Published online Jan 16, 2023. doi: 10.12998/wjcc.v11.i2.441
Peer-review started: October 9, 2022
First decision: December 13, 2022
Revised: December 21, 2022
Accepted: January 3, 2023
Article in press: January 3, 2023
Published online: January 16, 2023
Processing time: 94 Days and 21.6 Hours
Lung invasive mucinous adenocarcinoma (LIMA), formerly referred to as mucinous bronchioloalveolar carcinoma, is a rare disease that usually presents as bilateral lung infiltration, is unsuitable for surgery and radiotherapy, and shows poor response to conventional chemotherapy.
We report a 56-year-old Chinese man with a history of smoking and epidermal growth factor receptor mutation-positivity who was initially misdiagnosed as severe pneumonia, but was ultimately diagnosed as a case of invasive mucinous adenocarcinoma of the lung by computed tomography -guided percutaneous lung biopsy. Bronchorrhea and dyspnea were improved within 24 h after initiation of gefitinib therapy and the radiographic signs of bilateral lung consolidation showed visible improvement within 30 d. After more than 11 months of treatment, there is no evidence of recurrence or severe adverse events.
Although the precise mechanism of the antitumor effects of gefitinib are not clear, our experience indicates an important role of the drug in LIMA and provides a reference for the diagnosis and treatment of this disease.
Core Tip: Gefitinib improves severe bronchorrhea and prolongs the survival of a patient with lung invasive mucinous adenocarcinoma.
- Citation: Ou GC, Luo W, Zhang WS, Wang SH, Zhao J, Zhao HM, Qiu R. Gefitinib improves severe bronchorrhea and prolongs the survival of a patient with lung invasive mucinous adenocarcinoma: A case report. World J Clin Cases 2023; 11(2): 441-448
- URL: https://www.wjgnet.com/2307-8960/full/v11/i2/441.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i2.441
Lung cancer is the second most common cancer worldwide and the leading cause of cancer-related deaths, accounting for almost one-quarter of all cancer-related deaths[1]. Non-small cell lung cancer (NSCLC) is the main pathological type of lung cancer, accounting for about 80%–85% of all cases of lung cancer[2,3]. In the 2015 World Health Organization classification of lung tumors, lung invasive mucinous adenocarcinoma (LIMA) is classified as a variant of invasive lung adenocarcinoma, which accounts for approximately 5%–10% of lung adenocarcinomas[4]. The diagnostic hallmark of invasive mucinous adenocarcinoma (IMA) is tall columnar cell morphology with abundant intracellular/ extracellular mucus and invasive adenocarcinoma patterns (such as acinar, lepidic, papillary, and predominant patterns)[5]. The clinical presentation of LIMA is different from that of other subtypes of adenocarcinoma. LIMA typically shows diffuse lung involvement, which results in severe respiratory symptoms, such as bronchorrhea and dyspnea[6,7]. In addition, LIMA also tends to be resistant to conventional chemotherapy, and patients typically have a short survival time after detection of the cancer[8].
Recent years have witnessed important breakthroughs in the treatment of NSCLC, which has evolved from traditional surgery, chemotherapy, and radiotherapy to the era of molecular targeted therapy and immunotherapy. The development of epidermal growth factor receptor (EGFR) targeted tyrosine kinase inhibitors (TKIs) have helped facilitate the concept of personalized cancer therapy into a reality[9]. Several studies have demonstrated high antitumor activity of EGFR-TKIs against bronchioloalveolar carcinoma and the therapeutic response can be dramatic and long-lasting[10,11]. Gefitinib is a reversible EGFR targeted TKI that competes for Mg-ATP binding sites in the EGFR catalytic region, thereby blocking signaling, inhibiting cancer cell proliferation, and inhibiting mitogen-activated protein kinase activation, inducing cancer cell apoptosis[12].
Here, we report a patient with LIMA who was successfully treated with gefitinib. Bronchorrhea and dyspnea were improved within 24 h after treatment with gefitinib, and the radiographic signs of bilateral lung consolidation were visibly improved within 30 d. As of 11 mo after initiation of gefitinib, there are no signs of tumor progression.
On October 29, 2021, a 53-year-old man presented to the department of respiratory medicine at our hospital due to shortness of breath and excessive sputum production for 3 mo.
As shown in Figure 1, the patient expectorated large amount of white and foamy sputum (approximately 700 mL/d), accompanied by chest tightness and shortness of breath. He was earlier treated with oral amoxicillin and expectorants, but the results were not satisfactory.
The patient had no prior chronic disease, but had recently lost 2 kg of his body weight.
He was a chronic smoker (20 cigarettes per day for more than 10 years). The patient denied having a family history of lung cancer, hypertension, or coronary heart disease.
Vital signs at admission were as follows: Body temperature, 37.8°C; heart rate, 114/min; respiratory rate, 30/min; blood pressure, 147/110 mmHg; oxygen saturation, 90% (under nasal cannula 5 L/min oxygen inhalation). Physical examination revealed coarse crackles in both lungs.
Blood gas analysis showed respiratory failure with an oxygen partial pressure (PaO2) of 54 mmHg and oxygenation index of 129 mmHg. Conventional inflammation markers [C-reactive protein: 1.37 mg/mL (reference range, 0–10), procalcitonin: 0.05 ng/mL (< 0.05), white blood cell count: 10.9 × 109/L (4–10 × 109), absolute neutrophil count: 6.35 × 109/L (1.8–6.3 × 109), and neutrophil percentage: 58.3% (40%–75%)] were measured on the day of clinical examination. No obvious abnormalities were found in coagulation, sputum smear microscopy, sputum culture, tuberculosis bacilli gamma interferon release test, rheumatoid factor, liver and kidney function, tumor markers (carcinoembryonic antigen, cytokeratin, neuron-specific enolase), connective tissue related antibodies, or anti-neutrophil cytoplasmic antibody.
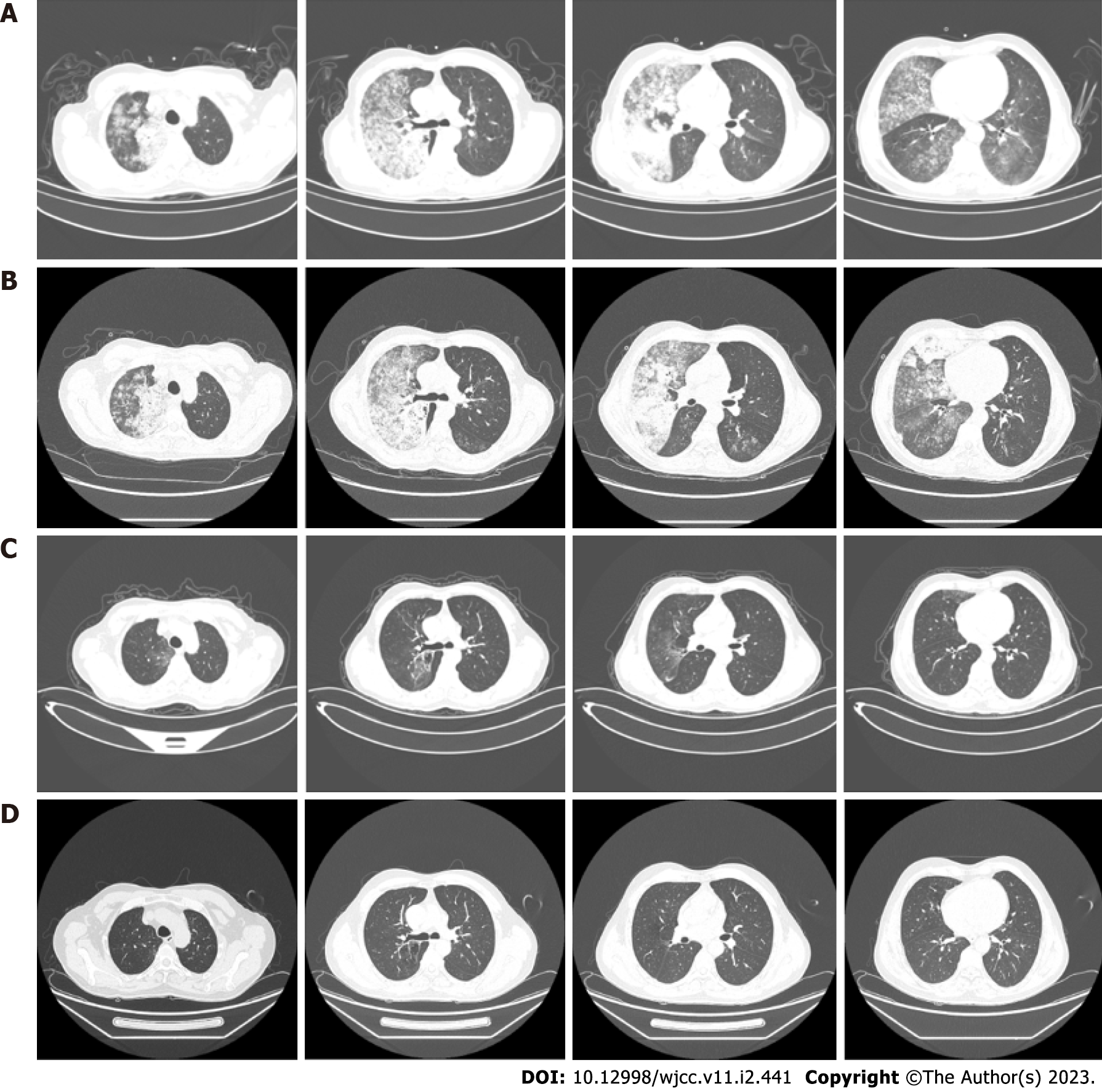
Chest high-resolution computed tomography scan showed large flaky hyperintense shadows in both lungs, especially in the right lung (Figure 2).
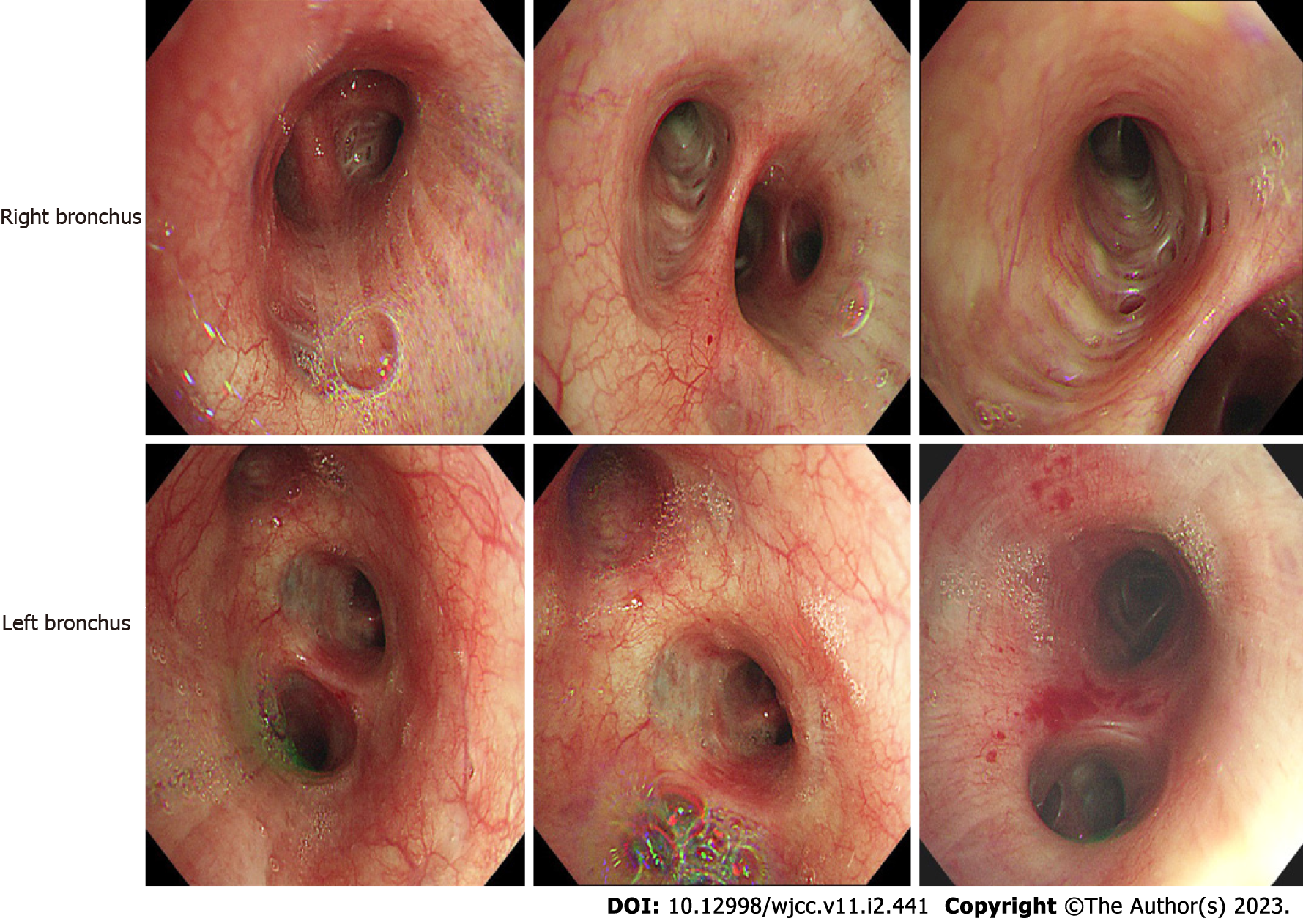
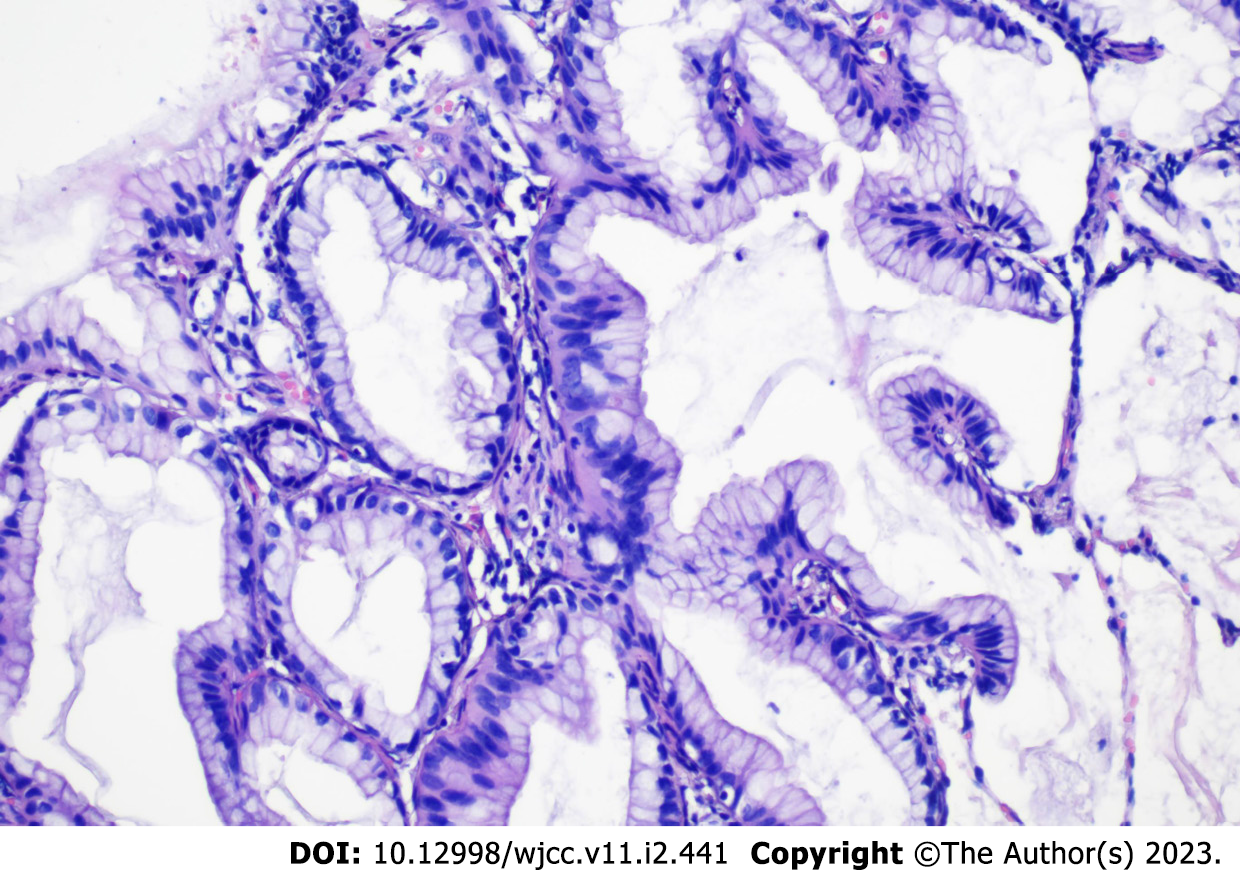
The clinical picture was suggestive of infectious pneumonia and he was administered routine anti-infection, expectorant, and antispasmodic treatment. However, the patient showed no symptomatic improvement after 3 d of treatment. Bronchoscopy was performed to further clarify the cause, which showed a lot of white secretions in the trachea (Figure 3). The right lower lobe was lavaged and sent for next-generation sequencing (NGS). However, NGS results showed no pathogens. Repeat chest computed tomography (CT) performed on the 7th day of treatment showed an increase in the lung solid lesions compared with those on admission, while there was no significant change in inflammatory marker levels. Subsequently, the patient underwent CT-guided percutaneous lung biopsy, which confirmed the diagnosis of LIMA (Figure 4). In addition, the tissue specimen was subjected to EGFR analysis. Systematic investigations revealed no metastases other than the lungs, and based on clinical and histological findings, the patient was diagnosed with stage IV disease. Fortunately, genetic testing revealed positive EGFR-21 mutation (c.2573T>G:p.L858R).
Other medications were discontinued, and treatment with an EGFR-tyrosine kinase inhibitor, gefitinib (250 mg/d) was commenced.
Twenty-four hours after initiation of treatment, the patient showed remarkable improvement in symptoms with alleviation of dyspnea and bronchorrhea. Arterial blood gas analysis showed a PaO2 of 81 mmHg and oxygenation index of 279 mmHg upon nasal inhalation of 2 L/min O2. The volume of sputum discharged decreased from 700 to 300 mL/d, and 3 d later it was further reduced to 100 mL/d. Chest CT scan showed remarkable improvement of infiltration 30 d (Figure 2) after treatment. After more than 11 mo of treatment (Figure 2), there was no evidence of recurrence or severe adverse events.
Adenocarcinoma is the most commonly diagnosed pathological subtype of NSCLC. LIMA was recognized as a distinct subtype of lung adenocarcinoma with unique biological properties, as well as a different prognosis compared with other lung adenocarcinoma subtypes[13]. Previous studies have shown that LIMA may originate from type II alveolar epithelial or Clara cell metaplastic lesions, and further develop atypical hyperplasia and carcinogenesis[14,15]. Tumor cells in IMAs are highly discrete, and show poor adhesion; These easily diffuse widely through alveolar pores and small airways, which is an important reason for their diffuse distribution[16,17]. In a study by Cha et al[18], 13.9% of IMAs were initially identified as pneumonia, which is much higher than the rate of initial misdiagnosis of invasive non-mucinous adenocarcinomas as pneumonia. Nie et al[19] analyzed the data of 40 patients with mucinous adenocarcinoma who underwent surgical resection; they found that 25% patients showed either pneumonia-like consolidation or multifocal patchy consolidation on chest CT. Invasive mucinous adenocarcinoma and pneumonia are not easy to distinguish due to the large overlap of clinical features, including cough and sputum symptoms[20].
This was also an important reason why this case was initially misdiagnosed as pneumonia. For middle-aged and elderly patients, those with pulmonary infiltrates that are not absorbed by anti-infection treatment, and who continue to experience cough with expectoration of large amount of white sputum, the possibility of pneumonia-type lung cancer should be considered and a pathological diagnosis obtained. Pathology is still the gold-standard for the diagnosis of the disease, and the accurate diagnosis of LIMA is a key imperative to initiate appropriate treatment and improve the prognosis.
Advances in the field of molecular profiling and targeted therapies have brought about a paradigm shift in the treatment landscape for NSCLC[21]. Lung adenocarcinoma patients with genetic mutations can achieve prolonged survival with corresponding targeted therapy. According to previous studies reporting gene mutation analysis in the context of LIMA, KRAs are the most common mutation type accounting for approximately 35%–75%, while the incidence of Tp53 mutation is approximately 46%; the incidence of EGFR mutation, ALK rearrangement, and BRAF mutation is very low[22-24]. However, several studies have demonstrated high antitumor activity of EGFR-TKIs against LIMA, and the therapeutic response can be dramatic and long-lasting. In 2013, López-González et al[25] described a 66-year-old woman diagnosed with right lung IMA who tested positive for EGFR mutation; treatment with gefitinib led to significant shrinkage of lesions, and the patient showed a long progression-free survival. Moreover, Pastorino et al[26] claimed that long-term gefitinib therapy may be effective in the management of atypical adenomatous hyperplasia and multifocal IMA presenting as diffuse ground glass opacities, when fluorescence in situ hybridization or molecular testing are indicative of sensitivity.
Our patient was EGFR mutation-positive for which the recommended treatment is gefitinib. This treatment resulted in a rapid reduction of bronchorrhea, associated with a dramatic improvement in dyspnea, hypoxia, and radiographic abnormalities. Gefitinib is an orally-active, selective EGFR-TKI that blocks signal transduction pathways implicated in the proliferation and survival of cancer cells[27]. Of note, some studies have reported contradictory findings about LIMA without EGFR mutation treated with gefitinib[28,29].
At present, the exact mechanism of action of targeted drugs for tumors is not fully understood. Owing to the low number of patients treated with these drugs, further research and follow-up studies are required to obtain more definitive evidence.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sánchez JIA, Colombia S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11909] [Article Influence: 2977.3] [Reference Citation Analysis (4)] |
| 2. | Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1607] [Cited by in RCA: 3075] [Article Influence: 439.3] [Reference Citation Analysis (0)] |
| 3. | Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. J Thorac Oncol. 2016;11:1653-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 434] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 4. | Zhang G, Wang X, Jia J, Zuo Z, Wang L, Gao S, Xue L, Xue Q. Development and validation of a nomogram for predicting survival in patients with surgically resected lung invasive mucinous adenocarcinoma. Transl Lung Cancer Res. 2021;10:4445-4458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Wang T. Double-mutant invasive mucinous adenocarcinoma of the lung in a 32-year-old male patient: A case report. World J Clin Cases. 2021;9:11078-11084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Shao K, Wang Y, Xue Q, Mu J, Gao Y, Wang B, Zhou L, Gao S. Clinicopathological features and prognosis of ciliated muconodular papillary tumor. J Cardiothorac Surg. 2019;14:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Shim HS, Kenudson M, Zheng Z, Liebers M, Cha YJ, Hoang Ho Q, Onozato M, Phi Le L, Heist RS, Iafrate AJ. Unique Genetic and Survival Characteristics of Invasive Mucinous Adenocarcinoma of the Lung. J Thorac Oncol. 2015;10:1156-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Kitazaki T, Fukuda M, Soda H, Kohno S. Novel effects of gefitinib on mucin production in bronchioloalveolar carcinoma; two case reports. Lung Cancer. 2005;49:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Manson GV, Ma PC. Response to pemetrexed chemotherapy in lung adenocarcinoma-bronchioloalveolar carcinoma insensitive to erlotinib. Clin Lung Cancer. 2010;11:57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Yano S, Kanematsu T, Miki T, Aono Y, Azuma M, Yamamoto A, Uehara H, Sone S. A report of two bronchioloalveolar carcinoma cases which were rapidly improved by treatment with the epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 ("Iressa"). Cancer Sci. 2003;94:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Kijima T, Suzuki M, Ueda K, Minami S, Takeda Y, Goya S, Matsuoka H, Kumagai T, Yoshida M, Osaki T, Tachibana I, Yokota S, Kawase I. Short-term gefitinib treatment brought about a long-term regression of bronchioloalveolar carcinoma without EGFR gene alterations: a case report. Oncol Res. 2007;16:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Kumar P, Mangla B, Javed S, Ahsan W, Musyuni P, Ahsan A, Aggarwal G. Gefitinib: An Updated Review of its Role in the Cancer Management, its Nanotechnological Interventions, Recent Patents and Clinical Trials. Recent Pat Anticancer Drug Discov. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Cai D, Li H, Wang R, Li Y, Pan Y, Hu H, Zhang Y, Gong R, Pan B, Sun Y, Chen H. Comparison of clinical features, molecular alterations, and prognosis in morphological subgroups of lung invasive mucinous adenocarcinoma. Onco Targets Ther. 2014;7:2127-2132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Kim YK, Shin DH, Kim KB, Shin N, Park WY, Lee JH, Choi KU, Kim JY, Lee CH, Sol MY, Kim MH. MUC5AC and MUC5B enhance the characterization of mucinous adenocarcinomas of the lung and predict poor prognosis. Histopathology. 2015;67:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Sumiyoshi S, Yoshizawa A, Sonobe M, Kobayashi M, Sato M, Fujimoto M, Tsuruyama T, Date H, Haga H. Non-terminal respiratory unit type lung adenocarcinoma has three distinct subtypes and is associated with poor prognosis. Lung Cancer. 2014;84:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Suzuki S, Aokage K, Hishida T, Yoshida J, Kuwata T, Yamauchi C, Tsuboi M, Ishii G. Interstitial growth as an aggressive growth pattern in primary lung cancer. J Cancer Res Clin Oncol. 2016;142:1591-1598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Masai K, Sakurai H, Sukeda A, Suzuki S, Asakura K, Nakagawa K, Asamura H, Watanabe SI, Motoi N, Hiraoka N. Prognostic Impact of Margin Distance and Tumor Spread Through Air Spaces in Limited Resection for Primary Lung Cancer. J Thorac Oncol. 2017;12:1788-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Cha YJ, Kim HR, Lee HJ, Cho BC, Shim HS. Clinical course of stage IV invasive mucinous adenocarcinoma of the lung. Lung Cancer. 2016;102:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Nie K, Nie W, Zhang YX, Yu H. Comparing clinicopathological features and prognosis of primary pulmonary invasive mucinous adenocarcinoma based on computed tomography findings. Cancer Imaging. 2019;19:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Koo HJ, Kim MY, Koo JH, Sung YS, Jung J, Kim SH, Choi CM, Kim HJ. Computerized margin and texture analyses for differentiating bacterial pneumonia and invasive mucinous adenocarcinoma presenting as consolidation. PLoS One. 2017;12:e0177379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Chi K, Sun W, Yang X, Wu J, Wang H, Liu X, Mao L, Zhou L, Huang X, Lin D. A prognostic classification based on the International Association for the Study of Lung Cancer histologic grading and immunoscore in KRAS-mutant invasive non-mucinous adenocarcinoma. Thorac Cancer. 2022;13:1050-1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Boland JM, Maleszewski JJ, Wampfler JA, Voss JS, Kipp BR, Yang P, Yi ES. Pulmonary invasive mucinous adenocarcinoma and mixed invasive mucinous/nonmucinous adenocarcinoma-a clinicopathological and molecular genetic study with survival analysis. Hum Pathol. 2018;71:8-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Righi L, Vatrano S, Di Nicolantonio F, Massa F, Rossi G, Cavazza A, Volante M, Votta A, Izzo S, Lo Iacono M, Ardissone F, Di Maio M, Novello S, Scagliotti GV, Papotti M. Retrospective Multicenter Study Investigating the Role of Targeted Next-Generation Sequencing of Selected Cancer Genes in Mucinous Adenocarcinoma of the Lung. J Thorac Oncol. 2016;11:504-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Watanabe H, Saito H, Yokose T, Sakuma Y, Murakami S, Kondo T, Oshita F, Ito H, Nakayama H, Yamada K, Iwazaki M. Relation between thin-section computed tomography and clinical findings of mucinous adenocarcinoma. Ann Thorac Surg. 2015;99:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | López-González A, Almagro E, Salas C, Varela A, Provencio M. Use of a tyrosine kinase inhibitor as neoadjuvant therapy for non-small cell lung cancer: A case report. Respir Med Case Rep. 2013;9:8-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Pastorino U, Calabrò E, Tamborini E, Marchianò A, Orsenigo M, Fabbri A, Sozzi G, Novello S, De Marinis F. Prolonged remission of disseminated atypical adenomatous hyperplasia under gefitinib. J Thorac Oncol. 2009;4:266-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749-5754. [PubMed] |
| 28. | Popat N, Raghavan N, McIvor RA. Severe bronchorrhea in a patient with bronchioloalveolar carcinoma. Chest. 2012;141:513-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Taja-Chayeb L, Candelaria M, Brom R, Trejo-Becerril C, Meza F, Duenas-Gonzalez A. Response to gefitinib in bronchioloalveolar carcinoma in the absence of EGFR mutation. Lung Cancer. 2005;50:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |