific immunomodulators to treat MRH refractory patients based on the overexpression of IL-6 in the giant multinucleated cells of the MRH inflammatory infiltrate, which play a vital role in improving joints and dermatological manifestations. Meanwhile, a recent case report mentions that using anakinra (IL-1 inhibition) allowed for control of the disease[1]. In our patient, we initially treated with prednisone, hydroxychloroquine, leflunomide and MTX, but the patient failed to respond to MTX, with new nodules detected on her hands and feet after 6 mo of treatment. Since the regimen was replaced with prednisone and CTX, the patient has had a striking improvement in symptoms. It has been reported that CTX was of significant benefit in 20% of cases, with complete arthritis resolution, and in 27% of cases of skin lesions in MRH. Additionally, partial arthritis and skin disease control was seen in 40 and 45% of cases, respectively[1]. However, the precise mechanism of CTX was not documented, but it can be postulated. CTX may regulate MRH-related immunological abnormalities since it is known to inhibit the production of the proinflammatory cytokines IL-6 and IL-12, which are secreted by macrophages and lymphocytes[18,19]. This disrupts these cytokine-based communication networks between macrophages and lymphocytes. The cytokines IL-6 and IL-12 always rely on the JAK-STAT pathway to lead to the formation of granulomas and erosion of arthritis. CTX does not rule out reversing the progression of MRH through the JAK-STAT pathway[19].
MRH and SLE are both multisystem diseases. The involvement of erosive arthritis and skin papulo
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan; Poddighe D, Kazakhstan; Tanaka H, Japan S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Mariotti EB, Corrà A, Lemmi E, Laschi L, Aimo C, Quintarelli L, Volpi W, Nacci F, Verdelli A, Ruffo di Calabria V, Guiducci S, Caproni M. Multicentric Reticulohistiocytosis Associated with an Early Form of Systemic Lupus Erythematosus: A Case Report of a Rare Disease, with Mini Review of the Literature. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Saba R, Kwatra SG, Upadhyay B, Mirrakhimov AE, Khan FN. Multicentric reticulohistiocytosis presenting with papulonodular skin lesions and arthritis mutilans. Case Rep Rheumatol. 2013;2013:201563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Abdirakhmanova A, Sazonov V, Mukusheva Z, Assylbekova M, Abdukhakimova D, Poddighe D. Macrophage Activation Syndrome in Pediatric Systemic Lupus Erythematosus: A Systematic Review of the Diagnostic Aspects. Front Med (Lausanne). 2021;8:681875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Dall'Ara F, Cavazzana I, Frassi M, Taraborelli M, Fredi M, Franceschini F, Andreoli L, Rossi M, Cattaneo C, Tincani A, Airò P. Macrophage activation syndrome in adult systemic lupus erythematosus: report of seven adult cases from a single Italian rheumatology center. Reumatismo. 2018;70:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Henderson LA, Cron RQ. Macrophage Activation Syndrome and Secondary Hemophagocytic Lymphohistiocytosis in Childhood Inflammatory Disorders: Diagnosis and Management. Paediatr Drugs. 2020;22:29-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 6. | Selmi C, Greenspan A, Huntley A, Gershwin ME. Multicentric reticulohistiocytosis: a critical review. Curr Rheumatol Rep. 2015;17:511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, Smolen JS, Wofsy D, Boumpas DT, Kamen DL, Jayne D, Cervera R, Costedoat-Chalumeau N, Diamond B, Gladman DD, Hahn B, Hiepe F, Jacobsen S, Khanna D, Lerstrøm K, Massarotti E, McCune J, Ruiz-Irastorza G, Sanchez-Guerrero J, Schneider M, Urowitz M, Bertsias G, Hoyer BF, Leuchten N, Tani C, Tedeschi SK, Touma Z, Schmajuk G, Anic B, Assan F, Chan TM, Clarke AE, Crow MK, Czirják L, Doria A, Graninger W, Halda-Kiss B, Hasni S, Izmirly PM, Jung M, Kumánovics G, Mariette X, Padjen I, Pego-Reigosa JM, Romero-Diaz J, Rúa-Figueroa Fernández Í, Seror R, Stummvoll GH, Tanaka Y, Tektonidou MG, Vasconcelos C, Vital EM, Wallace DJ, Yavuz S, Meroni PL, Fritzler MJ, Naden R, Dörner T, Johnson SR. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 885] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 8. | Farokhi A, van Vugt RM, Hoekzema R, Nurmohamed MT. Multicentric reticulohistiocytosis: a case report. BMC Res Notes. 2018;11:647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Trotta F, Colina M. Multicentric reticulohistiocytosis and fibroblastic rheumatism. Best Pract Res Clin Rheumatol. 2012;26:543-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Bennàssar A, Mas A, Guilabert A, Julià M, Mascaró-Galy JM, Herrero C. Multicentric reticulohistiocytosis with elevated cytokine serum levels. J Dermatol. 2011;38:905-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Pacheco-Tena C, Reyes-Cordero G, Ochoa-Albíztegui R, Ríos-Barrera V, González-Chávez SA. Treatment of multicentric reticulohistiocytosis with tocilizumab. J Clin Rheumatol. 2013;19:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Martínez-Martínez MU, Baranda-Cándido L, Abud-Mendoza C. Cutaneous papillomavirus infection in patients with rheumatoid arthritis or systemic lupus erythematosus. A case-control study. Lupus. 2013;22:948-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Rooney PJ. Hyperlipidemias, lipid storage disorders, metal storage disorders, and ochronosis. Curr Opin Rheumatol. 1991;3:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Horvath JR, Hoffman GS. Multicentric reticulohistiocytosis: a mimic of gout and rheumatoid arthritis. Cleve Clin J Med. 1999;66:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Saito K, Fujii K, Awazu Y, Nakayamada S, Fujii Y, Ota T, Tanaka Y. A case of systemic lupus erythematosus complicated with multicentric reticulohistiocytosis (MRH): successful treatment of MRH and lupus nephritis with cyclosporin A. Lupus. 2001;10:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Robak E, Sysa-Jedrzejowska A, Robak T. Cytokiny w układowym toczniu rumieniowatym [Cytokines in systemic lupus erythematosus]. Przegl Lek. 1996;53:623-626. [PubMed] |
| 17. | Azizah MR, Ainoi SS, Kuak SH, Kong NC, Normaznah Y, Rahim MN. The association of the HLA class II antigens with clinical and autoantibody expression in Malaysian Chinese patients with systemic lupus erythematosus. Asian Pac J Allergy Immunol. 2001;19:93-100. [PubMed] |
| 18. | Perini P, Calabrese M, Rinaldi L, Gallo P. Cyclophosphamide-based combination therapies for autoimmunity. Neurol Sci. 2008;29 Suppl 2:S233-S234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Theisen-Popp P, Pape H, Müller-Peddinghaus R. Interleukin-6 (IL-6) in adjuvant arthritis of rats and its pharmacological modulation. Int J Immunopharmacol. 1992;14:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |